ABSTRACT
The polyphenolic and oenological properties were evaluated in red wines from Italian varieties Ancellotta, Rebo, Nebbiolo, Barbera and Teroldego cultivated in a selected subtropical region. Ancellotta wines showed the highest concentrations of phenolics, particularly flavanols, flavonols and anthocyanins 3-O-glycosides. Grape variety rather than vintage had stronger influence on polyphenols. Wines from vintage 2012 showed the highest antioxidant activity and highest concentrations of polyphenols, mainly Ancellotta and Teroldego wines. The wines showed similar phenolic profile regarding the presence of phenolics, whereas their concentrations varied greatly among varieties and vintages. The results demonstrated good adaptability of these varieties and their potential to produce quality wines.
Introduction
The quality of red wines is dictated by its chemical composition and phenolic constituents that contribute to their typicality and sensorial characteristics such as colour, astringency and flavour.[Citation1] Phytochemical polyphenols are associated with the bioactive properties and antioxidant activity of grapes and wines.[Citation2,Citation3] These compounds are essentially influenced by grape variety, cultivation practices and climatic and soil conditions.[Citation4]
The evaluation of phenolic compounds in red wines is a valuable parameter in order to identify characteristics of a growing region, the influence of climate, cultivation and vinification techniques. It allows predicting the stability of these compounds during wine aging as well as finding the determinants for the expression of terroir.[Citation4,Citation5] Apart from the potential of producing unique wines, the cultivation of wine varieties in different growing regions is essential to sustain soil dynamics and agriculture activities as well as to investigate grapevine physiology and its environmental interactions.[Citation6,Citation7]
While several studies associate the phenolic composition with the identity of their wines, there is limited scientific information on the adaptation of wine varieties in diverse environments with regard to polyphenols synthesis and their influence on wine quality. Red wines from European varieties V. vinifera L. present distinct oenological characteristics, mostly related to grape cultivar and grapevine metabolism. The Italian red-skinned varieties Ancellotta, Rebo, Nebbiolo, Barbera and Teroldego are typically grown in North-West Italy and used to elaborate quality wines. In this context, their cultivation in a subtropical region could particularly influence the polyphenolic composition and oenological identity of their wines. The aim of this work was to investigate the antioxidant activity, the phenolic profile and oenological parameters of red wines elaborated from these varieties newly cultivated in a selected region of Southern Brazil, where the subtropical climate is predominant.
Materials and methods
Samples and location
Dry red wines were produced using the native Italian varieties Ancellotta, Rebo, Nebbiolo, Barbera and Teroldego from vintages 2011 and 2012, cultivated in the subtropical region of Campos Novos, Santa Catarina, Southern Brazil. The vineyards are located in the Experimental Station Epagri – Campos Novos Unit at latitude of 27°19’83”S and longitude of 50°49’18”W, situated in a plateau region at 965 metres above sea level. The region of Campos Novos is classified as “Region III” with mild climate, according to the Winkler Index (1668–1944 GDD). The average of minimum and maximum air temperatures reported for this region in the vintages of 2011 and 2012 ranged from 11–17°C to 22–28°C, respectively.[Citation8] The vineyards were planted in 2006, in a vertical trellis system. The seedlings of the Rebo variety were grafted on rootstock Kober 5BB; Ancellotta clone FEDIT 18, grafted on rootstock Paulsen 1103 ISV 1; Barbera clone AT 84, grafted rootstock 110R 151f; Nebbiolo clone CVT CN 142, rootstock 420A MI-Q14; and Teroldego clone SMA 133, rootstock Paulsen 1103 113F; all varieties were imported from Italy.
Microvinification
The Italian grapes were harvested at technical maturity (18–21°Brix), and the red wines were elaborated by microvinification. The grapes were mechanically separated from bunches and fed into stainless steel tanks with controlled temperature and agitation. Grape mash maceration was carried at 25°C for 10 days. Then, the must was separated by pressing and transferred to stainless steel tanks, when a commercial sulfiting agent was added to the must (10 mg/L free SO2) (Noxitan, Pascal Biotech, Paris), and selected Saccharomyces cerevisiae yeasts (20 g/100 kg) Fermol Rouge® (Pascal Biotech, France) were inoculated to promote alcoholic fermentation. The fermentation was carried out at 17°C for 15 days. After the fermentation was finished, the wines were cold-stabilised, and the free SO2 content was adjusted to 30 mg/L. The wines were then bottled and stored at 18 ± 1°C. The oenological parameters were determined according to established protocols of OIV.[Citation9]
Spectrophotometric analysis
The red wines were analysed for total monomeric anthocyanins using the pH-differential method.[Citation10] Polymeric and co-pigmented anthocyanins were determined as described by Levengood and Boulton.[Citation11] Colour intensity, colour tonality and colour density were determined according to Glories.[Citation12] The total phenolic content was assayed by the Folin-Cicalteu method.[Citation13] The polymerised and non-polymerised polyphenols were determined using the vanillin index.[Citation14] The content of ortho-diphenols was determined as described by Flanzy and Aubert.[Citation15] The content of tartaric ester was estimated according to Glories[Citation16], and total flavanols were determined by the DMACA method (p-dimethylaminocinnamaldehyde).[Citation17] The total antioxidant capacity of wines was determined by FRAP,[Citation18] DPPH,[Citation19]and ABTS[Citation20] methods. All analyses were carried out in triplicate on a UV-Vis spectrophotometer (Hitachi U-2010, CA, USA).
HPLC analysis of individual phenolics
Chromatographic analyses were carried out on a high-performance liquid chromatograph (Shimadzu, Kyoto, Japan) composed of a high-pressure pump, vacuum degasser, communicator system and LC-Solutions software, connected to a photodiode array detector (DAD) (Shimadzu). Separations were performed on a C18 reverse-phase column (4.6 × 250 mm, 5 µm) (Shimadzu). The wine samples were filtered through a 0.45 µm PTFE membrane filter (Millipore, Massachusetts, USA) and injected into the chromatograph. Flavonols, flavanols, cinnamic derivatives, tyrosol and trans-resveratrol were determined according to Ferreira-Lima et al.[Citation21] The hydroxybenzoic acids were determined according to Burin et al.,[Citation22] and anthocyanins 3-O-glycosides were determined according to Revilla et al.[Citation23]
Statistical analysis
Statistical analysis was conducted using Statistica 8.0 software (StatSoft, Tulsa, USA). Results were analysed by ANOVA, and statistical significance was assessed by Tukey HSD post hoc test (p < 0.05). All data were presented as the mean ± SD. The variability as influenced by grape variety and vintage was investigated by correlation analysis and principal component analysis (PCA).
Results and discussion
Oenological parameters, phenolic content and antioxidant activity of red wines
The oenological parameters of the wine samples are shown in . The phenolic content and antioxidant activity of the wines are shown in . The red wines elaborated with Italian grapes cultivated in the selected subtropical region showed mean values of pH of 3.7, titratable acidity of 4.9 g/L of tartaric acid, volatile acidity of 0.4 g/L of acetic acid and alcohol content of 11.7% (v/v). The alcohol content of wine samples was higher in wines of vintage 2012. This is probably due to differences in the content of sugars in grapes due to climate and harvest factors between vintages. Abundant or excessive rainfalls affect sugar accumulation in grape berries, decreasing alcohol formation during fermentation. In fact, during the vintage period of 2011, the rainfall was approximately 1100 mm in the region of Campos Novos, while during the vintage of 2012, the rainfall was lower, approximately 900 mm.[Citation8] The Ancellotta wines showed the highest colour intensity and colour density, with values up to 44.08 and 38.89, respectively, in wine samples from the vintage 2012. In comparison to the other wine varieties, the colour indexes were greatly lower in Nebbiolo wines.
Table 1. Oenological parameters of red wines produced from V. vinifera L. grapes cultivated in the subtropical region of Campos Novos, South Brazil, vintages 2011 and 2012.
Table 2. Phenolic content and antioxidant activity of Ancellotta, Rebo, Nebbiolo, Barbera and Teroldego wines produced from Italian grape varieties cultivated in a selected subtropical region, from vintages 2011 and 2012.
The spectrophotometric analyses showed the presence of polymerised and non-polymerised polyphenols, flavonoids, tartaric esters and ortho-diphenols in the wine samples (). The Ancellotta, Teroldego and Rebo wines presented higher levels of polyphenols, regardless of vintage. In relation to the vintage 2011, concentrations of total phenolics were higher in wines from the vintage 2012, with the exception of Teroldego wines. Anthocyanins in their monomeric, polymeric and copigmented forms were quantified in all varietal wines, whereas the polymeric compounds were predominant in all wines. Low concentrations of anthocyanins were observed in Nebbiolo wines, which is consistent with the lower indexes of colour parameters in these samples. According to Boulton,[Citation24] the colour of a red wine is also related to the natural capacity of the variety to form anthocyanin pigments in different climates. Indeed, the Nebbiolo variety native of the Piedmont Region in Italy is very susceptible to climate and soil variations.[Citation25] Hence, apart from viticulture practices and winemaking variations, this could explain the low concentrations of monomeric anthocyanins found for Nebbiolo wines in this study.
Importantly, all the wine samples showed high concentrations of polyphenols and high antioxidant activity. The antioxidant activity is a very relevant parameter to evaluate wine quality and its bioactive properties. For the analysed wines, the mean antioxidant activity ranged from 4.6 ± 1.7 mmol TEAC/L for Barbera wines from vintage 2011 to 10.8 ± 1.5 mmol TEAC/L for Ancellotta wines from vintage 2012. In relation to vintage 2011, the antioxidant activity of all varietal wines was higher for vintage 2012 that also had higher concentrations of total polyphenols. Phenolic compounds such as flavonols and flavanols, mainly quercetin and catechin, as well as anthocyanins are generally associated with the antioxidant properties of red wines.[Citation1,Citation4,Citation8] Indeed, the antioxidant activity was positively correlated with the phenolic content of all varietal wines (data not shown). All wines had abundant concentrations of monomeric and polymeric anthocyanins, and of both polymerised and non-polymerised polyphenols, determined as equivalents of catechin. The wines elaborated from the Ancellotta variety had the highest concentrations of polymerised polyphenols, total flavanols and total monomeric anthocyanins. Similarly, these wines showed the highest antioxidant activity, while the values were lower in Barbera and Nebbiolo wines.
The red wines from vintages 2011 and 2012 presented suitable oenological characteristics of V. vinifera wines. Regarding physical-chemical properties, the alcohol content, pH, titratable acidity and the content of sulphur dioxide verified for the wine samples are consistent with that of red wines.[Citation8,Citation26,Citation27] These parameters are essential to determine the oenological identity of wines. Hence, the adequacy of these parameters with regard to quality wines is a determinant factor when considering the potential of new growing regions, in this study, a selected region with subtropical climate.
Bioactive compounds of red wines and association between polyphenols and wine varieties
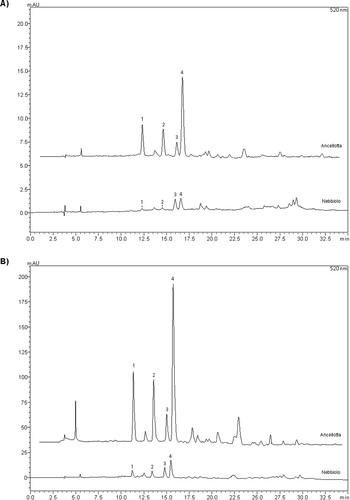
The individual phenolic profile of red wines is presented in . The flavanols (+)-catechin and (−)-epicatechin were the major compounds quantified in samples. These compounds were predominant in Ancellotta wines. Anthocyanins in their 3-O-monoglycoside forms were also quantified at high concentrations, with the exception of Nebbiolo wines in which these compounds were less abundant. The comparative chromatograms of the anthocyanins profile of Ancellotta and Nebbiolo wines are shown in . Malvidin 3-O-glycoside was the most abundant anthocyanin in all wine varieties. Gallic, protocatechuic and trans-caftaric acids were the most abundant phenolic acids, with the highest concentrations in Teroldego and Barbera wines, respectively. Other studies have found gallic acid as the predominant hydroxybenzoic acid in red wines.[Citation28,Citation29] The stilbene trans-resveratrol was also quantified in samples, with the highest concentrations in Barbera wines. In wines from the vintage of 2011, higher levels of trans-resveratrol were verified for Barbera, Rebo and Ancellotta wines, while for the vintage of 2012, the highest levels were determined in Barbera, Ancellotta and Teroldego wines. Indeed, trans-resveratrol is a phytoalexin produced in response to stress conditions, and its accumulation in grapes is influenced by climatic and cultivation factors as well as by grapevine physiology and grape variety.[Citation1,Citation3]
Table 3. Individual phenolic compounds (mg/L) in red wines produced from Italian grape varieties cultivated in a selected subtropical region, vintages 2011 and 2012.
Figure 1. Comparative chromatograms of anthocyanins in Ancellota and Nebbiolo red wines from vintages 2011 (A) and 2012 (B). Peaks: 1. delphinidin-3-O-glycoside (Retention time, RT = 12.24), 2. cyanidin-3-O-glycoside (RT = 14.54), 3. peonidin-3-O-glycoside (RT = 16.05), 4. malvidin-3-O-glycoside (RT = 16.68).
The flavonols myricetin, quercetin and kaempferol were quantified at higher concentrations in Ancellotta wines. Tyrosol, an important derivative of phenethyl alcohol originated from the fermentation process, was quantified in all wines. Interestingly, among the studied varieties, their wines showed similar phenolic profile regarding the presence of phenolic compounds, regardless of their concentrations. With the exception of cyanidin 3-O-glycoside, all phenolic compounds were quantified in all wines, regardless of vintage. Contrarily, for the majority of phenolic compounds, their concentrations in wines varied significantly between vintages. Indeed, regarding the 3-O-monoglycoside anthocyanins, their concentrations were significantly higher in wines from the vintage 2012 when compared to vintage 2011. The same was observed for tyrosol, kaempferol, (+)-catechin, caffeic and ellagic acids. These results indicate that cultivation and climatic factors, in this study represented by different vintages, greatly influence the chemical composition of grapes, thus, the phenolic concentrations in red wines. These findings corroborate that vintage and wine varieties particularly affect the polyphenolic composition of red wines, thereby influencing their bioactive and sensorial properties. These results are consistent with previous reports of differences in the phenolic composition of wines produced in different cultivation systems such as biodynamic and organic systems,[Citation30] and of red wines made from varietal grapes or from grapes grown in different growing regions.[Citation4,Citation29]
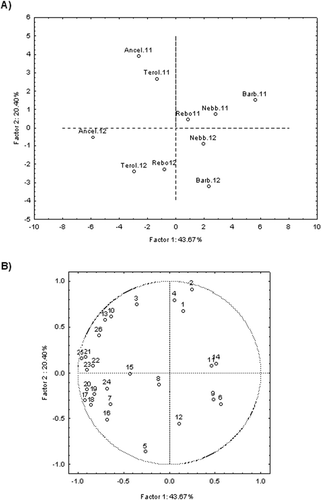
Further, in order to obtain more information on the bioactive composition of the new red wines as affected by vintage and grape variety, we assessed the influence of these factors on the polyphenolic profile and antioxidant activity of wines using the principal component analysis (PCA) (). The Factor 1 (PC1) explained 43.67% of data variability, while Factor 2 (PC2) explained 20.40%. The PC1 allowed the separation of Ancellotta and Teroldego wines from the other wine samples, while PC2 showed a clear distinction of wines in relation to vintage. The vintage 2011 was associated with the highest levels of polyphenols, total flavonoids, and total monomeric anthocyanins and with the highest antioxidant activity. In vintage 2012, the anthocyanins 3-O-monoglycosides, ellagic, trans-caftaric, caffeic and ferulic acids, and kaempferol were the predominant phenolics. When comparing the samples, some individual phenolic compounds such as trans-resveratrol and the phenolic acids gallic, protocatechuic, syringic, caffeic, trans-caftaric and ferulic were associated with Barbera, Nebbiolo and Rebo wines and clearly separated in PC1, which accounted for the most variation among wines (43.67%). Interestingly, these results suggest that wine variety rather than differences between vintages (2011 and 2012) had stronger influence on the phenolic contents and antioxidant activity of red wines.
Figure 2. Principal component analysis of phenolic profile and antioxidant activity of red wines Ancellotta (Ancel), Rebo (Rebo), Nebbiolo (Nebb), Barbera (Barb) and Teroldego (Terol) grown in a subtropical region as affected by vintages (2011/2012) and grape variety. A. Scores plot defined by PC1 and PC2. B. Loading biplot of variables. 1. Gallic acid, 2. Protocatechuic acid, 3. Vanillic acid, 4. Syringic acid, 5. Ellagic acid, 6. trans-caftaric acid, 7. Tyrosol, 8. Catechin, 9. Caffeic acid, 10. Epicatechin, 11. p-coumaric acid, 12. Ferulic acid, 13. Myricetin, 14. trans-resveratrol, 15. Quercetin, 16. Kaempferol, 17. Delphinidin, 18. Cyanidin, 19. Peonidin, 20. Malvidin, 21. Total polyphenols, 22. Total flavanols, 23. Total monomeric anthocyanins, 24. Antioxidant activity by ABTS assay, 25. Antioxidant activity by DPPH assay, 26. Antioxidant activity by FRAP assay.
Conclusion
The wines showed high content of phenolic compounds, particularly catechin, epicatechin and anthocyanins 3-O-glycosides, which are important bioactive compounds in grapes and wines. Ancellotta and Teroldego wines had the highest concentrations of total polyphenols, flavanols and total monomeric anthocyanins and showed the highest antioxidant activity, regardless of vintage. The red wines from typical Italian grapes grown in a selected subtropical region had particular oenological characteristics and a good bioactive potential regarding their polyphenolic composition. The differences among wine varieties and vintages were confirmed by PCA that showed that grape variety rather than vintage exerted predominant influence on the phenolic contents and antioxidant activity of red wines. Nevertheless, the Italian varieties showed good adaptation to subtropical cultivation and potential to produce quality wines.
LJFP_A_1344992_Graphical_Abstract.tif
Download TIFF Image (369.3 KB)Funding
This work was financially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.
Additional information
Funding
References
- Jackson, R.S. Chemical Constituents of Grapes and Wine. In Wine Science: Principles and Applications; Jackson, R.S., Ed.; Elsevier: San Diego, USA, 2008.
- Radovanovic, B.; Radovanovic, A.; Tomic, V. Relations Between the Phenolic Composition and Free Radical Scavenging, and Antibacterial Activities of Red Wines from Different Cultivars of Vitis vinifera L. International Journal of Food Properties 2012, 15, 725–735.
- Fernandez-Mar, M.I.; Mateos, R.; Garcia-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chemistry 2012, 13, 797–813.
- Stockham, K.; Sheard, A.; Paimin, R.; Buddhadasa, S.; Duong, S.; Orbell, J.D.; Murdoch, T. Comparative Studies on the Antioxidant Properties and Polyphenolic Content of Wine from Different Growing Regions and Vintages, A Pilot Study to Investigate Chemical Markers for Climate Change. Food Chemistry 2013, 140, 500–506.
- Coletta, A.; Trani, A.; Faccia, M.; Punzi, R.; Dipalmo, T.; Crupi, P.; Antonacci1, D.; Gambacorta, G. Influence of Viticultural Practices and Winemaking Technologies on Phenolic Composition and Sensory Characteristics of Negroamaro Red Wines. International Journal of Food Science & Technology 2013, 48, 2215–2227.
- Brillante, L.; Bois, B.; Lévêque, J.; Mathieu, O. Variations in Soil-Water Use by Grapevine According to Plant Water Status and Soil Physical-Chemical Characteristics – A 3D Spatio-Temporal Analysis. European Journal of Agronomy 2016, 77, 122–135.
- Torres, N.; Goicoechea, N.; Antolín, M.C. Antioxidant Properties of Leaves From Different Accessions of Grapevine (Vitis vinifera L.) cv. Tempranillo after Applying Biotic and/or Environmental Modulator Factors. Industrial Crops and Products 2016, 76, 77–85.
- Sartor, S.; Malinovski, L.I.; Caliari V.; Da Silva, A. L.; Bordignon-Luiz, M. T. Particularities of Syrah Wines from Different Growing Regions of Southern Brazil: Grapevine Phenology and Bioactive Compounds. Journal of Food Science and Technology 2017, 54, 1414–1424.
- Office International de la Vigne et du Vin. Recueil des Méthodes Internationales d’Analyse des Vins et des Moûts; Office International de la Vigne et du Vin: Paris, France, 1990.
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. In Current protocols in food analytical chemistry; Wrolstad, R. E., Ed.; John Wiley & Sons: New York, USA, 2001.
- Levengood, J.; Boulton, R. The Variation in the Color Due to Copigmentation in Young Cabernet Sauvignon Wines. In Red Wine Color; Waterhouse, A. L.; Kennedy, J. A., Ed.; American Chemical Society: Washington, US A, 2004.
- Glories, Y. La Coleur des Vins Rouges. Connaissance de la Vigne et du Vin 1984, 18, 253–271.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdicephosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Paronetto, L. Polifenoli e Tecnica Enologica; Selepress: Milan, Italy, 1977.
- Flanzy, M.; Aubert, S. Évaluation des Composés Phénoliques des Vins Blancs.Annales de Technologie Agricole 1969, 18, 27–44.
- Glories, Y. Recherches sur la Matière Colorante des Vins Rouges. Bulletin de La Societe Chimique 1978, 9, 2649–2652.
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of Pigment and Flavanol Content Wine Antioxidant Properties in Selected Aged Regional Wines from Greece. Journal of Food Composition and Analysis 2002, 15, 655–665.
- Benzie, I.F.F.; Strain, J.J. The Feric Reducing Ability of Plasma (FRAP) As a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Kim, Y.K.; Guo, Q.; Packer, L. Free Radical Scavenging Activity of Red Ginseng Aqueous Extracts. Toxicology 2002, 172, 149–156.
- Re, R.; Pellegrini, N.; Proteggemnte, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying and Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology & Medicine 1999, 26, 1234–1237.
- Ferreira-Lima, N.E.; Burin, V.M.; Bordignon-Luiz, M.T. Characterization of Goethe White Wines: Influence of Different Storage Conditions on the Wine Evolution during Bottle Aging. European Food Research and Technology 2013, 237, 509–520.
- Burin, V.M.; Costa, L.L.F.; Rosier, J.P.; Bordignon-Luiz, M.T. Cabernet Sauvignon Wines from Two Different Clones, Characterization and Evolution during Bottle Ageing. LWT- Food Science and Technology 2011, 44, 1931–1938.
- Revilla, I.; Pérez-Magarinõ, S.; González-SãoJosé, M.L.; Beltán, S. Identification of Anthocyanin Derivatives in Grape Skin Extracts and Red Wines by Liquid Chromatography with Diode Array and Mass Spectrometric Detection. Journal of Chromatography A 1999, 847, 83–90.
- Boulton, R. The Copigmentation of Anthocyanins and Its Role in the Color of Red Wine: A Critical Review. American Journal of Enology and Viticulture 2001, 52, 67–87.
- Guidoni, S.; Ferrandino, A.; Novello, V. Effects of Seasonal and Agronomical Practices on Skin Anthocyanin Profile of Nebbiolo Grapes. American Journal of Enology and Viticulture 2008, 59, 22–29.
- Cimino, F.; Sulfaro, V.; Trombetta, D.; Saija, A.; Tomaino, A. Radical-Scavenging Capacity of Several Italian Red Wines. Food Chemistry 2007, 103, 75–81.
- Tuberoso, C. I. G.; Serreli, G.; Congiu, F.; Montoro, P.; Fenu, M. A. Characterization, Phenolic Profile, Nitrogen Compounds and Antioxidant Activity of Carignano Wines. Journal of Food Composition and Analysis 2017, 58, 60–68.
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC Analysis of Diverse Grape and Wine Phenolics Using Direct Injection and Multidetection by DAD and Fluorescence. Journal of Food Composition and Analysis 2007, 20, 618–626.
- Torchio, F.; Urcan, D.E.; Lin, L.; Gerbi, V.; Giacosa, S.; Segade, S.R.; Pop, N.; Lambri, M.; Rolle, L. Influence of Different Withering Conditions on Phenolic Composition of Avanà, Chatus And Nebbiolo Grapes for the Production of ‘Reinforced’ Wines. Food Chemistry 2016, 194, 247–256.
- Parpinello, G.P.; Rombolà, A.D.; Simoni, M.; Versari, A. Chemical and Sensory Characterisation of Sangiovese Red Wines: Comparison Between Biodynamic and Organic Management. Food Chemistry 2015, 167, 145–152.
