ABSTRACT
Ipomoea batatas L. is widely used as a functional food in many countries. In this work, I. batatas leaf extracts (Soxhlet extract, microwave [MW] extract, and decoction extract) were characterized for the first time, and their total flavonoid and phenolic compound contents were measured by spectrophotometric and chromatographic analyses. These extracts were investigated for their antioxidant activities (free radical scavenging and reducing power assays), enzyme inhibitory activities (cholinesterase, tyrosinase, α-amylase, and α-glucosidase) and effects on inflammation pathways. Various bioactive secondary metabolites were identified; among them, chlorogenic acid appeared to be the most abundant. The decoction extract displayed the highest level of phenolics (89.26 mg GAE/g extract) and thus exhibited stronger antioxidant ability than the Soxhlet and MW extracts. Each preparation was tested in in vitro enzymatic assays, which revealed that all three extracts exhibited interesting inhibitory activities against acetylcholinesterase, α-glucosidase, and α-amylase. Moreover, in vitro and ex vivo anti-inflammatory activities were observed for the decoction extract. Collectively, these results indicated that I. batatas leaf can be considered a potential candidate for the development of functional foods to combat the symptoms of metabolic disorders, such as type II diabetes mellitus.
Introduction
In recent years, plant-based products have been considered to be major sources for developing new drugs and functional products due to the negative effects of synthetics. Currently, many studies related to natural products have had substantial impacts in pharmaceutical and food fields.[Citation1] Ipomoea batatas L., which is commonly known as sweet potato, is a dicotyledonous plant belonging to the Convolvulaceae family, of which the edible part is its tuberous root.[Citation2] Native to the tropical regions of the Americas, it is distinguished by its particularly soft flesh and nutrient content. Additionally, I. batatas is acknowledged as a necessary food to fight malnutrition due to its high nutritional value and ease of cultivation and productivity. For example, it is cultivated for its high vitamin A content in Africa, where it is estimated that more than 3 million children suffer from diseases caused by the lack of this essential vitamin.[Citation3] Used worldwide as a side dish with fish or meat, sweet potato is also a classic winter street food in the Middle East, China, and the Philippines, where it is a staple in stews and soups.[Citation4] In addition to being rich in vitamins, its root contains complex carbohydrates, dietary fiber, and other micronutrients, including essential minerals, such as potassium, manganese, iron, and calcium.[Citation5] It is also naturally free of fat and cholesterol, low in sodium, and less calorically dense than traditional potatoes.[Citation6] Despite the abundance of research about its tuberous roots as a nutraceutical product useful for the management of diabetes mellitus,[Citation7,Citation8] the phytochemical composition and biological properties of I. batatas leaves have scarcely been investigated.[Citation9–Citation11] However, the high radical scavenging activity of these leaves and their ability to suppress the oxidation of low-density lipoprotein (LDL) have been well documented.[Citation12–Citation14] In this study, we report the qualitative high-performance liquid chromatography (HPLC) analysis of three preparations, including their in vitro antioxidant activities; enzymatic inhibitory profiles against α-glucosidase, tyrosinase, α-amylase, and cholinesterases (ChEs) (butyrylcholinesterase [BChE] and acetylcholinesterase [AChE]); and anti-inflammatory activity (reactive oxygen species [ROS], nitrite, inducible nitric oxide synthase [iNOS] gene expression and prostaglandin E2 [PGE2] reduction). Thus, the present study may offer new and innovative perspectives on I. batatas leaves for the development of new functional products endowed with promising pharmacological features.
Materials and methods
Plant material
Fresh leaves of I. batatas L. were manually collected in October 2015 in Anguillara Veneta (Northern Italy). The plant was authenticated by a senior professor of botany in our Department of Pharmacy. All herbal material was frozen in liquid nitrogen, minced and freeze-dried using lyophilization (VirTis, Gardiner, NY, US). The dry raw material was ground to a fine powder, passed through a 40-mesh to obtain uniform granulometry, and stored in a vacuum box in the dark at 4 °C until extraction.
Reagents and standards
Analytical standards of gallic acid, catechin, chlorogenic acid, p-OH benzoic acid, vanillic acid, epicatechin, syringic acid, 3-OH benzoic acid, 3-OH-4-MeO benzaldehyde, p-coumaric acid, rutin, sinapic acid, t-ferulic acid, naringin, 2,3-di-MeO benzoic acid, benzoic acid, o-coumaric acid, quercetin, t-cinnamic acid, and naringenin (purity ≥95%) were purchased from Sigma-Aldrich (Milan, Italy). The stock standard solution was prepared by dissolving 10 mg of the analyte in 10 mL of methanol and stored in a glass-stoppered bottle at 4 °C in the dark. Standard working solutions at various concentrations were prepared daily by the appropriate dilution of aliquots of the stock solutions in water. HPLC-grade methanol and acetonitrile were purchased from Sigma-Aldrich (Milan, Italy), while HPLC-grade acetic acid (99–100%) was purchased from J.T. Baker B.V. (Deventer, Holland). For sample preparation and chromatographic analysis, deionized water ≥ 18 MΩ•cm resistivity purified with a Milli-Q system (Millipore, Bedford, USA) was used. All solvents and solutions were filtered through a 0.45-µm polytetrafluoroethylene (PTFE) filter from Supelco (Bellefonte, PA, USA) before use. 5,5-Dithio-bis(2-nitrobenzoic) acid) (DTMB), AChE (electric eel AChE, Type-VI-S, EC 3.1.1.7), BChE (horse serum BChE, EC 3.1.1.8), acetylthiocholine iodide (ATCI), butyrylthiocholine chloride (BTCl), galantamine hydrobromide (from Lycoris sp., ≥94%), α-amylase (porcine pancreas, EC 3.2.1.1), α-glucosidase (from Saccharomyces cerevisiae, EC 3.2.1.20), 4-nitrophenyl-α-D-glucopyranoside (PNPG), tyrosinase (from mushroom, EC 1.14.18.1), L-2,3-dihydroxyphenylalanine (L-DOPA), kojic acid (≥99%) and acarbose (≥95%) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Soxhlet extraction of I. batatas leaves
For the Soxhlet extractions, 500 mg of freeze-dried herbal material was inserted into a paper filter vessel and introduced into the Soxhlet extractor. Then, the extraction was performed with 50 mL of 60% MeOH in water. The Soxhlet extractor was allowed to reflux for 6 h or until at least three cycles of extraction had been completed. The solution was filtered and freeze-dried.
Microwave (MW) extraction of I. batatas leaves
MW-assisted extraction was performed on 500 mg of raw material inserted in a glass-sealed vial and suspended in 20 mL of 60% MeOH in water. The temperature was thermostated at 80 °C, the power of the instrument was fixed at 60 W, and the extraction time was 5 min. The resulting suspension was filtered and freeze-dried.
Decoction preparation
For each sample, 500 mg of fresh leaves was freeze-dried, suspended in 20 mL of 60% MeOH in water and boiled for 5 min. The leaves were filtered and freeze-dried again.
Antioxidant properties
Antioxidant assays (2,2-diphenyl-1-picrylhydrazyl [DPPH] and 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid [ABTS]) radical scavenging results and the reducing power in terms of the cupric reducing antioxidant capacity [CUPRAC] and ferric reducing ability of plasma [FRAP]), were reported in our previous papers.[Citation15,Citation16] The antioxidant capacities were expressed as trolox equivalents (mmolTEs/g extract). The total phenolic content was also determined by the Folin–Ciocalteu method [Citation17] and expressed as gallic acid equivalents (mgGAEs/g extract), whereas the total flavonoid content was determined using the AlCl3 method[Citation18] and expressed as rutin equivalents (mg REs/g extract).
ChE inhibition
The ChE inhibitory activity was measured using Ellman’s method, as reported previously.[Citation19] An aliquot of sample solution (50 µL, 2 mg/mL) was mixed with 125 µL of DTNB and AChE or BChE solution (25 µL) in Tris-HCl buffer (pH 8.0) in a 96-well microplate and incubated for 15 min at 25 °C. The reaction was initiated by the addition of ATCI or BTCl (25 µL). A blank was prepared by adding sample solution to all reaction reagents without the enzyme(s) (AChE or BChE) solution. The sample and blank absorbance values were recorded at 405 nm after 10 min of incubation at 25 °C. The absorbance of the blank was subtracted from that of the sample, and the ChE inhibitory activity was expressed as milligrams of galantamine equivalents (mg GALAEs/g extract).
α-Amylase inhibition
The α-amylase inhibitory activity was measured using the Caraway–Somogyi iodine/potassium iodide (IKI) method.[Citation20] The sample solution (25 µL, 2 mg/mL) was mixed with α-amylase solution (50 µL) in phosphate buffer (pH 6.9 with 6-mM sodium chloride) in a 96-well microplate and incubated for 10 min at 37 °C. After pre-incubation, the reaction was initiated by the addition of starch solution (50 µL, 0.05%). Similarly, a blank was prepared by adding sample solution to all reaction reagents without enzyme solution. The reaction mixture was incubated for 10 min at 37 °C and stopped by adding HCl (25 µL, 1 M). This was followed by the addition of the IKI solution (100 µL). The sample and blank absorbance values were recorded at 630 nm. The absorbance of the blank was subtracted from that of the sample, and the α-amylase inhibitory activity was expressed as millimoles of acarbose equivalents (mmol ACAEs/g extract).
α-Glucosidase inhibition
The α-glucosidase inhibitory activity was measured as described previously. [Citation20] The sample solution (50 µL) was mixed with glutathione (50 µL, 2 mg/mL), 50 µL of α-glucosidase solution in phosphate buffer (pH 6.8), and 50 µL of 10-mM PNPG solution in a 96-well microplate and incubated for 15 min at 37 °C. A blank was prepared by adding the sample solution to all reaction reagents without enzyme solution. The reaction was stopped by the addition of sodium carbonate (50 µL, 0.2 M). The sample and blank absorbance values were recorded at 400 nm. The absorbance of the blank was subtracted from that of the sample, and the α-glucosidase inhibitory activity was expressed as millimoles of acarbose equivalents (mmol ACAEs/g extract).
Tyrosinase inhibition
The tyrosinase inhibitory activity was measured using a modified dopachrome method with L-DOPA as the substrate, as reported previously[Citation20] with slight modifications. The sample solution (25 µL, 2 mg/mL) was mixed with tyrosinase solution (40 µL) and phosphate buffer (100 µL, pH 6.8) in a 96-well microplate and incubated for 15 min at 25 °C. The reaction was initiated by the addition of L-DOPA (40 µL). A blank was prepared by adding sample solution to all reaction reagents without the enzyme (tyrosinase) solution. The sample and blank absorbance values were recorded at 492 nm after incubating for 10 min at 25 °C. The absorbance of the blank was subtracted from that of the sample, and the tyrosinase inhibitory activity was expressed as milligrams of kojic acid equivalents (mg KAEs/g extract).
HPLC-photodiode array (PDA) instrument configuration
A stock solution was prepared for each sample with 1 mg in 1 mL of eluent, and HPLC analyses were performed on a Waters liquid chromatograph equipped with a PDA detector and a C18 reversed-phase column to separate the mixture. The column was thermostated at 30 ± 1 °C, and the elution was performed using a water–acetonitrile mobile phase (93:7, with 3% acetic acid). The complete separation of standards was achieved in 60 min. The ultraviolet/visible (UV/Vis) acquisition wavelength was set in the range of 200–500 nm. Quantitative analyses were performed at the maximum wavelength for each compound according to a validated procedure.[Citation19]
Molecular modeling
Enzymes preparation
Molecular modeling studies were performed to elucidate the possible inhibition of α-glucosidase by chlorogenic acid; docking and molecular dynamics results for chlorogenic acid with tyrosinase were previously published by our group.[Citation21] The crystallographic enzyme structure was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB)[Citation22]: α-glucosidase (PDB code: 3AXI).[Citation23] The enzyme was prepared for docking using Swiss-PDB viewer[Citation24] to correct errors in the crystal structures and finalized with the Wizard Preparation tool embedded in Maestro 10.0.6.[Citation25]
Ligand preparation
The chlorogenic acid structure was downloaded from the zinc database,[Citation26] neutralized at neutral pH and minimized. This ligand was used in docking experiments without further modifications.
Docking experiments
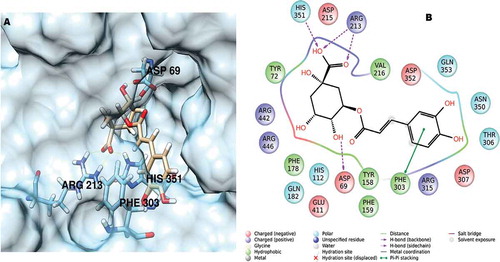
Docking experiments were performed with Gold suite 6 software[Citation21] using the scoring function GoldScore, as reported previously.[Citation21] The docking grid was automatically calculated in a radius of 10 Å from the center of the crystallographic ligand. The best docking position found for chlorogenic acid in complex with α-glucosidase is depicted in .
ROS production
ROS generation was assessed using an ROS-sensitive fluorescence indicator: 2ʹ,7ʹ-dichlorodihydrofluorescein in diacetate (DCFH-DA). When DCFH-DA is introduced into viable cells, it penetrates the cell and becomes deacetylated by intracellular esterases to form DCFH, which can react quantitatively with ROS within the cell and be converted to 2ʹ,7ʹ-dichlorofluorescein (DCF), which can be detected by a fluorescence spectrophotometer. To determine any intracellular effects on ROS production, cells were seeded in a black 96-well plate (1.5 x 104 cells/well) in medium containing a scalar concentration of extracts. Immediately after seeding, cells were stimulated for 1 h with H2O2 (1 mM). After the cells were incubated with DCFH-DA (20 μM) for 30 min, the fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorescence microplate reader.
PGE2 radioimmunoassay (RIA)
The PGE2 levels (pg/mL) were measured by RIA, as reported previously.[Citation27,Citation28] Briefly, specific anti-PGE2 antibodies were developed in rabbit, and the cross-reactivity against other prostanoids was <0.3%. One hundred microliters of prostaglandin standard or sample was incubated overnight at 4 °C with3H-prostaglandin (3000 cpm/tube; NEN) and antibody (final dilution: 1:120000) in 1.5 mL of 0.025-M phosphate buffer. Free and antibody-bound prostaglandins were separated by the addition of 100 μL 5% bovine serum albumin and 100 μL 3% charcoal suspension, followed by centrifugation for 10 min at 4,000 x g at 5 °C and decanting of the supernatants into the scintillation fluid (Ultima Gold™, Perkin Elmer) for β emission counting. The detection limit of the assay method was 0.6 pg/mL.
Ex vivo studies
Male C57BL6 mice were housed in Plexiglas cages (40 cm × 25 cm × 15 cm), one mouse per cage, in acclimatized colony rooms (22 ± 1 °C; 60% humidity) on a 12-h/12-h light/dark cycle (light phase: 07:00–19:00 h). The mice were given free access to tap water and food 24 h/day throughout the study, with no fasting periods. The mice were fed a standard laboratory diet (3.5% fat, 63% carbohydrate, 14% protein, and 19.5% other components without caloric value; 3.20 kcal/g). The housing conditions and experimentation procedures were all strictly in accordance with the European Union ethical regulations on animal care for scientific research. According to the recognized ethical principles of “Replacement, Refinement and Reduction of Animals in Research”, liver specimens were obtained as residual material from vehicle-treated mice that were randomized in our previous experiments. These experiments were approved by the Local Ethical Committee (G. D’Annunzio University) and Italian Health Ministry (project n.955/2016-PR). The mice were sacrificed through CO2 inhalation (100% CO2 at a flow rate of 20% of the chamber volume per min), and liver specimens were immediately collected and maintained in a humidified incubator with 5% CO2 at 37 °C for 4 h in Dulbecco’s modified Eagle medium (DMEM) buffer with added bacterial lipopolysaccharide (LPS) (10 µg/mL) (incubation period), as described previously.[Citation29] During incubation, tissues were treated with scalar concentrations of I. batatas leaf extract (100–1000 μg/mL). Finally, liver specimens were assayed for nitrite determination and iNOS gene expression.
Nitrite measurement
Nitrites are stable nitric oxide end products that are commonly used as an index of in vivo nitric oxide production. Liver specimens were homogenized for 2 min with a Potter–Elvehjem homogenizer in 2 mL of DMEM buffer. The homogenate was centrifuged at 4,500 g for 10 min, and nitrite production was determined in the supernatant fraction by mixing 50 µL of the assay buffer with 50 µL of Griess reagent (1.5% sulfanilamide in 1-M HCl plus 0.15% N-(1-naphthyl) ethylenediamine dihydrochloride in distilled water, v/v). After 10 min of incubation at room temperature, the absorbance at 540 nm was determined, and the nitrite concentrations were calculated from a sodium nitrite standard curve. All measurements were performed in triplicate.
INOS gene expression analysis
Immediately after incubation, liver specimens were rapidly removed, dissected and stored in RNAlater solution (Ambion, Austin, TX) at −20 °C until further processing. Total RNA was extracted from the liver using TRI Reagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s protocol. Contaminating DNA was removed using 2 units of RNase-free DNase 1 (DNA-free kit, Ambion, Austin, TX), according to the manufacturer’s instructions. The RNA solution was quantified at 260 nm using a spectrophotometer (BioPhotometer, Eppendorf, Germany), and its purity was assessed by the ratio of the readings at 260 and 280 nm. The quality of the extracted RNA samples was also determined by electrophoresis through agarose gels and staining with ethidium bromide, followed by visualization under UV light. One microgram of total RNA extracted from each sample in a 20-μL reaction volume was reverse-transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Reactions were incubated in a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) initially at 25 °C for 10 min, then at 37 °C for 120 min, and finally at 85 °C for 5 s. Gene expression was determined through quantitative real-time polymerase chain reaction (PCR) using TaqMan probe-based chemistry (Applied Biosystems, Foster City, CA, USA). Reactions were performed in MicroAmp Fast Optic 96-well Reaction Plates (Applied Biosystems, Foster City, CA, USA) with an ABI PRISM 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). PCR primers and TaqMan probes were obtained from Applied Biosystems (Assays-on-Demand Gene Expression Products; Mm00440502 mL for iNOS gene, and Mm00607939 s1 for β-actin gene). β-actin was used as the housekeeping gene. In accordance with the manufacturer’s instructions, each amplification reaction was performed with 10 μL of TaqMan Fast Universal PCR Master Mix (2X), No AmpErase UNG (Applied Biosystems, Foster City, CA), 1 μL of primer probe mixture, 1 μL of cDNA, and 8 μL of nuclease-free water. The thermal cycling conditions (fast operational mode) were as follows: 95 °C for 20 s, followed by 40 cycles of amplification at 95 °C for 1 s and 60 °C for 20 s. Data were elaborated using Sequence Detection System (SDS) software version 2.3 (Applied Biosystems, Foster City, CA, USA). The comparative 2−ΔΔCt method was used to quantify the relative abundance of mRNA and determine the relative changes in individual gene expression (relative quantification), as reported previously.[Citation30] This method uses a calibrator sample to compare gene expression in different samples. The values obtained reflect the changes in gene expression in the sample of interest by comparison with the calibrator sample, after normalization to the housekeeping gene.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA). Means ± standard deviations (SDs) were determined for each experimental group. The antioxidant and enzyme inhibitory effects ( and ) were analyzed using one-way analysis of variance (ANOVA) (p < 0.05). For pharmacological in vitro and ex vivo evaluations, data were analyzed by ANOVA followed by a Newman–Keuls comparison multiple test to assess the concentration–response relationship. Statistical significance was set at p < 0.05. The number of animals randomized into each experimental group was calculated using the “Resource Equation” [Citation31], according to the guidelines suggested by the “National Centre for the Replacement, Refinement and Reduction of Animals in Research” (NC3RS) and reported at the following web site: https://www.nc3rs.org.uk/experimental-designstatistics.
Table 1. Phenolic compounds of I. batatas leaf extracts (µg/mg extract)*.
Table 2. Antioxidant properties of I. batatas leaf extracts*.
Table 3. Enzyme inhibitory effects of I. batatas extracts*.
Results and discussion
Phytochemical composition
The three extracts were first analyzed using a validated HPLC protocol to assess the presence and amount of the most representative secondary metabolites (). Among the twenty phenolic compounds studied, in terms of the total extracted amount, the most abundant compound across all preparations was chlorogenic acid. In addition, biologically active compounds, such as epicatechin, catechin, and o-coumaric acid, were detected in discrete quantities. Furthermore, the Soxhlet and decoction extraction techniques achieved better recovery of these important metabolites than MW-assisted extraction (25-fold higher).
To facilitate comparison, the total phenolic and flavonoid contents in the studied extracts were determined by Folin–Ciocalteu and AlCl3 methods, respectively, to obtain wider information about the extracts’ chemical compositions. shows that the decoction extract contained the highest phenolic content (89.26 mgGAEs/g extract), and the total flavonoid content exhibited the following decreasing order: Soxhlet (25.33 mgREs/g extract) > decoction (17.71 mgREs/g extract) > MW (7.74 mgREs/g extract).
Antioxidant properties
The antioxidant abilities of I. batatas leaf extracts were evaluated using four different methods, including free radical scavenging (DPPH and ABTS) and reducing power (CUPRAC and FRAP) assays. The decoction extract exhibited the best results in all of the antioxidant assays. For example, the free radical inhibitory activities of the decoction extract (2.84 mmolTEs/g extract for DPPH and 3.05 mmolTEs/g extract for ABTS) were approximately 3-fold higher than those of the Soxhlet extract (0.93 mmolTEs/g extract for DPPH and 0.96 mmolTEs/g extract for ABTS). In addition, the reducing power determined by the CUPRAC and FRAP assays can be ranked as follows: decoction > MW > Soxhlet (). This result may be explained by the higher concentration of phenolics in the decoction extract. Consistent with our results, several researchers have reported a linear correlation between the phenolic contents and antioxidant abilities of plant extracts. [Citation32–Citation34]
Enzyme inhibition evaluation
We extended our studies to the evaluation of the inhibitory effects of I. batatas leaf extracts against ChEs (AChE and BChE), tyrosinase, α-amylase, and α-glucosidase. The results are summarized in . The AChE inhibition activity of the I. batatas leaf Soxhlet extract (0.71 mgGALAEs/g extract) was very similar to that of the MW extract (0.70 mgGALAEs/g extract). In contrast, the decoction extract exhibited the weakest AChE inhibitory activity (0.66 mgGALAEs/g extract). However, the studied I. batatas leaf extracts showed strong selectivity and no inhibitory effects on BChE. This result can be only partially explained by the chlorogenic acid content, which accounts for most of the total in all extracts, as reported by other authors.[Citation35,Citation36] For α-amylase and, especially, α-glucosidase, the Soxhlet extract showed the strongest inhibitory effects, followed by the MW and decoction extracts. Based on previous reports,[Citation37,Citation38] the higher levels of chlorogenic acid in these extracts may contribute to their observed α-amylase and α-glucosidase inhibitory effects. Conversely, only the Soxhlet extract exhibited an inhibitory effect against tyrosinase (22.48 mg KAE/g extract).
In silico evaluation
To rationalize these interesting results, molecular modeling studies were performed to investigate the effects of chlorogenic acid on α-glucosidase, which revealed that the compound fitted well in the enzymatic pocket of the enzyme, analogous to the crystallographic ligand maltose, with complete superimposition of the sugar moiety of chlorogenic acid on that of maltose (). The best position found for chlorogenic acid is stabilized by a network of hydrogen bonds with His351, Arg213, and Asp69 residues and an additional π–π stack involving Phe303. Our results are in full agreement with literature data,[Citation37,Citation38] indicating that chlorogenic acid may act as a competitive, reversible inhibitor of α-glucosidase. Therefore, strong evidence suggests that a portion of the hypoglycemic activity found in the popularly used decoction of I. batatas leaves is related to an elevated level of chlorogenic acid. Indeed, this compound appears to be involved in several metabolic pathways (e.g., improved glucose tolerance, insulin resistance and secretion, and inhibition of mitochondrial carbonic anhydrases VA and VB).[Citation39,Citation40]
Together with caffeic acid, chlorogenic acid is the widest-distributed hydroxycinnamic acid in plant tissues and exerts inhibitory effects on α-amylase and α-glucosidase. I. batatas leaves are also rich in flavonols, such as catechin (3.26 µg/mg in Soxhlet extract) and epicatechin (3.11 µg/mg in decoction extract), which have strong antioxidant activities. Thus, high amounts of chlorogenic acid, together with catechin and epicatechin, might explain the α-glucosidase inhibition activity observed since these compounds are implicated in the attenuation of peroxide production and act as free radical scavengers. [Citation41] Furthermore, the Soxhlet extract possessed tyrosinase inhibitory activity, possibly due to its kojic acid content, as reported previously.[Citation42] These results clearly indicate that both the level of chlorogenic acid in this extract and the (synergistic or antagonist) interactions involving other phenolic components are responsible for the tyrosinase inhibitory effects observed, as reported previously.[Citation43]
Ex vivo evaluation
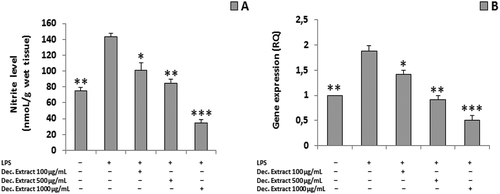
To assess the safety and pharmacological activity of the traditional homemade formulation of this plant, we further evaluated the decoction extract using a panel of ex vivo and in vivo assays, as reported below. We observed significant reductions in ROS production and PGE2 release (ANOVA, p < 0.0001; ***p < 0.001 vs H2O2 group) following treatment with the decoction extract (100–1000 µg/mL) ( and ). We also found significant concentration-dependent reductions in the LPS-stimulated nitrite level and iNOS gene expression in liver specimens treated with the decoction extract (100–1000 μg/mL) (ANOVA, p < 0.0001; *p < 0.5, **p < 0.01, ***p < 0.001 vs LPS group) ().
Figure 2. ROS level in the decoction (Dec.) extract per dose (ANOVA, p < 0.0001; ***p < 0.001 vs H2O2 group).
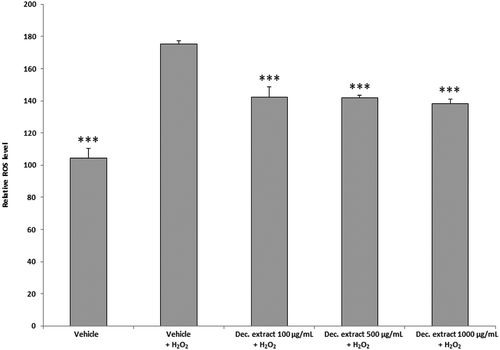
Figure 3. PGE2 level in the decoction (Dec.) extract per dose (ANOVA, p < 0.0001; ***p < 0.001 vs H2O2 group).
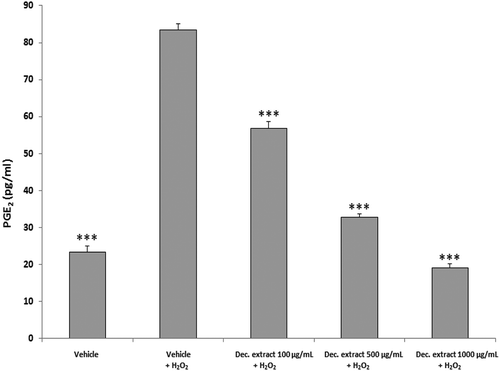
Figure 4. (A) Nitrite level in the decoction (Dec.) extract per dose. (B) iNOS gene expression in the decoction (Dec.) extract per dose (ANOVA, p < 0.0001; *p < 0.5, **p < 0.01, ***p < 0.001 vs LPS group).
Preliminary reports indicated the capabilities of potato leaf extracts and their isolated components, such as IbACP peptide, to stimulate the activity of caspase-3 and -9, which are both involved in apoptosis.[Citation44,Citation45] In addition, the inhibitory effect on cell viability induced by the decoction extract treatment is in agreement with the significant reduction in PGE2 activity observed, which promotes intestinal tumorigenesis. [Citation46] In liver specimens challenged with LPS inflammatory stimulus, we also detected reductions in the nitrite level and iNOS gene expression by the decoction extract (100 µg/mL), which corroborates the hypothesis that anti-inflammatory activity is induced by I. batatas leaves. In fact, our findings of reduced PGE2 levels and iNOS gene expression are consistent with the inhibition of cyclooxygenase 2 (COX-2) and iNOS gene expression induced by chlorogenic acid. [Citation47] We cannot rule out the possibility that the observed reduction in the nitrite level was also dependent on the total content of polyphenols in the extract as these compounds could act as free radical scavengers, hydrogen-donating compounds, and/or singlet oxygen quenchers.[Citation14]
The reported results indicate that a protective effect can be achieved by the traditional use of I. batatas leaf extracts as valuable food supplements for the management of the glycemic level by inhibiting α-glucosidase and α-amylase and concurrently limiting the burden of inflammation and oxidative stress associated with type II diabetes mellitus.
Conclusion
This study represents the first exhaustive analysis of the total flavonoid/phenolic contents, antioxidant and anti-inflammatory activities and enzyme inhibition properties of three different extracts (Soxhlet extract, MW extract and decoction extract) of I. batatas leaves. The extracts were characterized by high levels of chlorogenic acid, catechin, and epicatechin, which could be responsible for the observed biological activities. To better corroborate the results obtained using homemade decoction extracts as co-adjuvants in the treatment of type II diabetes mellitus, we demonstrated the useful interactions in the active site of α-glucosidase using molecular modeling studies. Decoction is the most appropriate technique to obtain a promising functional food.
Additional information
Funding
References
- Bauer, A.; Brönstrup, M. Industrial Natural Product Chemistry for Drug Discovery and Development. Nat Prod Rep. 2014, 31, 1, 35–60. DOI:10.1039/C3NP70058E.
- Islam, S. Nutritional and Medicinal Qualities of Sweetpotato Tops and Leaves; Cooperative Extension Service, Chicago, IL, 2014.
- Motsa, N. M.; Modi, A. T.; Mabhaudhi, T. Sweet Potato (Ipomoea Batatas L.) as a Drought Tolerant and Food Security Crop. S. Afr. J. Sci. 2015, 111, 11–12, 1–8. DOI:10.17159/sajs.2015/20140252.
- Islam, S. Sweetpotato (Ipomoea Batatas L.) Leaf: Its Potential Effect on Human Health and Nutrition. J. Food Sci. 2006, 71, 2, R13–R121. DOI:10.1111/j.1365-2621.2006.tb08912.x.
- Truong, V. D.; McFeeters, R.; Thompson, R.; Dean, L.; Shofran, B. Phenolic Acid Content and Composition in Leaves and Roots of Common Commercial Sweetpotato (Ipomea Batatas L.) Cultivars in the United States. J. Food Sci. 2007, 72, 6, C343–C349. DOI:10.1111/j.1750-3841.2007.00415.x.
- Huang, D.-J.; Chen, H.-J.; Hou, W.-C.; Lin, C.-D.; Lin, Y.-H. Sweet Potato (Ipomoea Batatas [L.] Lam ‘Tainong 57ʹ) Storage Root Mucilage with Antioxidant Activities in Vitro. Food Chem. 2006, 98, 4, 774–781. DOI:10.1016/j.foodchem.2005.07.018.
- Ludvik, B.; Neuffer, B.; Pacini, G. Efficacy of Ipomoea Batatas (Caiapo) on Diabetes Control in Type 2 Diabetic Subjects Treated with Diet. Diabetes Care. 2004, 27, 2, 436–440. DOI:10.2337/diacare.27.2.436.
- Ludvik, B. H.; Mahdjoobian, K.; Waldhaeusl, W.; Hofer, A.; Prager, R.; Kautzky-Willer, A.; Pacini, G. The Effect of Ipomoea Batatas (Caiapo) on Glucose Metabolism and Serum Cholesterol in Patients with Type 2 Diabetes. Diabetes Care. 2002, 25, 1, 239–240. DOI:10.2337/diacare.25.1.239.
- Islam, M. S.; Yoshimoto, M.; Terahara, N.; Yamakawa, O. Anthocyanin Compositions in Sweetpotato (Ipomoea Batatas L.) Leaves. Biosci. Biotechnol. Biochem. 2002, 66, 11, 2483–2486. DOI:10.1271/bbb.66.2483.
- Lee, C.-L.; Lee, S.-L.; Chen, C.-J.; Chen, H.-C.; Kao, M.-C.; Liu, C.-H.; Chen, J.-Y.; Lai, Y.-T.; Wu, Y.-C. Characterization of Secondary Metabolites from Purple Ipomoea Batatas Leaves and Their Effects on Glucose Uptake. Molecules. 2016, 21, 6, 745. DOI:10.3390/molecules21060745.
- Pochapski, M. T.; Fosquiera, E. C.; Esmerino, L. A.; Dos Santos, E. B.; Farago, P. V.; Santos, F. A.; Groppo, F. C. Phytochemical Screening, Antioxidant, and Antimicrobial Activities of the Crude Leaves’ Extract from Ipomoea Batatas (L.) Lam. Pharmacogn Mag. 2011, 7, 26, 165. DOI:10.4103/0973-1296.80682.
- Ijaola, T.; Osunkiyesi, A.; Taiwo, A.; Oseni, O.; LanreIyanda, Y.; Ajayi, J.; Oyede, R. Antidiabetic Effect of Ipomoea Batatas in Normal and Alloxan-Induced Diabetic Rats. Iosr-Jac. 2014, 7, 5, 16–25. DOI:10.9790/5736-07521625.
- Nagai, M.; Tani, M.; Kishimoto, Y.; Iizuka, M.; Saita, E.; Toyozaki, M.; Kamiya, T.; Ikeguchi, M.; Kondo, K. Sweet Potato (Ipomoea Batatas L.) Leaves Suppressed Oxidation of Low Density Lipoprotein (LDL) in Vitro and in Human Subjects. J Clin Biochem Nutr. 2011, 48, 3, 203. DOI:10.3164/jcbn.10-84.
- Zhang, L.; Tu, Z.-C.; Yuan, T.; Wang, H.; Xie, X.; Fu, Z.-F. Antioxidants and Α-Glucosidase Inhibitors from Ipomoea Batatas Leaves Identified by Bioassay-Guided Approach and Structure-Activity Relationships. Food Chem. 2016, 208, 61–67. DOI:10.1016/j.foodchem.2016.03.079.
- Zengin, G.; Menghini, L.; Malatesta, L.; De Luca, E.; Bellagamba, G.; Uysal, S.; Aktumsek, A.; Locatelli, M. Comparative Study of Biological Activities and Multicomponent Pattern of Two Wild Turkish Species: Asphodeline Anatolica and Potentilla Speciosa. J Enzyme Inhib Med Chem. 2016, 31, sup1, 203–208. DOI:10.1080/14756366.2016.1178247.
- Zengin, G.; Uysal, S.; Ceylan, R.; Aktumsek, A. Phenolic Constituent, Antioxidative and Tyrosinase Inhibitory Activity of Ornithogalum Narbonense L. from Turkey: A Phytochemical Study. Ind Crops Prod. 2015, 70, 1–6. DOI:10.1016/j.indcrop.2015.03.012.
- Slinkard, K.; Singleton, V. L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 1, 49–55.
- Zengin, G.; Ceylan, R.; O Guler, G.; Carradori, S.; Uysal, S.; Aktumsek, A. Enzyme Inhibitory Effect and Antioxidant Properties of Astragalus Lagurus Extracts. Curr Enzym Inhib. 2016, 12, 2, 177–182. DOI:10.2174/1573408012666160127231058.
- Locatelli, M.; Zengin, G.; Uysal, A.; Carradori, S.; De Luca, E.; Bellagamba, G.; Aktumsek, A.; Lazarova, I. Multicomponent Pattern and Biological Activities of Seven Asphodeline Taxa: Potential Sources of Natural-Functional Ingredients for Bioactive Formulations. J Enzyme Inhib Med Chem. 2017, 32, 1, 60–67. DOI:10.1080/14756366.2016.1235041.
- Zengin, G. A Study on in Vitro Enzyme Inhibitory Properties of Asphodeline Anatolica: New Sources of Natural Inhibitors for Public Health Problems. Ind Crops Prod. 2016, 83, 39–43. DOI:10.1016/j.indcrop.2015.12.033.
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D. C.; Crisan, G.; S., Rohn Functional Constituents of Wild and Cultivated Goji (L. Barbarum L.) Leaves: Phytochemical Characterization, Biological Profile, and Computational Studies. J Enzyme Inhib Med Chem. 2016 32(1), 153–168.
- Berman, H. M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T. N.; Weissig, H.; Shindyalov, I. N.; Bourne, P. E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 1, 235–242. DOI:10.1093/nar/28.1.235.
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Steric Hindrance by 2 Amino Acid Residues Determines the Substrate Specificity of Isomaltase from Saccharomyces Cerevisiae. J. Biosci. Bioeng. 2011, 112, 6, 545–550. DOI:10.1016/j.jbiosc.2011.08.016.
- Guex, N.; Peitsch, M. C. SWISS‐MODEL and the Swiss‐Pdb Viewer: An Environment for Comparative Protein Modeling. Electrophoresis. 1997, 18, 15, 2714–2723. DOI:10.1002/(ISSN)1522-2683.
- Release, S. 4: Maestro. (2015). (Version 10.4); Schrödinger, LLC: New York, NY, 2015.
- Irwin, J. J.; Sterling, T.; Mysinger, M. M.; Bolstad, E. S.; Coleman, R. G. ZINC: A Free Tool to Discover Chemistry for Biology. J Chem Inf Model. 2012, 52, 7, 1757–1768. DOI:10.1021/ci3001277.
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol Inhibits Isoprostane Production in Young and Aged Rat Brain. J. Bio. Regulators Homeostatic Agents. 2010, 24, 4, 441.
- Verratti, V.; Brunetti, L.; Tenaglia, R.; Chiavaroli, A.; Ferrante, C.; Leone, S.; Orlando, G.; Berardinelli, F.; Di Giulio, C.; Vacca, M. Physiological Analysis of 8-ISO-PGF2 Alpha: A Homeostatic Agent in Superficial Bladder Cancer. J. Biol. Regul. Homeost. Agents. 2011, 25, 1, 71.
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytotherapy Res. 2016, DOI:10.1002/ptr.5655.
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Leone, S.; Di Michele, P.; Shohreh, R.; Vacca, M. Obestatin Inhibits Dopamine Release in Rat Hypothalamus. Eur. J. Pharmacol. 2010, 641, 2, 142–147. DOI:10.1016/j.ejphar.2010.05.059.
- Charan, J.; Kantharia, N. How to Calculate Sample Size in Animal Studies? J. Pharmacol. Pharmacotherapeutics. 2013, 4, 4, 303. DOI:10.4103/0976-500X.119726.
- Abootalebian, M.; Keramat, J.; Kadivar, M.; Ahmadi, F.; Abdinian, M. Comparison of Total Phenolic and Antioxidant Activity of Different Mentha Spicata and M. Longifolia Accessions. Ann. Agric. Sci. 2016, 61, 175–179. DOI:10.1016/j.aoas.2016.10.002.
- Daud, M. N. H.; Fatanah, D. N.; Abdullah, N.; Ahmad, R. Evaluation of Antioxidant Potential of Artocarpus Heterophyllus L. J33 Variety Fruit Waste from Different Extraction Methods and Identification of Phenolic Constituents by LCMS. Food Chem. 2017, 232, 621–632. DOI:10.1016/j.foodchem.2017.04.018.
- Muddathir, A.; Yamauchi, K.; Batubara, I.; Mohieldin, E.; Mitsunaga, T. Anti-Tyrosinase, Total Phenolic Content and Antioxidant Activity of Selected Sudanese Medicinal Plants. South. Afr. J. Bot. 2017, 109, 9–15. DOI:10.1016/j.sajb.2016.12.013.
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y. Neuroprotective Effects of Chlorogenic Acid on Scopolamine-Induced Amnesia via Anti-Acetylcholinesterase and Anti-Oxidative Activities in Mice. Eur. J. Pharmacol. 2010, 649, 1, 210–217. DOI:10.1016/j.ejphar.2010.09.001.
- Oboh, G.; Agunloye, O. M.; Akinyemi, A. J.; Ademiluyi, A. O.; Adefegha, S. A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-Oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem. Res. 2013, 38, 2, 413–419. DOI:10.1007/s11064-012-0935-6.
- Narita, Y.; Inouye, K. Kinetic Analysis and Mechanism on the Inhibition of Chlorogenic Acid and Its Components against Porcine Pancreas Α-Amylase Isozymes I and II. J. Agric. Food Chem. 2009, 57, 19, 9218–9225. DOI:10.1021/jf9017383.
- Oboh, G.; Agunloye, O. M.; Adefegha, S. A.; Akinyemi, A. J.; Ademiluyi, A. O. Caffeic and Chlorogenic Acids Inhibit Key Enzymes Linked to Type 2 Diabetes (In Vitro): A Comparative Study. J Basic Clin Physiol Pharmacol. 2015, 26, 2, 165–170. DOI:10.1515/jbcpp-2013-0141.
- Liu, X.; Li, M.; Tan, B.; Chen, H.; Lu, Y. Inhibitory Effects of Chlorogenic Acid and Isochlorogenic Acid from Purple Sweet Potato Leaves on Α-Glucosidase. Mod. Food Sci. Technol. 2014, 3, 103–107.
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A. P.; De Salvador, R. F.; Monti, S. M.; Buonanno, M.; Zengin, G.; Angeli, A. Microwave-Assisted Extraction, HPLC Analysis, and Inhibitory Effects on Carbonic Anhydrase I, II, VA, and VII Isoforms of 14 Blueberry Italian Cultivars. J Enzyme Inhib Med. Chem. 2016, 31, sup4, 1–6. DOI:10.1080/14756366.2016.1214951.
- Mazzio, E. A.; Harris, N.; Soliman, K. F. Food Constituents Attenuate Monoamine Oxidase Activity and Peroxide Levels in C6 Astrocyte Cells. Planta Med. 1998, 64, 7, 603–606. DOI:10.1055/s-2006-957530.
- Masuda, T.; Fujita, N.; Odaka, Y.; Takeda, Y.; Yonemori, S.; Nakamoto, K.; Kuninaga, H. Tyrosinase Inhibitory Activity of Ethanol Extracts from Medicinal and Edible Plants Cultivated in Okinawa and Identification of a Water-Soluble Inhibitor from the Leaves of Nandina Domestica. Biosci. Biotechnol. Biochem. 2007, 71, 9, 2316–2320. DOI:10.1271/bbb.70249.
- Mazlan, N. A.; Mediani, A.; Abas, F.; Ahmad, S.; Shaari, K.; Khamis, S.; Lajis, N. Antioxidant, Antityrosinase, Anticholinesterase, and Nitric Oxide Inhibition Activities of Three Malaysian Macaranga Species. The Scientific World J. 2013, 2013.
- Chang, V. H.-S.; Yang, D. H.-A.; Lin, -H.-H.; Pearce, G.; Ryan, C. A.; Chen, Y.-C. IbACP, a Sixteen-Amino-Acid Peptide Isolated from Ipomoea Batatas Leaves, Induces Carcinoma Cell Apoptosis. Peptides. 2013, 47, 148–156. DOI:10.1016/j.peptides.2013.02.005.
- Lee, S.-L.; Chin, T.-Y.; Tu, S.-C.; Wang, Y.-J.; Hsu, Y.-T.; Kao, M.-C.; Wu, Y.-C. Purple Sweet Potato Leaf Extract Induces Apoptosis and Reduces Inflammatory Adipokine Expression in 3T3-L1 Differentiated Adipocytes. Evidence-Based Complement. Altern. Med. 2015, 2015.
- Hawcroft, G.; Ko, C.; Hull, M. Prostaglandin E2-EP4 Receptor Signalling Promotes Tumorigenic Behaviour of HT-29 Human Colorectal Cancer Cells. Oncogene. 2007, 26, 21, 3006–3019. DOI:10.1038/sj.onc.1210113.
- Hwang, S. J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 1, 81–90. DOI:10.1007/s00011-013-0674-4.