ABSTRACT
Nanomaterials are being applied due to their excellent characteristics, such as the high surface area-to-volume ratio, excellent physicochemical properties, and biological compatibility. Chitosan is an amino polysaccharide and a versatile, second-most abundant natural polymer. In this presented work, chitosan was purified from Penaeus vannamei from the Persian Gulf and the homogeneity level was used a combination of three procedures: deproteinization, demineralization, and deacetylation. Chitosan nanoparticles were synthesized by the ionization gelation technique. P. vannamei protease immobilized on the surface of the positively charged chitosan nanoparticles through ionic or electrostatic interaction. The immobilized enzyme exhibited better tolerance to variations in the medium pH and temperature, enhanced pH and temperature stability, improved storage stability, and good reusability compared with the free enzyme. The Michaelis–Menten kinetic constant (Km), the maximum reaction velocity (Vmax), and the catalytic efficiency (kcat) for native P. vannamei protease were 2.5 µM, 87 µM. min−1, and 132 min−1, respectively, whereas for immobilized enzyme, Km, Vmax, and kcat values were 2.7 µM, 83 µM. min−1, and 128 min−1, respectively. The effect of both soluble and immobilized P. vannamei protease on the clarification of orange juice was also analysed.
Introduction
Enzyme is a large biological molecule that regulates chemical reaction in numerous biological processes, including signal transduction, gene expression, immune response, metastasis, metabolism, pharmaceutical and medical fields, food and environmental industry, biofuel area, life science studies as well as biosensors.[Citation1–Citation7] The interest in biocatalysts for chemical production continues to grow because they generally have high stereo, chemo, and region-selectivity. They also offer an efficient and environmentally friendly catalyst without the need of high pressures, temperatures, and harsh chemical environments. However, even with these advantages, the practical use of enzymes is limited. While enzyme exhibits efficient catalytic activity under mild conditions, that is, at ambient temperatures and aqueous media, the stability and activity are also limited to operation under those conditions. The fragile nature, high cost, and high loadings required for commercial production limit the use of soluble enzyme.[Citation8–Citation10] Enzyme immobilization is one of the strategies to overcome these problems. In most industrial applications, immobilized enzymes, which feature reusability and improved stability, exhibit greater potential application than their free forms.
The catalytic behaviour of immobilized enzymes strongly depends on the properties of their carriers, such as material types, structures, and compositions.[Citation11–Citation14] Nanomaterials can serve as excellent supporting materials for enzyme immobilization, because they offer the ideal characteristics for balancing the key factors that determine the efficiency of biocatalysts, including surface area, mass transfer resistance, effective enzyme loading, and the functionality of biocatalysts, showing great potential for applications in bioconversion, biosensors, and biomedical field.[Citation15–Citation17] In polymer composites conjugated with nanoparticles, a uniform dispersion of nanoparticles leads to a very large matrix/filler interfacial area, which changes the molecular mobility, the relaxation behaviour, and the consequent thermal and mechanical properties of the material.[Citation18] Several natural polymer materials like cellulose, alginate, chitin, collagen, carrageenan, chitosan, starch, sepharose, pectin, and other natural polymer materials are commonly used as support materials. Chitin and chitosan are the second most available biopolymers after cellulose. Chitosan is the N-deacetylated product of chitin, which is a major component of arthropod and crustacean shells such as lobsters, crabs, shrimps, cuttlefishes, and lower plant and animals.[Citation19,Citation20] As a natural resource, chitosan exhibits unique interesting properties such as biocompatibility, biodegradability, and nontoxicity. For these reasons, chitosan polymer is used in several important applications in the food packaging, agriculture, biomedical and cosmetics domains, wastewater treatment, as chromatographic support, in enzyme immobilization, and as a carrier for controlled drug delivery.[Citation21,Citation22] In addition, chitosan is economically attractive because chitin is the most abundant natural polymer after cellulose. However, chitosan is macromolecular, which significantly marks its application. To overcome this drawback, the use of chitosan-fabricated nano-chitosan is effective.[Citation23–Citation25] Chitosan nanoparticles are natural materials with excellent physicochemical, antimicrobial, and biological properties, which make them a superior environmentally friendly material and they possess bioactivity that does not harm humans. Due to these unique properties, chitosan nanoparticles are being used in a vast array of widely different products and applications, ranging from pharmaceutical, tissue engineering and food packaging, biosensing and diagnostics, enzyme immobilization, and waste water treatment.[Citation18] Enzymes bound to solid surfaces are often less active than the corresponding enzymes in aqueous solutions; the decrease in the activity of the bound enzymes is often attributed to the loss of the enzyme structure, resulting from the unfavourable interactions between the enzyme and the surface functions of the solid matrix. Such enzyme–solid interactions also play a major role in the denaturation, stability, refolding, and degradation of the bound enzyme.[Citation26]
The sources of the protease enzyme of Penaeus vanamei have been studied for the first time in a previous work, and a novel thermostable protease was purified and characterized. The purified protease showed a single band on native and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with a molecular weight of 24 kDa on SDS-PAGE. The enzyme displayed broad catalytic activity over a variety of conditions, with maximal activity around pH 7 and 80°C. Activation energy for the enzymatic activity was 6.50 kcal mol−1 K−1.[Citation1]
To the best of our knowledge, studies of P. vannamei protease immobilized on chitosan nanoparticles have not been reported. In the present work, chitosan nanoparticles were prepared by the ionization gelation methodology. P. vannamei protease was immobilized over chitosan nanoparticle through a non-covalent approach and conditions for the immobilization and characterizations of the immobilization enzyme were specified. Additionally, the pH stability, thermostability, reusability, and kinetic properties of the nano-enzyme were also investigated and the data were compared with those obtained utilizing its soluble form.
Materials and methods
Chemicals
Protease enzyme isolated from P. vannamei has been purified according to a previous procedure.[Citation1] All other chemicals were of reagent grade and purchased from Merck (Darmstadt, Germany).
Specimen collection and extraction of chitosan
P. vannamei, caught from the Persian Gulf, was immediately frozen and transported to the enzymology laboratory in the University of Hormozgan, Bandar Abbas, Iran. Their shells and operculum were removed. All these waste were placed in ziploc bags and refrigerated overnight. The weight of the crushed shrimp’s exoskeletons wet samples was taken by placing them on foil paper. The samples were dried in an oven at 70°C until achieving constant weight.
A combination of three procedures – deproteinization, demineralization, and deacetylation – will provide chitosan. Around 5 g of shrimp shell waste was treated with 4% NaOH at room temperature for 24 h. The alkali was drained from the shells and washed with distilled water repeatedly until the pH dropped to neutral. This process causes the deproteinization of shells. For demineralization to yield chitin, the deproteinized shells were treated with 4% HCl at room temperature for 12 h. The acid from the chitin as drained off, washed with distilled water, and finally dried at room temperature. The process was repeated with 2% NaOH and 1% HCl. The chitin obtained still has a slight pink hue. Further decolourization can be achieved by soaking chitin in 1% potassium permanganate for 30 min, followed by 1% oxalic acid for 30 min to 2 h. The decolourized chitin can be deacetylated to form chitosan by treating with 65% NaOH for 3 days at room temperature. Alkali should be drained off and washed repeatedly with distilled water until the pH is lowered. Chitosan can be further dried at room temperature and stored.
Preparation of the chitosan nanoparticles
Chitosan nanoparticles were prepared by the ionization gelation technique.[Citation27,Citation28] In brief, 20 mg chitosan was dissolved in 40 ml of 2.0% (v/v) acetic acid. Around 2 0 ml of 0.75 mg/ml TPP was dropped into a beaker. Chitosan nanoparticles could be stably stored in distilled water. The morphological characterizations of the chitosan nanoparticles were evaluated by a scanning electron microscope.
Enzyme immobilization procedure
Around 10 mg of chitosan nanoparticles was previously incubated with 50 mM phosphate buffer pH 7.5 for 4 h. After filtration, the nanoparticles were added into 5 ml of 50 mM phosphate buffer pH 7 containing a certain amount of P. vannamei protease. The mixture was stirred in a shaker at 20◦C for 1 h and was subsequently stood at 4◦C for 1 h. The chitosan nanoparticles were collected and the supernatant was removed. The chitosan nanoparticles were washed several times with phosphate buffer (50 m M, pH 7.0) until protein in the washing could not be detected. The immobilized P. vannamei protease was then filtered and stored in phosphate buffer pH 7.0 at 4◦C.
Determination of immobilized protein amount
The amount of immobilized enzyme was estimated by subtracting the amount of protein determined in the supernatant after immobilization from the amount of protein used for immobilization. The protein content in solution was determined by the method of Bradford.[Citation29]
Enzymatic activity assay and determination of protein concentration
Protease activity was determined at room temperature in 50 mM phosphate buffer containing 2 mM EDTA, pH 7, using casein as the substrate. One unit of protease is defined as the amount of enzyme that hydrolyses casein and the liberation of ammonia to produce equivalent absorbance to 1 μmol of tyrosine/min with tyrosine as standard.
The pH profiles and pH stability of free and immobilized P. vannamei protease
The effect of pH on the activity of native and modified P. vannamei protease was determined by assaying the enzyme activity at different pH values ranging from 2.0 to 12.0 at room temperature, and the buffer systems of 50 mM sodium acetate for pH 2–6, phosphate for pH 7, Tris-HCl for pH 8–9, and sodium carbonate for pH 10–12. The residual enzymatic activity was determined as described above.
For determination of the pH stability, free and immobilized P. vannamei protease was incubated in buffer solutions at pH 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 at room temperature for 1 h separately. Then, the activity of P. vannamei protease was measured and the relative activity was calculated as a percentage of this highest activity (the highest values represent 100% of the enzyme activity).
Influence of temperature on enzyme activity and stability
The effect of temperature on the activity of immobilized native and modified P. vannamei protease was determined by assaying the enzyme activity in a standard procedure mentioned above. The temperature was varied from 20◦C to 90°C at pH 7.5. Thermal stability of the immobilized enzyme was determined by measuring the residual activity after incubating the enzyme for 1 h in a circulating water bath at 30◦C, 40◦C, 50◦C, 60◦C, 70◦C, 80◦C, and 90°C at pH 7.5. Heated samples were cooled immediately in ice water, and the residual enzymatic activity was determined as described above. Activity of the same enzyme solution kept on ice in the thermal stability experiments was considered as control (100%).
Determination of storage and recycling stability
For determination of the storage stability, free and immobilized P. vannamei protease was stored at room temperature for a certain period of time. The storage stability was compared by storage efficiency defined as
For determination of the recycling stability, the immobilized P. vannamei protease was collected after each reaction, washed with distilled water, and then utilized in the next cycle. The recycling stability of the immobilized enzyme was evaluated by measuring the enzyme activity in each successive reaction cycle.
Determination of kinetic parameters of free and immobilized P. vannamei protease
Kinetic parameters (Km, kcat and Ka = kcat/Km) have been determined according to the Michaelis–Menten equation, fitting experimental data by a nonlinear regression procedure (GraphPad Prism 5.0, GraphPad software, Inc.). The goodness-of-fit of each data set to its best-fit theoretical kinetic curve was assessed as the square of the correlation coefficient (R2). Km (Michaelis–Menten constant) is equal to the substrate concentration when the initial velocity is one-half of the maximum one (Vmax), indicating catalysis efficiency. kcat (turnover number) relates to the number of substrate molecules converted into product, in a unit of time, by an enzyme molecule fully saturated with substrate. Moreover, the affinity constant (Ka = kcat/Km) appeared useful in order to compare the free and immobilized enzyme catalytic efficiency.
Juice clarification studies
The enzymatic activity of the native and immobilized P. vannamei protease towards the clarification of turbidity was investigated using orange as a model juice. Aliquots of orange juice were poured into glasses and the reaction was initiated by adding 0.7% (w/v) of the free or immobilized enzyme. The contents were vigorously shaken and placed in a water bath at 50°C and incubated for 1 h. After treatment, the nanoparticles were separated from the reaction solution, filtered through a Whatman filter paper (No. 1). The respective filtrate solutions were stored at 4°C in the dark for up to 15 days. The turbidity of the solution was measured immediately after the clarification treatment (day 0), and after 1, 7, and 15 days of storage at 4°C. All experiments were performed using three duplicates. Data presented in the figures correspond to mean values with standard errors.
Results and discussion
Effect of immobilization on pH profile and pH stability
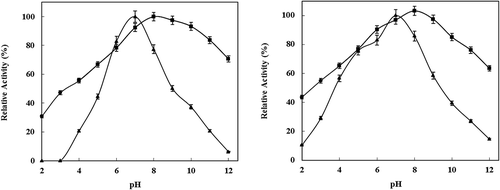
The effect of pH on the activity of free and immobilized P. vannamei protease in casein hydrolysis was determined in the pH range 2.0–12.0 and the results are presented in . Soluble enzyme showed maximum activity at pH 7 with a sharp decrease in activity below pH 4 and above pH 8. The immobilized enzyme showed less susceptibility to pH change and showed improved activity in the pH range 3–9. The immobilization of the enzyme affected the pH profile of P. vannamei protease by shifting the optimal pH to a more alkali level (pH 8–9). This phenomenon can be explained that when the surrounding of enzymes is gathered cations, the number of cations around the enzyme would increase, and thus the microenvironment around the enzyme catalytic zone would be slightly alkaline and hence the optimal pH value of the immobilized enzyme is shifted from 7.0 to 8 compared with the free form. On the other hand, the drop in the relative enzyme activity at lower pH was less significant than it was for the free enzyme, likely due to the protection afforded to the enzyme by the chitosan nanoparticles matrix. Thus, the immobilization process can also protect the enzyme from alkaline and severe acidic media. shows the effect of different pH values on the stability of soluble and immobilized P. vannamei protease. The pH stabilities of free and immobilized P. vannamei protease were investigated in the medium with pH value between 3 and 12 at room temperature for 1 h (). The maximum activity of P. vannamei protease was taken as 100%. The optimum pH values of free and immobilized enzyme were 7.0 and 8.0, respectively. As can be seen, the optimum pH of the resultant nano-enzyme shifted from 7.0 to 8.0, which was mainly due to the charge changes of the chitosan nanoparticles surface. As a result, the microenvironment had some changes accordingly, which induced the shifting of the optimal pH. Additionally, the immobilized enzyme retained more than 30 and 64% of its activity at pH 3 and 12.0, respectively. Under the same conditions, a nearly complete inactivation of native P. vannamei protease was observed. The increased enzyme stability on the chitosan nanoparticles was attributed to the decreased lateral interactions between the P. vannamei protease molecules and the nanoscale supports. The strong resistance of the chitosan nano- enzyme against the acidic and alkaline changes in medium was tentatively ascribed to the confinement effect of the carrier. The wide range of stability attained after immobilization might be due to the low diffusional limitations or the secondary interaction between the enzyme and the support. Extreme pH could induce free enzyme unfolding, but the confinement of the chitosan nanoparticles matrix inhibited to some extent the unfolding–refolding motions of the enzyme inside. The expansion of pH stability should be attributed to the strong electrostatic interaction formed between chitosan nanoparticles and P. vannamei protease at the experimental pH range, which is one of the principal forces to maintain the active conformation of enzymes. The high pH stability of nano-P. vannamei protease is important in the use of this enzyme in industrial applications.
Figure 1. a) Effect of pH on the activities of free (▲) and immobilized (■) enzyme. The activity at optimal pH was taken as 100%. b) Irreversible inactivation of free (▲) and immobilized (■) P. vannamei protease after incubation for different times at pH 3.0–12. The activity of untreated enzyme was taken as 100%.
The temperature profile, thermal stability, and thermodynamic study of soluble and immobilized P. vannamei protease
The effect of temperature on the activity of free and immobilized enzyme was studied by keeping the reaction vessel in a thermostat at different temperatures ranging from 30 to 90°C. Temperature has a greater impact on the activity of both free and immobilized enzyme as shown in . For the free enzyme, the activity drops suddenly when the reaction temperature was varied from its optimum value of 60°C. The immobilized enzyme was found to be stable at an optimum temperature of about 70°C, as the free form showed only a 40% reduction in activity at this reaction temperature. This increase in optimum temperature and activity stability on a wide range of temperatures for the immobilized enzyme might be due to the change in the conformational integrity of the enzyme structure by the immobilization method.[Citation30–Citation32] Immobilization of P. vannamei protease on chitosan nanoparticles caused an increase in enzyme rigidity, which is commonly reflected by the increase in stability towards denaturation by raising the temperature.
Figure 2. a) Effect of temperature on the activities of free (▲) and immobilized (■) enzyme. The activity at optimal temperature was taken as 100%. b) Irreversible thermoinactivation of free (▲) and immobilized (■) P. vannamei protease at 30–90°C. The activity of the same enzyme solution, kept on ice, was considered as the control (100%).
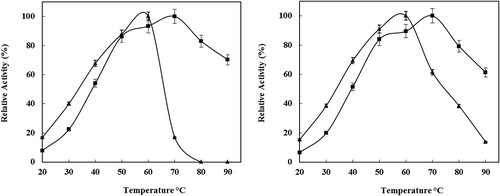
Thermal stability was investigated by incubating free and immobilized P. vannamei protease on chitosan nanoparticles at temperatures ranging from 30◦C to 90°C for 1 h and then determining the activity at optimum reaction temperature. The effect of temperature on the stability of free and immobilized enzyme is illustrated in . Free P. vannamei protease has shown high stability at 60°C, and immobilized biocatalysis has high stability at 40–90°C. There was no activity loss for the immobilized P. vannamei protease at 70°C. However, the free P. vannamei protease activity decreased at the same temperature. At 70°C, immobilized P. vannamei protease on chitosan nanoparticles retained an activity of about 100%, whereas the activity retained by the free P. vannamei protease was only 17%. This result could be explained by the restricted conformational mobility of the molecules after immobilization.[Citation33,Citation40] The significant improvement of the thermal stability might be due to the movement constraints imposed and the protective function by the rigid chitosan nanoparticles matrix. The activity was low at the incubation time of 1 h, which could be ascribed to the effect of mass transfer. Enhanced thermal stability is one of the most common properties desired as output from a protein engineering study and is often an important economic factor.[Citation34]
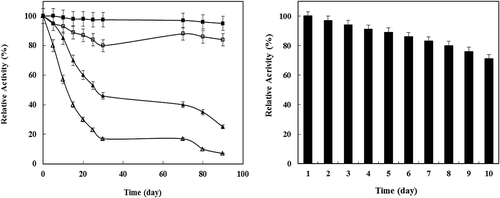
Storage stability and reusability
The high cost of enzymes for industrial use and the time required to immobilize them have led to increased interest in the storage stability of these enzymes.[Citation35] In general, an enzyme in solution is not stable during storage, and its activity is gradually reduced. The stability of P. vannamei protease was enhanced upon immobilization. The activity of the free enzyme dropped significantly faster than that of the immobilized P. vannamei protease under the same storage conditions after 20 days (). As the number of storage days increased, the P. vannamei protease immobilized on the chitosan nanoparticles support exhibited higher stability, which can be attributed to the limited conformational changes in enzyme molecules in the matrix of chitosan nanoparticles. The potential application of the immobilized P. vannamei protease was further investigated by examining the reusability. When comparing the performance of immobilized biocatalysts, intended for preparative or industrial use, characterization of their operational stabilities is very important. The operational stability of immobilized P. vannamei protease in the current study was evaluated in a repeated batch process. shows the effect of repeated use on the activity of immobilized P. vannamei protease. The P. vannamei protease immobilized chitosan beads retained a specific activity of 90% after five reuses. It should be noted that this high operational stability could significantly reduce the operation cost in practical applications.
Kinetic parameters of the free and immobilized P. vannamei protease
P. vannamei protease and its immobilized form revealed a Michaelis–Menten type of kinetic when hydrolysing casein. The kinetic parameters were calculated from the Michaelis–Menten plot. As indicated in , the Km values of the free and immobilized P. vannamei protease were 253 and 261 µM, respectively, which indicates that immobilized P. vannamei protease has a lower binding affinity for the substrate than the free enzyme. The increase of Km value indicated that the enzyme immobilized on chitosan nanoparticles had a lower affinity for binding substrate than free enzyme. This may be due to the steric effect arising from the structural rigidity of the entire enzyme structure, which was distorted after immobilization. Moreover, the slight increase in Km value may be due to diffusion limitation, the steric hindrance of the active site, and/or the loss of enzyme flexibility necessary for substrate binding by immobilization.[Citation36,Citation37] The decrease in kcat could be due to the restricted diffusion of the substrate to the active site and the higher structural rigidity of the immobilized enzyme. Increase in Km and decrease in kcat values upon immobilization, which are common in most immobilized enzymes, have been frequently reported.[Citation7,Citation37,Citation38]
Table 1 Kinetic parameters for free and immobilized forms of P. vannamei protease.
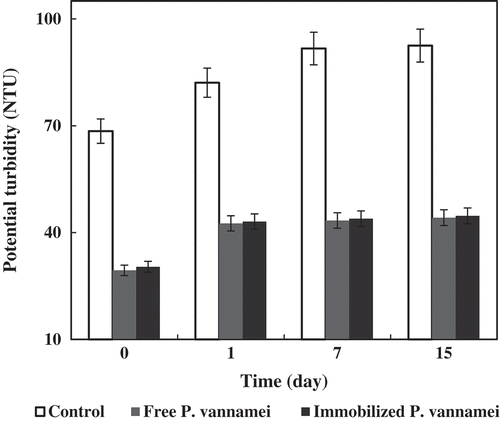
Effect of free and immobilized P. vannameiprotease treatment on turbidity in orange juice
The performance of the free and immobilized P. vannamei protease for the clarification of pomegranate juice is herein discussed (). The turbidity-reducing effect of either form of the enzymes, after the treatment, at day 0 was statistically significant. The turbidity-lowering effect of the protease treatment was maintained until the end of the cold storage period, at day 15. The protease catalyses the hydrolysis of the proteins in the juice and prevents the formation of complexes between the positively charged proteins and the negatively charged pectin. The insolubility and turbidity effects, induced by these complexes, resulting from the association of proteins with cell materials and/or phenolic compounds, could be alleviated under proteolytic actions .[Citation38,Citation39] Both forms of protease effectively decreased the development of turbidity during cold storage.
Conclusion
The catalytic efficacy of P. vannamei protease by immobilizing onto marine chitosan nanoparticles, with concomitant enhancement in enzyme immobilization efficiency, activity, and storage stability, opens up new avenues in biotechnological applications. This immobilization strategy furnished good reusability, so the immobilized P. vannamei protease can be successively reused 10 times without significant loss of its catalytic performance. Furthermore, the immobilized enzyme showed efficiency in orange juice clarification, which was better than the free form. This process of enzyme immobilization is simple, robust, and applicable to other enzymes for potential applications in the industry. This marine chitosan nanoparticles material may be a promising material for the immobilization of other industrially important enzymes.
Funding
Financial support from the University of Hormozgan is gratefully acknowledged.
Additional information
Funding
References
- Dadshahi, Z.; Homaei, A.; Zeinali, F.; Sajedi, R.H.; Khajeh, K. Extraction and Purification of a Highly Thermostable Alkaline Caseinolytic Protease from Wastes Litopenaeus Vanamei Suitable for Food and Detergent Industries. Food Chemistry [Internet]. Elsevier Ltd 2016, 202, 110–115. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0308814616301030
- Syshchyk, O.; Skryshevsky, V.A.; Soldatkin, O.O.; Soldatkin, A.P. Enzyme Biosensor Systems Based on Porous Silicon Photoluminescence for Detection of Glucose, Urea and Heavy Metals. Biosensors Bioelectronics [Internet]. Elsevier 2015, 66, 89–94. doi: 10.1016/j.bios.2014.10.075
- Xiaoyan, Z.; Yuanyuan, J.; Zaijun, L.; Zhiguo, G.; Guangli, W. Improved Activity and Thermo-Stability of the Horse Radish Peroxidase with Graphene Quantum Dots and Its Application in Fluorometric Detection of Hydrogen Peroxide. Spectrochimica Acta Particle A Molecular Biomolecular Spectroscopic [Internet]. Elsevier B.V. 2016, 165, 106–113. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1386142516301603
- Homaei, A.; Stevanato, R.; Etemadipour, R.; Hemmati, R. Purification, Catalytic, Kinetic and Thermodynamic Characteristics of a Novel Ficin from Ficus Johannis. Biocatal Agricultural Biotechnology [Internet]. Elsevier Ltd 2017, 10, 360–366. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1878818117300920
- Homaei, A. Purification and Biochemical Properties of Highly Efficient Alkaline Phosphatase from Fenneropenaeus Merguiensis Brain. Journal Molecular Catalysis B Enzymes [Internet]. Elsevier B.V. 2015, 118, 16–22. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1381117715001216
- Zeinali, F.; Homaei, A.; Kamrani, E. Sources of Marine Superoxide Dismutases: Characteristics and Applications. International Journal of Biological Macromolecule [Internet]. Elsevier B.V. 2015, 79, 627–637. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0141813015003943
- Rouzbehan, S.; Moein, S.; Homaei, A.; Moein, M.R. Kinetics of Α-Glucosidase Inhibition by Different Fractions of Three Species of Labiatae Extracts: A New Diabetes Treatment Model. Pharmaceutical Biology [Internet]. Informa Healthcare USA, Inc 2017, 55, 1483–1488. Available from: https://www.tandfonline.com/doi/full/10.1080/13880209.2017.1306569
- Tran, D.N.; Balkus, K.J. Perspective of Recent Progress in Immobilization of Enzymes. ACS Catalysis 2011, 1, 956–968.
- Homaei, A.; Samari, F. Investigation of Activity and Stability of Papain by Adsorption on Multi-Wall Carbon Nanotubes. International Journal of Biological Macromolecule [Internet] 2017. [cited 2017 Apr 20]. Available from: http://www.sciencedirect.com/science/article/pii/S0141813017305160
- Homaei, A.A.; Mymandi, A.B.; Sariri, R.; Kamrani, E.; Stevanato, R.; Etezad, S.-M.; et al. Purification and Characterization of a Novel Thermostable Luciferase from Benthosema Pterotum. Journal of Photochemistry and Photobiological B [Internet]. Elsevier B.V. 2013, 125, 131–136. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23811161
- Xu, R.; Tang, R.; Zhou, Q.; Li, F.; Zhang, B. Enhancement of Catalytic Activity of Immobilized Laccase for Diclofenac Biodegradation by Carbon Nanotubes. Chemical Engineering Journal [Internet]. Elsevier B.V. 2015, 262, 88–95. doi: 10.1016/j.cej.2014.09.072
- Homaei, A.; Barkheh, H.; Sariri, R.; Stevanato, R. Immobilized Papain on Gold Nanorods as Heterogeneous Biocatalysts. Amino Acids [Internet] 2014, 46, 1649–1657. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24658998
- Homaei, A.; Etemadipour, R. Improving the Activity and Stability of Actinidin by Immobilization on Gold Nanorods. International Journal of Biological Macromolecule [Internet] 2015, 72, 1176–1181. Available from: http://www.sciencedirect.com/science/article/pii/S0141813014007053
- Homaei, A. Enzyme Immobilization and Its Application in the Food Industry. In Advance Food Biotechnology, 1st Edn [Internet].; Rai, R.; Eds.; John Wiley & Sons, Ltd., 2015; 145–164. Available from: http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1118864557.html
- Feng, W.; Ji, P. Enzymes Immobilized on Carbon Nanotubes. Biotechnology Advancement [Internet]. Elsevier Inc. 2011, 29, 889–895. doi: 10.1016/j.biotechadv.2011.07.007
- Wang, Q.; Zhou, L.; Jiang, Y.; Gao, J. Improved Stability of the Carbon Nanotubes-Enzyme Bioconjugates by Biomimetic Silicification. Enzyme Microbiological Technology [Internet]. Elsevier Inc. 2011, 49, 11–16. doi: 10.1016/j.enzmictec.2011.04.007
- Ansari, S.A.; Husain, Q. Potential Applications of Enzymes Immobilized On/In Nano Materials: A Review. Biotechnology Advancement [Internet]. Elsevier Inc. 2012, 30, 512–523. doi: 10.1016/j.biotechadv.2011.09.005
- Malmiri, H.J.; Ali, M.; Jahanian, G.; Berenjian, A. Potential Applications of Chitosan Nanoparticles as Novel Support in Enzyme Immobilization. American Journal of Biochemistry and Biotechnology. 2012, 8, 203–219.
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Progress Polymer Sciences 2006, 31, 603–632.
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas-Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; et al. Functionalization of Chitosan by a Free Radical Reaction: Characterization, Antioxidant and Antibacterial Potential. Carbohydrate Polymer 2017, 155, 117–127.
- Zheningt, A.; Juningq, I.; Lus, H. Preparation of Chitosan Nanoparticles as Carrier for Immobilized Enzyme. Applied Biochemistry and Biotechnology 2007, 136, 77–96.
- Aljawish, A.; Muniglia, L.; Klouj, A.; Jasniewski, J.; Scher, J.; Desobry, S. Characterization of Films Based on Enzymatically Modified Chitosan Derivatives with Phenol Compounds. Food Hydrocolloids 2016, 60, 551–558.
- Gouda, M.; Elayaan, U.; Youssef, M.M. Synthesis and Biological Activity of Drug Delivery System Based on Chitosan Nanocapsules. Advancement Nanoparticles [Internet]. Scientific Research Publishing 2014, 3, 148–158. [cited 2016 Sep 12]. doi: 10.4236/anp.2014.34019
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and Characterization of Chitosan Microspheres as Drug Carrier for Prednisolone Sodium Phosphate as Model for Anti-Inflammatory Drugs. Journal Control Release [Internet]. Elsevier 1996, 39, 17–25. [cited 2016 Sep 12]. Available from: http://linkinghub.elsevier.com/retrieve/pii/0168365995001298
- Tian, X.X.; Groves, M.J. Formulation and Biological Activity of Antineoplastic Proteoglycans Derived from Mycobacterium Vaccae in Chitosan Nanoparticles. The Journal of Pharmacy and Pharmacology 1999, 51, 151–157.
- Shi, W.; Wei, M.; Jin, L.; Li, C. Calcined Layered Double Hydroxides as a “Biomolecular Vessel” for Bromelain: Immobilization, Storage and Release. Journal Molecular Catalysis B Enzymes 2007, 47, 58–65.
- Rudzinski, W.E.; Palacios, A.; Ahmed, A.; Lane, M.A.; Aminabhavi, T.M. Targeted Delivery of Small Interfering RNA to Colon Cancer Cells Using Chitosan and PEGylated Chitosan Nanoparticles. Carbohydrate Polymer [Internet]. 2016, 147, 323–332. [cited 2016 Sep 12]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27178938
- Tang, Z.-X.; Qian, J.-Q.; Shi, L.-E. Characterizations of Immobilized Neutral Lipase on Chitosan Nano-Particles. Materials Letters [Internet]. 2007, 61, 37–40. [cited 2016 Sep 12]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0167577X06004587
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analysis Biochemical [Internet]. 1976, 72, 248–254. [cited 2014 Jul 9]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/942051
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent Advances in Enzyme Immobilization Techniques: Metal-Organic Frameworks as Novel Substrates. Coord Chemical Reviews 2016, 322, 30–40.
- Wu, L.; Wu, S.; Xu, Z.; Qiu, Y.; Li, S.; Xu, H. Modified Nanoporous Titanium Dioxide as a Novel Carrier for Enzyme Immobilization. Biosensors Bioelectronics 2016, 80, 59–66.
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes. Advancement Food Nutritional Researcher 2016, 79, 179–211.
- Xiao, A.; Xu, C.; Lin, Y.; Ni, H.; Zhu, Y.; Cai, H. Preparation and Characterization of Κ-Carrageenase Immobilized onto Magnetic Iron Oxide Nanoparticles. Electronic Journal of Biotechnology 2016, 19, 1–7.
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From Protein Engineering to Immobilization: Promising Strategies for the Upgrade of Industrial Enzymes. International Journal Molecular Sciences [Internet]. 2013, 14, 1232–1277. [cited 2016 Oct 12]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23306150
- Wu, C.; Xu, C.; Ni, H.; Yang, Q.; Cai, H.; Xiao, A. Preparation and Characterization of Tannase Immobilized onto Carboxyl-Functionalized Superparamagnetic Ferroferric Oxide Nanoparticles. Bioresource Technology 2016, 205, 67–74.
- Homaei, A.; Saberi, D. Immobilization of Α-Amylase on Gold Nanorods: An Ideal System for Starch Processing. Process Biochemistry (Barking, London, England) [Internet]. 2015, 50:1394–9. Available from: http://www.sciencedirect.com/science/article/pii/S1359511315300118
- Homaei, A.;. Enhanced Activity and Stability of Papain Immobilized on CNBr-activated Sepharose. International Journal of Biological Macromolecule [Internet]. 2015, 75, 373–377. Available from: http://www.sciencedirect.com/science/article/pii/S0141813015000665
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of Protein−Polyphenol Haze in Beverages; Journal of Agricultural and Food Chemistry 1996, 44, 1997–2005.
- Pinelo, M.; Zeuner, B.; Meyer, A.S. Juice Clarification by Protease and Pectinase Treatments Indicates New Roles of Pectin and Protein in Cherry Juice Turbidity. Food Bioproduction Processing 2010, 88, 259–265.
- Homaei, A.; Etemadipour, R. Improving the Activity and Stability of Actinidin by Immobilization on Gold Nanorods. International Journal of Biological Macromolecule [Internet]. Elsevier B.V. 2015, 72, 1176–1181. doi: 10.1016/j.ijbiomac.2014.10.029