ABSTRACT
Fluorescence spectroscopy and computational methods were used to study the interaction between anthocyanins from sour cherries extract and bovine β-lactoglobulin. The experimental tests indicated that the number of binding sites was lower than 1, suggesting weak binding of anthocyanins by β-lactoglobulin, most probably as the result of the multitude of compounds present in the extract. Intrinsic and extrinsic fluorescence experiments have highlighted blue- and red-shifts in maximum emission. In agreement with the fluorescence spectroscopy findings, the in silico results suggested better affinity of the protein preliminary treated at high temperature for the anthocyanin. The molecular docking tests performed using the cyanidin 3-rutinoside as the ligand, one of the major components of anthocyanin extract indicated that the driving forces leading to complex formation mainly involve diamino and nonpolar neutral amino acids on the surface of the protein. The stability of the complex appeared to be mainly ensured by hydrophobic interactions and hydrogen bonds.
Introduction
The amino acid composition of whey proteins (high proportion of Cys, Trp, branched amino acids), their ability to bind various ligands, and the presence of bioactive peptides within their sequence give them excellent nutritional properties.[Citation1] Additionally, whey proteins have important techno-functional properties in food systems such as their ability to act as emulsifiers, gelling, and texturing agents.[Citation2] The globular protein β-lactoglobulin (β-LG) is found in the whey fraction of the milk of many mammals. β-LG is a small globular protein with 162 amino acid residues, having a molecular mass of 18,400 Da. The protein is classified as a member of the lipocalin-protein family because of its high affinity to bind small hydrophobic ligands.[Citation3] In cow milk, β-LG is present as a noncovalent dimer, but the dimeric structure is not required for binding of biologically relevant molecules like retinol.[Citation4]
The tertiary structure of β-LG is dominated by the β-barrel, and consists of nine anti-parallel β-sheets and a major α-helix at the C-terminal end of the polypeptide chain.[Citation5] The β-barrel forms a hydrophobic cavity inside the protein, where hydrophobic compounds can be bound.[Citation6] The β-barrel consists of two β-sheets, where strands A to D form one sheet, and strands E to H form the other (with some participation from strand A, facilitated by a 90° bend at Ser21). The loop EF, that connects strands E and F at the open end of the β-barrel, acts as a gate.[Citation3,Citation7] Harvey et al.[Citation8] suggested that β-LG contains three binding sites for different hydrophobic molecules: the first one is in the central cavity, known as calyx, the second is the surface cleft which lies between the α-helix and the surface of the barrel, and the third one is located at the monomer/monomer interface.
Epidemiological, clinical and animal studies showed that regular consumption of polyphenol-rich foods ensure the prevention of cardiovascular and neurodegenerative diseases, osteoporosis and, possibly, cancer[Citation9]. Anthocyanins are a group of naturally occurring phenolic compounds, which are responsible for the attractive colours of many fruits (particularly in berries) and related products derived from them. These polyphenolic substances are glycosides of polyhydroxy- and polymethoxy-derivatives of 2-phenyl benzopyrylium or flavilium salts. Anthocyanins are becoming increasingly important as antioxidants,[Citation10,Citation11] and they play a potentially important role in human health.[Citation12] However, anthocyanins easily degrade during food processing and storage, being highly sensitive to factors such as: light, pH, temperature, presence of oxygen and enzymes.[Citation13] Nevertheless, the use of anthocyanins in the development of food colorants and healthy and/or functional ingredients has been limited because of their low stability under given environmental conditions and interaction with other compounds in the food matrix. Attempts to improve the bioavailability of phenolics have been reported by developing complexes with different carriers, such as: β-LG,[Citation3,Citation14] serum albumin,[Citation15] whey proteins,[Citation16] α-lactalbumin,[Citation17] and liposomes.[Citation18] Therefore, the binding studies correlated with molecular dynamics and docking tests are needed as prerequisites for better understanding the ligand-receptor interactions from the microencapsulation perspective. Microencapsulation is a technique aimed to protect the biological active compounds through coating, requiring the correct choice of the wall material, the core release form and the encapsulation method.
Sour cherries (Prunus cerasus L) are rich in phenolic compounds, which display a broad spectrum of health promoting effects, such as antioxidant and anti-inflammatory activities,[Citation19] inhibited intestinal tumour in ApcMin mice and reduced proliferation of human colon cancer cells.[Citation20] Fluorescence spectroscopy, which is a useful method to study the ligand-protein interaction since it evaluates the binding parameters and establish the forces involved in interaction, was successfully used to study the interaction between different polyphenolics such as naringenin, hesperetin and apigenin with bovine serum albumin,[Citation21] naringenin with β-LG,[Citation14] curcumin with β-LG,[Citation3] pelargonidin with whey proteins,[Citation22] etc.
Heating (depending on time, temperature, rate of heating) might induce important structural alterations of proteins conformation that affect their functionality. Therefore, the knowledge of the binding ability of β-LG is of great importance for proper understanding of the functional component-protein interactions, and therefore for estimating the potential therapeutic and/or technological applications.[Citation23] The interaction between anthocyanins extract from sour cherries (ASC) skins and heat treated bovine β-LG was here investigated based on quenching experiments. In our previous report, the major anthocyanins in sour cherries extract were identified as cyanidin-3-rutinoside (8.20 ± 0.201 mg/100 g d.w.), followed by peonidin-3-glucoside (3.81 ± 0.068 mg/100 g d.w.) and cyanidin-3-glucoside (0.93 ± 0.004 mg/100 g d.w.).[Citation24]
Furthermore, in order to deepen understanding the effect of thermal treatment on the β-LG-ASC complex in relation with protein structural changes, the in situ fluorescence spectroscopy involving the use of intrinsic and extrinsic intensity fluorescence, phase diagram, synchronous spectra, three-dimensional fluorescence spectroscopy and quenching experiments was performed. Finally, in silico tests were employed to complement the experimental results. In particular, molecular dynamics simulations and molecular docking were used to provide details on affinity and binding site of β-LG for cyanidin 3-rutinoside, one of the major components of anthocyanin extract from sour cherry.[Citation25] The computational techniques are crucial for understanding the functionality of different compounds within complex matrices. They offer the possibility to mimic complex experimental set-ups while providing atomic level details, which enrich the knowledge on the mechanisms of the interaction between different molecules in model systems. Our study aimed to provide detailed descriptions of the interactions mechanism between anthocyanins from sour cherries skins extract and the major protein from whey from the perspective of developing new functional composites for food applications.
Materials and methods
Materials
β-LG (purity > 90%, genetic variants A and B) from bovine milk, 1-anilino-8-naphtalenesulphonic acid (ANS), acrylamide and potassium iodide (KI) were purchased from Sigma (Sigma–Aldrich Co., St. Louis, MO). Sour cherries (Prunus cerasus) belonging to Timpurii de Cluj variety were purchased from the local market (Galați) in the months of June - July of the year 2015. Fruit samples were washed using a cherries:water ratio of 1:2 (w/w). The skins and seeds were manually separated from the pulp, washed with distilled water and then blotted on paper towels to remove any residual pulp. Skins were freeze-dried (Alpha 1-4 LD plus, Martin Christ, Germany) and stored at –20°C until analyses.
Anthocyanins extraction from sour cherries skins
The extraction of phenols from freeze-dried sour cherry was performed according to the procedure described by Turturică et al.[Citation26] In brief, 1 g of freeze-dried sour cherry skins were homogenized with 8 mL of ethanol (70%) and placed on an orbital shaker at room temperature for 4 h. The supernatant was collected and centrifuged at 11800 × g, and 10°C for 10 min. The supernatant was then concentrated under reduced pressure at 35°C to dryness (AVC 2-18, Christ, UK). The solutions were obtained by dissolving 1 g of extract in 10 mL of MilliQ water.
Phytochemicals analysis
Total monomeric anthocyanins (TAC) content in sour cherries extract was estimated according to our earlier report.[Citation26]
Preparation of the β-LG-ASC complex
To obtain protein solution, β-LG was weighed and dissolved in 20 mM Tris-HCl buffer solution (pH 7.5) at a concentration of 1 mg/ml. β-LG-ASC complex was prepared by simple mixing of the two components. The ASC extract was added to the protein solution to reach a final protein/ASC molar ratio of 1:1.
Heat treatment
Plastic tubes (1 cm diameter) were filled with 0.2 mL of β-LG solutions containing 1 mg/mL− protein solution in 20 mM Tris HCl buffer at pH 7.5. The samples were heated at different temperatures ranging from 25 to 100°C for 15 min using a thermostatic water bath (Digibath-2 BAD 4, Raypa Trade, Barcelona, Spain). The protein was then cooled by introducing the tubes in ice water, such as to avoid further thermal denaturation. In order to investigate the effect of the thermal treatment on the complex, 0.2 mL of the β-LG-ASC complex were heated as described above.
Prior to fluorescent measurements, a volume of 100 µL of thermally treated β-LG/β-LG-ASC complex solutions were suspended in 2.5 mL 20 mM Tris buffer, pH 7.5.
Quenching experiments
All the fluorescence investigations were performed on a LS-55 luminescence spectrometer (Perkin Elmer Life Sciences, Shelton, CT, USA) equipped with the software Perkin Elmer FL Winlab. For the quenching experiments with ASC, the excitation wavelength was set at 292 nm while the emission spectra were collected from 310 nm to 400 nm, with increments of 0.5 nm. Both the excitation and emission slit widths were set at 10 nm. A volume of 100 µL of thermally treated protein solution was suspended in 2.5 mL Tris buffer of 20 mM concentration at pH 7.5 and titrated by successive addition of ASC. The Stern–Volmer constants, binding constants, number of binding sites and thermodynamic parameters were calculated as previously reported.[Citation23]
Fluorescence spectroscopy investigations of the β-LG-ASC complex
Intrinsic and extrinsic fluorescence, phase diagram, synchronous spectra, 3-dimensional spectral measurements and quenching experiments using acrylamide and KI of the β-LG-ASC complex were performed as previously described by Aprodu et al.[Citation27] The fluorescence quenching data were analysed by fitting to the Stern–Volmer equation as described by Dumitraşcu et al.[Citation23]
Set up of the in silico investigations
In order to estimate the particularities of the interaction between β-LG and anthocyanins at single molecule level, molecular mechanics, molecular dynamics and docking tests were performed using as models the three dimensional structure of β-LG from RCSB Protein Data Bank (PDB ID: 4DQ3,[Citation28] and of cyanidin 3-rutinoside (CYR) molecule. The effect of the thermal treatment on β-LG structure was simulated after optimizing the protein model using steepest-descent algorithm. The molecular dynamics was employed for heating the solvated protein at 25°C and 90°C through weak coupling each component of the system to a Berendsen thermostat to control the temperature. Further equilibrating dynamics was long enough to reduce any significant temperature and energy oscillations. Only the temperatures indicated by the experimental approach as significant for β-LG interaction with anthocyanin were considered in the in silico test. The molecular mechanics and molecular dynamics simulations were performed by means of GROMACS 4.6 package[Citation29] on an Intel® Core™ 2 CPU 6300 1.86 GHz processor-based machine running Linux. Gromos43a1 force field was used to define the topology, and periodic boundary conditions were applied along the xyz coordinates.
The β-LG molecules equilibrated at different temperatures were then used as receptor in the shape complementarity-based docking procedure, whereas the ligand was CYR molecule. The top ten rigid-body docking solutions generated by PatchDock algorithm[Citation30] were further used as input for the FireDock algorithm, resulting in the refinement of the β-LG – CYR complexes through adjusting the relative orientation of the interfacing atoms of the two molecules. A final ranking was carried out, taking into account the binding energy values which include softened attractive and repulsive van der Waals energy.[Citation31] The best fits were then used as models for investigating the interactions between thermally treated β-LG and CYR molecules. The LigPlot+ tool[Citation32] was employed for checking the atomic level contacts between the two molecules within each complex.
Results and discussion
Fluorescence quenching mechanism of heat treated β-LG by ASC
The interactions between β-LG and ASC were examined by following the influence of increasing concentration of ASC on the fluorescence intensity spectra of β-LG preliminary treated at temperatures ranging from 25°C to 100°C for 15 min. Regardless of the intensity of the thermal treatment, the fluorescence of the protein was significantly quenched with increasing the ligand concentration ().
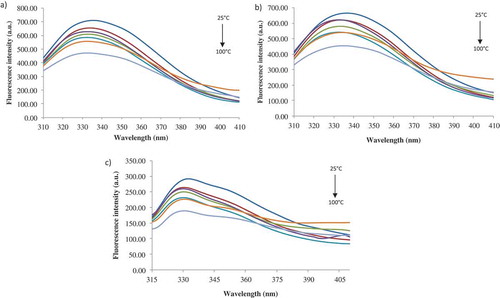
Figure 1. The fluorescence spectra of the interaction between β-LG thermally treated at 25°C (a), 50°C (b) and 90°C (c) and ASC. The ASC concentration (from a-f) varied from 0 to 0.093 µM 25°C.
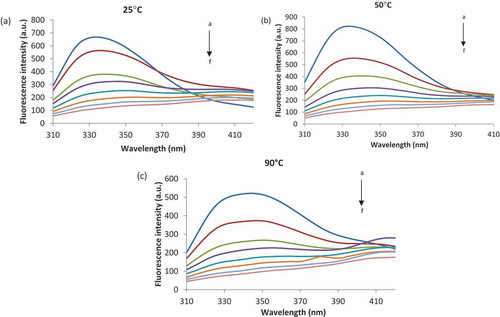
The addition of 0.093 µM of ASC to native β-LG () led to a red shift of 75 nm in maximum position (λmax). In the temperature range of 50°C to 90°C ( and ), significant red-shifts between 78 nm and 76 nm were found. These results suggest that interactions between β-LG and ASC lead to modifications in the polarity of the environment around Trp residues present in the polypeptide chains.[Citation33] Furthermore, the appreciable spectral red shift suggests that Trp residues in the protein became more exposed to the hydrophilic solvent as a result of the interaction with ASC.[Citation34]
The Stern–Volmer plots for the quenching of β-LG fluorescence by ASC are depicted in , and the estimated Stern–Volmer constant (KSV) at different temperatures are listed in . The lowest KSV value was calculated for the sample treated at 100°C (0.23 ± 0.03 × 106 M−1) and the highest at 80°C (0.59 ± 0.15 × 106 M−1), suggesting that the degree of exposure of Trp to ASC is increasing with the temperature up to 80°C, and decreases afterwards at even higher temperatures. To distinguish static and dynamic quenching, the comparison of the values of KSV at different temperatures was performed. It can be seen that the values of KSV decreased with the increase of temperature, representing the static quenching mechanism. Gholami and Bordbar[Citation14] explained that at higher temperatures due to faster motions, dissociation of weakly bound complexes increases, and consequently, the Stern–Volmer constants decrease. These authors reported KSV values for binding of naringenin to bovine β-LG of 0.1370 × 106 M−1 at 25°C, whereas Li et al.[Citation3] found KSV values of 6.0 x104 M−1 for curcumin binding to β-LG at pH 7.0. Arroyo-Maya et al.[Citation22] suggested also a decrease in KSV values at lower temperatures (25-45°C).
Table 1. The binding parameters of the β-LG heat treated at different temperatures by ASC.
Figure 2. The Stern–Volmer plots for the quenching of β-LG, preliminary treated at different temperatures, with ASC.
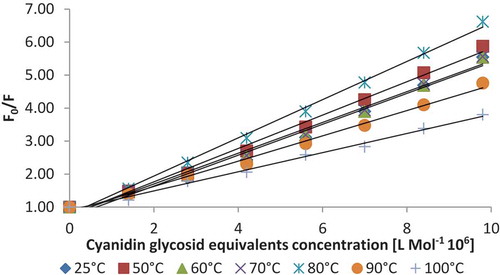
In order to find the equilibrium between free and bound molecule, the apparent binding constant (Ka) values and the number of binding sites (n) for the quenching process at different temperatures were derived from the slope and intercept of log[(Fo- F)/F] vs. log [Q], and the obtained results are listed in . The Ka values did not changed significantly in the whole temperature range studied (p >0.05), varying from 1.25 ± 0.22 × 1014 M−1 at 25°C to 1.18 ± 0.16 × 1014 M−1 at 100°C. At each tested temperature, the n value was lower than 1, suggesting that the weak binding of ASC to β-LG might be due to the multitude of compounds present in the ASC, that might compete to the binding sites. However, the n values increased with increasing temperature up to 100°C, from 0.60 ± 0.11 to 0.88 ± 0.03, indicating the increase of β-LG affinity for ASC.
The values of Ka and n obtained by Mohammadi et al.[Citation35] were 2.49 × 1012 M−1 s−1 and 0.85, whereas Li et al.[Citation3] reported values of 6.0 × 1012 M−1 s−1 and n of 1.1, when studying the binding of curcumin to β-LG. Gholami and Bordbar[Citation14] suggested Ka values of 0.5685 × 106 M−1 and n value of 1.11.
Thermodynamic parameters give information necessary to understand the molecular forces that drive the complex formation. The values of enthalpy (ΔH) and entropy (ΔS) changes acquired from the slopes and intercepts at the origin of the fitted lines, and the free energy (ΔG) are listed in . When plotting the natural logarithm of Ka versus T−1 two linear relationships were obtained, one in the temperature range of 25°C–70°C, and the second in the temperature range between 80°C and 100°C. The ΔG values for ASC binding were positive and negative in the two temperature range (), indicating that the binding process was nonspontaneous and spontaneous, respectively. In the temperature range of 25°C–70°C, the positive values of ΔH and negative for ΔS indicates that the reaction between ASC and β-LG is endothermic with a decrease in entropy. At higher temperatures, the reaction is endothermic with a positive entropy change. This sort of reaction is reactant-favoured at low temperatures and product-favoured at high temperatures. Ross and Subramanian[Citation36] have quantified the sign and magnitude of the thermodynamic parameters related with various individual kinds of interactions that may take place in the protein association process, which can be easily resolved as: (i) ΔH > 0 and ΔS > 0, hydrophobic force; (ii) ΔH<0 and ΔS<0, van der Waals’ force and hydrogen bonding; (iii) ΔH < 0 and ΔS > 0, electrostatic interactions. Therefore, from the thermodynamic characteristics summarized in , the positive ΔH and ΔS values suggest that hydrophobic force plays the major role in the β-LG and ASC binding interaction at higher temperatures. The positive values of ΔH and ΔS indicate that the binding of ASC and β-LG is mainly entropy-driven, and the enthalpy is unfavourable for it. Thus, it can be concluded that the hydrophobic force enacted a major role in the interaction, but it did not mean that the electrostatic interaction was omitted.
Table 2. The thermodynamic parameters for the association between heat treated β-LG and ASC.
In silico investigations on anthocyanin binding to β-LG
The influence of the β-LG thermal treatment on the single molecule interaction with CYR molecule was further investigated through the in silico approach. The protein model was heated up to 25, and 90°C, and then equilibrated through molecular dynamics steps until the potential energy oscillations became lower than 0.1%. The particularities of the interaction and affinity between β-LG and CYR molecules were checked on the top scoring refined assembly models after performing docking simulations. Regardless of the simulated temperature, upon the interaction with the anthocyanin the secondary structure of the β-LG molecules was well preserved. In agreement with the findings of Stănciuc et al.,[Citation37] in the 25–90°C temperature range, the amount of aminoacids involved in β-LG strands content (26.6–29.7%) prevailed over the helical one (7–9.5%). Due to the folding events governing the thermal induced behaviour of β-LG, the temperature increase from 25 to 90°C caused the slight decrease of both total surface volume of the β-LG - CYR complex ().
Table 3. Characteristics of the complex formed between β-LG equilibrated at 25°C and 90°C and CYR molecules.
Three different ligand binding sites have been previously suggested for β-LG molecule. As reviewed by Roufik et al.[Citation38] the most common one is the internal cavity defined by the β-barrel, although ligand binding to a hydrophobic pocket located on the protein surface in a groove between the α-helix and the β-barrel, or to an exposed patch close to Trp19-Arg124 residues was also reported. Because of the large volume, CYR molecule cannot enter the internal cavity of β-LG. The potential β-LG binding pockets for CYR, probed through molecular docking after simulating the thermal treatment at 25°C and 90°C, are located on the same side of the protein, and share four amino acids (Leu58, Asn90, Ile71, and Met107) from a large hydrophobic cavity on surface of the protein.
In agreement with the fluorescence spectroscopy measurements indicating the improvement of the affinity between β-LG and CYR molecules with the temperature, a significant increase of the binding energy () was obtained when equilibrating the protein at 90°C, with respect to the 25°C. Higher attractive van der Waals energy contribution to the total binding energy was attained at 90°C (), mainly because of the rearrangements within the local conformation of the CYR binding site, causing the exposure of new functional groups and even of amino acids partially buried within the core of the receptor molecule in native state. Although increasing the temperature used to preliminary treat the protein resulted in the slight increase of the total interaction surface (from 619.60 to 623.20 Å2), significant changes within the range of amino acids involved in the interaction with CYR were noticed (). Some of the newly exposed residues, namely Lys60, Asn109 and Ser116, established hydrogen bonds with the ligand at distances ranging between 2.18 and 3.17Å (), therefore contributing to the stabilization of the β-LG–CYR complex at high temperature. An addition 2.35Å long hydrogen bond involving Asn90, one of the residues shared by the two binding pockets, was identified when treating the protein at 90°C. No hydrogen bonds were found to contribute to the global binding energy in the complex equilibrated at low temperature.
Figure 3. Atomic level details on binding between of CYR to the β-LG molecule equilibrated at 25°C (a) and at 90°C (b). Only the β-LG amino acids placed in close hydrophobic contacts with CYR atoms are represented by an arc with spokes radiating towards the ligand atoms they contact. The figure was drawn using LigPlot+.32
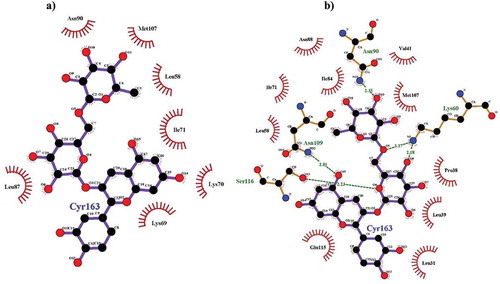
According to Morgan et al.[Citation39] β-LG molecules contains 16 free amino groups that can act as binding site for potential covalent ligands. An improvement of the contacts established by the CYR molecule with these residues was observed at high temperature, offering the premises for getting highly stable complexes. In are provided the maps of the atomic level contacts established between the β-LG treated at 25°C and 90°C and CYR molecules. Analysing the contacts established between the two molecules one can see that, in addition to the Asn90 residue found to preserve its relative position in respect to the ligand and the hydrophobic interactions regardless of the simulated temperature, four additional residues, namely Lys60, Asn88, Asn109 and Gln115 provided free amino groups to be involved in stabilizing the interface within the assembly at high temperature. In agreement with the findings of Sahihi et al.[Citation40] who studied the interaction between β-LG and three different polyphenol flavonoids (quercetin, quercitrin, and rutin), the phenolic groups in the structure of CYR molecule play key role in the binding process. In fact, Sahihi et al.[Citation40] showed that the number of hydrogen bonds connecting the flavonoids by its receptor protein increased with the OH groups in the ligand structure.
Moreover, the β-LG conformational changes induced by the thermal treatment favoured the general exposure of some nonpolar neutral amino acids, normally residing toward the hydrophobic core of the protein. Therefore the nonpolar interactions involving the newly exposed Leu31, Leu39, and Ile84 amino acids might have been served as driving force for the binding of the CYR ligand to the thermally treated protein.
Intrinsic fluorescence of the complex
Fluorescence measurement is a useful approach to study the interactions between ligands and proteins because the fluorophore is sensitive to the polarity of its surrounding environment.[Citation41] At excitation wavelength of 280 nm, both Tyr and Trp have fluorescence emission, whereas at 292 nm only the Trp shows fluorescence. In general though, the intrinsic fluorescence of Tyr and Trp containing proteins is complex and strongly depends on the environment and photophysics of these residues, especially of Trp. Therefore, in our study, in order to get a complete view of the intrinsic fluorescence of the β-LG-ASC complex, three excitation wavelengths were used to evaluate the stability of the complex in terms of fluorescence intensity and maximum emission wavelengths, as follow: 274, 280, and 292 nm. gives the heat induced structural changes of β-LG-ASC complex monitored by emission spectrum at different excitation wavelengths. At 25°C, the emission peaks (λmax) of hydrophobic residue of β-LG were located at 335, 336, and 332 nm, when excited at 274, 280, and 292 nm. Aprodu et al.[Citation27] reported values for λmax of 336, 340, and 327 nm for the complex formed between β-LG and carotenoids from sea buckthorn at 25°C. In its native form at neutral pH, β-LG presents a typical maximum position around 334 nm[Citation36] when excited at 292 nm. As it can be observed, the 2 nm blue-shifts in λmax were recorded by adding the ASC, due to the conformational changes in the tertiary structure of the protein. The intrinsic fluorescence of β-LG is given by the internal Trp19, while Trp61 is exposed to the solvent and its fluorescence is totally quenched. This is possible due to the location of Trp61 near to a disulphide bond (Cys66-Cys160) or near to the guanidine group of Arg124, or due to self-quenching with another Trp61 from another monomer. Tyr42 and Tyr102 residues are buried, while Tyr20 and Tyr99 are exposed.[Citation42]
Figure 4. The heat-induced structural changes of β-LG-ASC complex monitored as fluorescence spectra at excitation wavelength of 274 nm (a), 280 nm (b) and 292 nm (c).
Native β-LG has one well defined binding site per monomer, and depending on ligand and binding conditions, one or more secondary sites were reported.[Citation1] The main binding site of native β-LG for hydrophobic ligands is formed by the calyx of the protein,[Citation43] whereas several studies[Citation44,Citation45,Citation46] indicated secondary binding sites on the protein monomer for retinol and protoporphyrine IX, and palmitic acid and retinoid, respectively.
The thermal treatment led to a significant decrease in fluorescence intensity up to 100°C. When excited a 274 nm, a maximum decrease in fluorescence intensity (with about 34%) was found at 100°C (). In the whole temperature range studied, the λmax was blue shifted from 335 nm at 25°C to 332 nm at 100°C, suggesting a decrease in the polarity of Tyr microenvironment. Heating at 100°C caused also a decrease with about 32% () and 35% () of the fluorescence intensity when exciting the protein at 280 nm and 292 nm, respectively. Bhattacharjee and Das[Citation47] reported that increase of temperature may cause efficient thermal deactivation of fluorophores causing intensity to decrease. β-LG has 15 lysine residues each having a primary amine (ε-NH2) group, which are considered to be a quencher of fluorescence. The drop of Trp fluorescence intensity possibly reflects tertiary structural alteration of the protein in such a way that the amine groups effectively quench the fluorescence of β-LG.[Citation47]
When excited at 280 nm, a blue-shift of 3 nm was found in the temperature range of 50°–80°C, followed by a 2 nm red-shift at higher temperatures. Red-shifts of 2 nm were found in the whole temperature range, when excited at 292 nm. Trp and Tyr residues are assigned to be buried in the protein core when λmax is equal or lower than 330 nm, and exposed to solvent when λmax is higher than 330 nm. A fully exposed Trp in an aqueous solution is expected to have λmax over 350 nm.[Citation48] The decrease in fluorescence intensity together with blue-shifts in λmax could be explained by the effect of heating on protein aggregation by inducing the formation of intermolecular bonds.[Citation49]
Extrinsic fluorescence of the complex formed between anthocyanins and β-LG
β-LG binds to the hydrophobic probe ANS which is sensitive to proteins conformation and enhances its fluorescence indicating the presence of surface hydrophobic pockets.[Citation50] The ANS fluorescence intensity of (un)-treated β-LG-ASC complex was measured by exciting the samples at 365 nm and collecting the emission between 400 and 600 nm. A slight increase in ANS fluorescence intensity up to 100°C was observed, accompanied by a blue-shift from 519 nm at 25°C to 517 nm at 60°C, followed by 2 nm red-shifts at 70–90°C. For most proteins, λmax of ANS bound to hydrophobic pockets lies in the 480–490 nm range, whereas ANS exposed to free water has λmax well above 500 nm. A further increase in temperature up to 100°C resulted in a blue-shift of ANS-fluorescence of 2 nm, suggesting that the hydrophobic residues were exposed to a non-polar environment. Therefore, thermal treatment up to 90°C caused conformational changes resulting in a more flexible structure, in which the hydrophobic regions initially buried in the protein core were exposed and become more accessible for binding with ANS.
Phase diagram
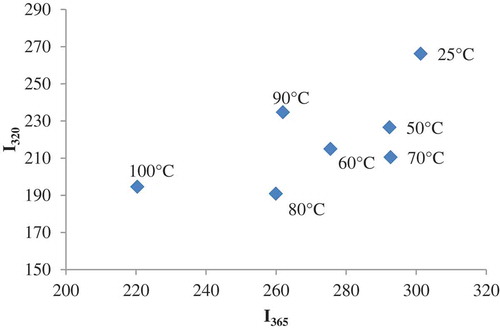
In , the phase diagram of heat induced structural changes of β-LG-ASC complex obtained by plotting Iλ1 versus Iλ2, (where I1 and I2 are the spectral intensity values measured at wavelengths 320 nm and 365 nm) used to detect partially folded species and hidden intermediates is presented.[Citation51] A linear plot involves an all-or-none transition between the two conformations, while a non-linear plot reflects the sequential character of structural transformations.[Citation52] The correlation was nonlinear, suggesting the presence of several structurally distinct conformations induced by heating. The thermal denaturation process of β-LG was described as a multistep mechanism and highly dependent on the protein concentration and pH.[Citation53] At neutral pH and physiological concentrations (<5 wt%), the dissociation of the dimer is coupled with a conformational transition to an R-type state, around 40–55°C. The native (N) to the R-state transition was associated with a single anomalous carboxyl group which appears to be buried in the hydrophobic interior of the protein in conformation N, and becomes exposed to the surface in conformation R.[Citation54] A progressive loss of β-sheet structure was observed with increasing temperature, while an abrupt loss of the helical conformation was detected near 65°C. Stănciuc et al.[Citation37] suggested significant structural rearrangement of the protein between 70°C and 80°C, associated with protein unfolding, and at 90°C attributed to protein folding. The formation of new native-like strands such as antiparallel β-sheets and β-turns at 90°C was also suggested.
Synchronous spectra
In order to investigate the nature of the microenvironment in the vicinity of the fluorophore groups within the complex during thermal denaturation, synchronous spectra of the β-LG-ASC complex were performed. This technique involves scanning simultaneously the excitation and emission monochromators while maintaining a fixed wavelength difference (Δλ) between them. The characteristic features of the Tyr and Trp residues were obtained after setting the Δλ at 15 nm and 60 nm, respectively. The synchronous spectra of the complex at different temperatures at Δλ of 15 and 60 nm are shown in and b. The shifts in the position of λmax correspond to changes of polarity around the chromophore molecules. The addition of ASC caused a blue-shift in λmax from 300 to 274 nm in case of Tyr and from 280 nm to 275 nm in case of Trp, indicating that binding sites are located in the close proximity of Tyr and Trp residues and had an effect on their microenvironment.[Citation27]
Figure 6. Synchronous fluorescence spectra registered at Δλ = 15 nm (a) and Δλ = 60 nm (b) for β-LG-ASC complex at different temperatures.
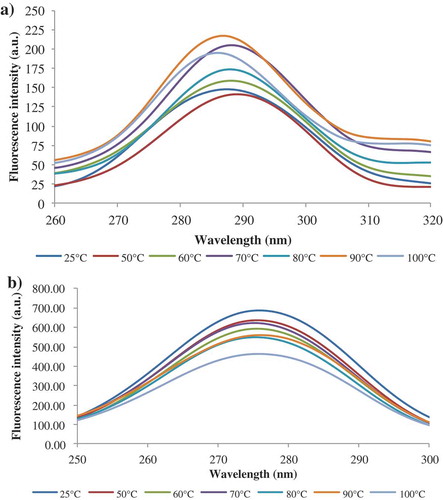
At Δλ of 15 nm and 50°C, the complex presented a significant 15 red-shift in λmax, whereas a further increase in temperature up to 100°C caused a blue shift of 3 nm (). In , a small 1 nm red-shift for Trp residues was observed at temperatures higher than 90°C. Therefore, it can be concluded that the heat treatment induced conformational changes that led to exposure of Tyr residues at lower temperatures, and partial exposure of Trp residues at higher temperatures.
Three-dimensional fluorescence spectroscopy
The changes of three dimension fluorescence spectra of β-LG-ASC complex are listed in . Peak A denotes the Raleigh scattering peak (λex = λem), the strong peak 1 and 2 mainly reveals the spectral characteristics of Trp and Tyr residues, whereas peak B is the second-order scattering peak (λem = 2 λex). As it can be seen from , the fluorescence intensity of peak 1 in the three-dimensional fluorescence spectra of the complex changes more significantly than peak 2, especially at higher temperatures. The decrease in peak 2 intensity reveals an increase in polarity surrounding the Trp and Tyr residues, with decreasing the exposure of some hydrophobic regions. The blue-shifts in λmax revealed that heat treatment induced the folding of the polypeptides chains.
Table 4. Characteristic parameters of the three-dimensional fluorescence spectra of β-LG-ASC complex.
Quenching experiments
Acrylamide and KI are external quenchers (charged and non-charged) used to analyse the solvent accessibility and the polarity of the microenvironment close to Trp residues. Acrylamide quenches the fluorescence of exposed and partially exposed Trp residues, while KI quenches only the exposed Trp located at or near to the surface of the molecules. In case of both quenchers, the fluorescence intensity of the complex decreases with increasing the concentration of the quenchers. The Stern–Volmer constants (KSV) of β-LG-CSB complex with acrylamide and KI at different temperature values are shown in . Acrylamide quenching yielded a linear Stern–Volmer plot, which implies that simple collisional is responsible for fluorescence quenching. For both quenchers, the KSV values showed no significant differences (p > 0.05). However, for acrylamide, a sharp increase at 50°C, followed by a slight decrease at higher temperatures () was observed, which can be attributed to the fact that the Trp becomes less accessible to the acrylamide. It can be concluded that the accessibility of Trp residues to the quencher was significantly modified by heating up to 50°C.
Table 5. The Stern–Volmer quenching constant (KSV) of the β-LG-ASC complex at different temperatures.
Conclusion
Fluorescence spectroscopy and molecular docking were employed in this study in order to describe the interaction mechanism between anthocyanin’s from sour cherries skins extract and bovine β-lactoglobulin and the thermal stability of the complex. The purpose was to understand in great detail the protein-ligand interactions from the perspective of developing new functional composites with applications in food industry. The studies revealed that, anthocyanins have the ability to bind with β-lactoglobulin via a static quenching process. The number of binding sites was lower than 1, suggesting that the weak binding of anthocyanins to β-lactoglobulin might be due to the multitude of compounds present in the extract. The thermodynamic parameters suggested the role of hydrophobic interactions. The phase diagram suggested the presence of more than two structurally distinct molecular species induced by thermal treatment. Intrinsic and extrinsic fluorescence experiments have highlighted blue- and red-shifts in maximum emission, suggesting sequential folding and unfolding with increasing temperature. The synchronous spectra indicated the exposure of Tyr residues at lower temperatures, and partial exposure of Trp residues at higher temperatures. The spectroscopic results on β-lactoglobulin affinity for anthocyanin after the thermal treatment were further confirmed with molecular docking study, in which the cyanidin 3-rutinoside was docked to the whey protein equilibrated through molecular dynamics at different temperatures. Regardless of the simulated temperature, the potential β-lactoglobulin binding pockets preferred by the cyanidin 3-rutinoside shared a large hydrophobic cavity located on protein surface. The interactions established between the two components of the assembly were mostly hydrophobic, with significant participation of the hydrogen bonds at high temperature, involving mainly the newly exposed amino acids from β-lactoglobulin molecules and the OH groups of the ligand.
Funding
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS-UEFISCDI, project number PN-II-RU-TE-2014-4-0115.
Additional information
Funding
References
- Le Maux S.; Bouhallab S.; Giblin L.; Brodkorb A.; Croguennec T. Bovine β-lactoglobulin/Fatty Acid Complexes: Binding, Structural, and Biological Properties. Dairy Science Technology 2014, 94, 409–426.
- Smithers G.W. Whey and Whey Proteins-from ‘Gutter-to-Gold’. International Dairy Journal 2008, 18, 695–704.
- Li M.; Maa Y.; Ngadi M.O. Binding of Curcumin to β-lactoglobulin and Its Effect on Antioxidant Characteristics of Curcumin. Food Chemistry 2013, 141, 1504–1511.
- Iametti S.; Scaglioni L.; Mazzini S.; Vecchio G.; Bonomi F. Structural Features and Reversible Association of Different Quaternary Structures of β-lactoglobulin. Journal of Agricultural and Food Chemistry 1998, 46, 2159–2166.
- Brownlow S.; Cabral J.H.; Cooper R.; Flower D.R.; Yewdall S.J.; Polikarpov I.; Sawyer L. Bovine β-lactoglobulin at 1.8 Å Resolution – Still an Enigmatic Lipocalin. Structure 1997, 5, 481–495.
- Kuwata K.; Hoshino M.; Forge V.; Era S.; Batt C.A.; Goto Y. Solution Structure and Dynamics of Bovine β-Lactoglobulin A. Protein Science 1999, 8, 2541–2545.
- Edwards P.B.; Creamer L.K.; Jameson G.B. Structure and Stability of Whey Proteins. In Milk Proteins – From Expression to Food; Thompson, A.; Boland, M.; Singh, H.; Eds.; Elsevier: New York, 2009; 165–166.
- Harvey B.J.; Bell E.; Brancaleon L. A Tryptophan Rotamer Located in a Polar Environment Probes pH-dependent Conformational Changes in Bovine Beta-lactoglobulin A. The Journal of Physical Chemistry B 2007, 111, 2610–2620.
- Nucar A.; Maselli P.; Giliberti V.; Carbonaro M. Epicatechin-induced Conformational Changes in β-lactoglobulin B Monitored by FT-IR Spectroscopy. Springer Plus 2013, 2, 661.
- Mazza G. Health aspects of natural colors. In Natural Food and Colorants Science and Technology; Lauro, G.J.; Francis, F.J.; Eds.; Marcel Decker: New York, 2000, pp 289–314.
- Wang W.D.; Xu S.Y. Degradation Kinetics of Anthocyanins in Blackberry Juice and Concentrate. Journal of Food Engineering 2007, 82, 271–275.
- Konczak I.; Zhang W. Anthocyanins – More than Nature’s Colours. Journal of Biomedicine and Biotechnology 2004, 5, 239–240.
- Fennema O.R. Food Chemistry, 4th ed.; CRC Press: New York, 1996.
- Gholami S.; Bordbar A.K. Exploring Binding Properties of Naringenin with Bovine β-Lactoglobulin: A Fluorescence, Molecular Docking and Molecular Dynamics Simulation Study. Biophysical Chemistry 2014, 33–42.
- Soares S.; Mateus N.; de Freitas V. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary r-Amylase (HSA) by Fluorescence Quenching. Journal of Agricultural and Food Chemistry 2007, 55, 6726–6735.
- Rodríguez S.D.; von Staszewski M.; Pilosof A.M.R. Green Tea Polyphenols-Whey Proteins Nanoparticles: Bulk, Interfacial and Foaming Behavior. Food Hydrocolloids 2015, 50, 108–115.
- Al-Hanish A.; Stanic-Vucinic D.; Mihailovic J.; Prodic I.; Minic S.; Stojadinovic M.; Radibratovic M.; Milcic M.; Cirkovic Velickovic T. Noncovalent Interactions of Bovine α-Lactalbumin with Green Tea Polyphenol, Epigalocatechin-3-Gallate. Food Hydrocolloids 2016, 61, 241–250.
- Bonechi C.; Martini S.; Ciani L.; Lamponi S.; Rebmann H.; Rossi C.; Ristori S. Using Liposomes as Carriers for Polyphenolic Compounds: The Case of Trans-Resveratrol. PLoS One 2012, 7, e41438.
- Seeram N.P.; Momin R.A.; Nair M.G.; Bourquin L.D. Cyclooxygenase Inhibitory and Antioxidant Cyanidin Glycosides in Cherries and Berries. Phytomedicine 2001, 8, 362–369.
- Kang S.Y.; Seeram N.P.; Nair M.G.; Bourquin L.D. Tart Cherry Anthocyanins Inhibit Tumor Development in ApcMin Mice and Reduce Proliferation of Human Colon Cancer Cells. Cancer Letters 2003, 194, 13–19.
- Bi S.; Yan L.; Pang B.; Wang Y. Investigation of Three Flavonoids Binding to Bovine Serum Albumin Using Molecular Fluorescence Technique. Journal of Luminescence 2012, 132, 132–140.
- Arroyo-Maya I.J.; Campos-Terán J.; Hernández-Arana A.; McClements D. J. Characterization of Flavonoid-Protein Interactions Using Fluorescence Spectroscopy: Binding of Pelargonidin to Dairy Proteins. Food Chemistry 2016, 213, 431–439.
- Dumitrașcu L.; Stănciuc N.; Aprodu I. New Insights Into Xanthine Oxidase Behavior upon Heating Using Spectroscopy and In Silico Approach. International Journal of Biological Macromolecules 2016, 88, 306–312.
- Oancea A-M.; Turturică M.; Bahrim G.; Râpeanu G.; Stănciuc N. Phytochemicals and Antioxidant Activity Degradation Kinetics during Thermal Treatments of Sour Cherry Extract. LWT - Food Science and Technology 2017, 82, 139–146.
- Blando F.; Gerardi C.; Nicoletti I. Sour Cherry (Prunus cerasus L) Anthocyanins as Ingredients for Functional Foods. Journal of Biomedicine and Biotechnology 2004, 5, 253–258.
- Turturică M., Stănciuc N., Bahrim G., Râpeanu G. Effect of Thermal Treatment on Phenolic Compounds from Plum (Prunus domestica) Extracts – A Kinetic Study. Journal of Food Engineering 2016, 171, 200–207.
- Aprodu I.; Ursache F.M.; Turturică M.; Râpeanu G.; Stănciuc N. Thermal stability of the Complex Formed between Carotenoids from Sea Buckthorn (Hippophae rhamnoides L.) and Bovine β-lactoglobulin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2017, 173, 562–571.
- Loch J.I.; Bonarek P.; Polit A.; Riès D.; Dziedzicka-Wasylewska M.; Lewiński K. Binding of 18-Carbon Unsaturated Fatty Acids to Bovine β-lactoglobulin-Structural and Thermodynamic Studies. International Journal of Biological Macromolecules 2013, 57, 226–231.
- Hess B.; Kutzner C.; van der Spoel D.; Lindahl E. GROMACS 4: Algorithms for Highly Efficient, Load-balanced, and Scalable Molecular Simulation. Journal of Chemical Theory and Computation 2008, 4(3), 435–447.
- Schneidman-Duhovny D.; Inbar Y.; Nussinov R.; Wolfson H.J. PatchDock and SymmDock: Servers for Rigid and Symmetric Docking. Nucleic Acids Research 2005, 33(suppl. 2), W363–W367.
- Andrusier N.; Nussinov R.; Wolfson H.J. FireDock: Fast Interaction Refinement in Molecular Docking. Proteins: Structure, Functions, Bioinformatics 2007, 69(1), 139–159.
- Laskowski R.A.; Swindells M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. Journal of Chemical Information and Modeling 2011, 51(10), 2778–2786.
- Lakowicz J. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, 1999.
- Klajnert B.; Stanisławska L.; Bryszewska M.; Pałecz B. Interactions between PAMAM Dendrimers and Bovine Serum Albumin. Biochimica et Biophysica Acta: Proteins Proteomics 2003, 1648, 115–126.
- Mohammadi F.; Bordbar A.K.; Divsalar A.; Mohammadi K.; Saboury A. Interaction of Curcumin and Diacetylcurcumin with the Lipocalin Member β-lactoglobulin. Protein Journal 2009, 28, 117–123.
- Ross P.D.; Subramanian S. Thermodynamics of Protein Association Reactions: forces Contributing to Stability. Biochemistry 1981, 20, 3096–3102.
- Stănciuc N.; Aprodu I.; Râpeanu G.; Bahrim G. Fluorescence Spectroscopy and Molecular Modeling Investigations on the Thermally Induced Structural Changes of Bovine β-lactoglobulin. Innovative Food Science and Emerging Technologies 2012, 15, 50–56.
- Roufik S.; Gauthier S.F.; Leng X.; Turgeon S.L. Thermodynamics of Binding Interactions between Bovine β-lactoglobulin A and the Antihypertensive Peptide β-Lg f142-148. Biomacromolecules 2006, 7, 419–426.
- Morgan F.; Molle D.; Henry G.; Venien A.; Leonil J.; Peltre G.; Bouhallab S. Glycation of Bovine β-lactoglobulin: Effect on the protein structure. International Journal of Food Science & Technology 1999, 34, 429–435.
- Sahihi M.; Heidari-Koholi Z.; Bordbar A. K. The interaction of Polyphenol Flavonoids with β-Lactoglobulin: Molecular Docking and Molecular Dynamics Simulation Studies. Journal of Macromolecular Science B 2012, 51, 2311–2323.
- Liang L.; Tajmir-Riahi H.; Subirade M. Interaction of β-lactoglobulin with Resveratrol and Its Biological Implications. Biomacromolecules 2007, 9, 50–56.
- Liang L.; Subirade M. Study of the Acid and Thermal Stability of β-lactoglobulin-ligand Complexes Using Fluorescence Quenching. Food Chemistry 2012, 132, 2023–2029.
- Kontopidis G.; Holt C.; Sawyer L. Invited Review: β-lactoglobulin: Binding Properties, Structure, and Function. Journal of Dairy Science 2004, 84, 785–796.
- Dufour E.; Genot C.; Haertlé T. Beta-lactoglobulin Binding Properties during Its Folding Changes Studied by Fluorescence Spectroscopy. Biochimica et Biophysica Acta - Protein Structure and Molecular Enzymology 1994, 1205, 105–112.
- Dufour E.; Hoa G.H.B.; Haertlé T. High-pressure Effects on β-lactoglobulin Interactions with Ligands Studied by Fluorescence. Biochimica et Biophysica Acta - Protein Structure and Molecular Enzymology 1994, 1206, 166–172.
- Narayan M.; Berliner L.J. Mapping Fatty Acid Binding to β-lactoglobulin: Ligand Binding is Restricted by Modification of Cys 121. Protein Science 1998, 7, 150–157.
- Bhattacharjee C.; Das K.P. Thermal Unfolding and Refolding of β-lactoglobulin. An Intrinsic and Extrinsic Fluorescence Study. European Journal of Biochemistry 2000, 267, 3957–3964.
- Eftink M.R. Fluorescence Techniques for Studying Protein Structure. Methods of Biochemical Analysis 1991, 35, 127–205.
- Rodrigues R.M.; Martins A.J.; Ramos O.L.; Malcata F.X.; Teixeira J.A.; Vicente A.A. Influence of Moderate Electric Fields on Gelation of Whey Protein Isolate. Food Hydrocolloids 2015, 43, 329–339.
- Das K.P.; Kinsella J.E. Effect of Heat Denaturation on the Adsorption of β-lactoglobulin at the Oil/Water Interface and on the Coalescence Stability of Emulsions. Journal of Colloid and Interface Science 1990, 139, 551–560.
- Kuznetsova I.; Turoverov K.; Uversky V. Use of the Phase Diagram Method to Analyze the Protein Unfolding-Refolding Reactions: Fishing Out the “Invisible” Intermediates. Journal of Proteome Research 2004, 3, 485–494.
- Kataeva I. A.; Uversky V.N.; Brewer J.M.; Schubot F.; Rose J.P.; Wang B.C.; Ljungdahl L.G. Interactions between Immunoglobulin-Like and Catalytic Modules in Clostridium thermocellum Cellulosomal Cellobiohydrolase CbhA. Protein Engineering, Design and Selection 2004, 17, 759–769.
- Blanch E.W.; Hecht L.; Barron L.D. New Insight into the pH-dependent Conformational Changes in Bovine β-lactoglobulin from Raman Optical Activity. Protein Science 1999, 8, 1362–1367.
- Seo J.A.; Hédoux A.; Guinet Y.; Paccou L.; Affouard F.; Lerbret A.; Descamps M. Thermal Denaturation of β-Lactoglobulin and Stabilization Mechanism by Trehalose Analyzed from Raman Spectroscopy Investigations. The Journal of Physical Chemistry B 2010, 114, 6675–6684.