ABSTRACT
The purpose of the study was to investigate the effect of extraction conditions to determine anthocyanin compounds in strawberry fruit. Anthocyanin profile of this fruit were determined via high-performance liquid chromatography-electrospray ionization-mass spectrometry. Flow rate of mobile phase, fragmentor potential, injection volume, and column temperature were applied to be optimum values as 0.5 mL min−1, 110 V, 5 µL, and 30°C, respectively. The extraction process was the important step in the isolation and identification of anthocyanins. For this purpose, anthocyanin contents in strawberry fruit were examined in terms of solvent type, extraction time, and liquid:solid ratio (v:w). The best extraction conditions in this fruit were found to be methanol as solvent, 30 min as extraction time and 1:1 as liquid:solid ratio (v:w). The results showed that the anthocyanin contents of strawberry fruit change by altering extraction conditions.
Introduction
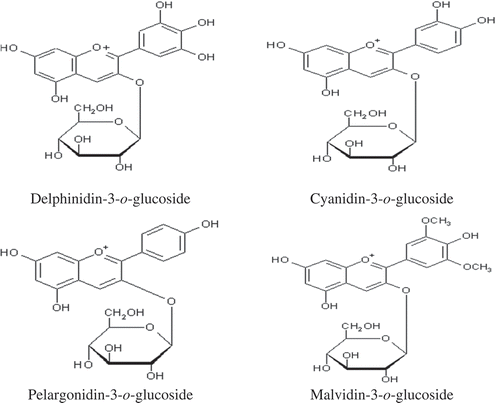
The basic structures of the anthocyanins are the anthocyanidins, and if anthocyanidins are found in the form of glycoside, they are identified as anthocyanins.[Citation1] Anthocyanins are naturally occurring polyphenol compounds which give orange, red, purple, and blue colour to many fruits, vegetables, grains, flowers, and plants. In recent years, there has been increasing interest in anthocyanin pigments owing to their potential use as natural food colouring and also association with a protective effect against many chronic diseases.[Citation2] As stated in the literature, these compounds can increase flexibility of the collagen fibres in blood vessel walls, making the structure more firm and preventing cholesterol from accumulating and hardening. It is also recommended that it aids to prevent cardiovascular diseases and cancers, sustains normal circulation of the human blood system, and both prevents type II diabetes and regulate blood sugar concentration in diabetic patients.[Citation3] The amount of anthocyanin in ripe fruits changes depending on especially temperature, light, and climatic factors.[Citation4–Citation6] Glycosylation of anthocyanidins generally takes place at the 3-position with glucose, arabinose, and galactose, the most common sugar moieties. Therefore, the most common anthocyanins in fruit contain cyanidin-3-glucoside, delphinidin-3-glucoside, peonidin-3-glucoside, pelargonidin-3-glucoside, and petunidin-3-glucoside, cyanidin 3-galactoside, cyanidin 3-arabinoside plus the single diglycoside cyanidin 3-rutinoside. Cyanidin 3-glucoside is the most abundant one in fruits and plants. Unlike this compound, other anthocyanins have limited distribution.[Citation6]
Strawberry is a fruit, rich in anthocyanin, ellagic acid, ellagitannins, gallotannins, proanthocyanidins, quercetin, catechin, ascorbic acid, folic acid, and minerals. This fruit contains a significant amount of beneficial molecules such as lignans, flavonoids, and sesquiterpenoids.[Citation7,Citation8] These molecules show biological activity against many chronic and degenerative diseases. Strawberry plays a role in metabolic syndrome and reduces the levels of specific biomarkers associated with cardiovascular disease, glycaemia, controlling the body’s energy metabolism, antitumor activity in oesophageal, lung and colon cancer, and reducing mental decline.[Citation7,Citation8] Extraction is one of the most important steps in isolation, determination, and use of anthocyanins.[Citation9] The regaining of anthocyanins is usually accomplished via a solvent-extraction procedure. In this procedure, solvent type, solvent concentration, liquid-to-solid ratio, temperature, and time are significant factors to be optimized.[Citation2,Citation10]
In the present study, the contents of anthocyanin compounds in strawberry fruit were examined. For this aim, the effects of solvent type, extraction time, and liquid:solid ratio (v:w) were explored so as to determine the anthocyanin contents in strawberry. Anthocyanin contents in this fruit were analysed via high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS). Anthocyanin contents of this fruit were determined qualitatively and quantitatively.
Materials and method
Reagents and standards
Acetone, acetonitrile, methanol, ethanol, and formic acid were purchased from Sigma (Germany). Hydrochloric acid was purchased from Merck (Darmstadt, Germany). Delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside standards were obtained from Extrasynthese (Genay, France) as chloride salts. All used solvents were in HPLC grade, and other used chemicals were of analytical grade. Ultrapure water was obtained from water purification system (Millipore Direct Q). Stock solutions of anthocyanins (500 mg L−1) were prepared with 0.1% HCl in methanol, and standard solutions were freshly prepared from these stock solutions in every week and stored in dark at −20°C. These standard solutions were used for calibration curve.
Apparatus
For quantitative and qualitative analyses of delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside, standards were used by Agilent 1200 HPLC-MS system. General structures of these anthocyanins are presented in . The HPLC-ESI-MS system consists of an autosampler, a binary pump, a temperature-controlled column oven, coupled to an Agilent 1200 LC/Quadrupole 6110 MS detector operated in selected ion monitoring and full ion scanning (SCAN) mode equipped with ESI source. C18-HPLC column was used. A binary gradient elution was performed.
Sample preparation
Strawberries, which were grown in Elazig, were purchased from different local markets of Elazig, Turkey, during their harvesting season in 2011. These samples were washed firstly using tap water then with deionized water. After that, the samples were put in plastic bags, passed through nitrogen gas, and preserved in darkness in freezer at −20°C until the time of analysis.
Analytical procedure
Deep-frozen strawberries were thawed and ground with a domestic mixer to obtain a homogeneous mixture. About 5 g of strawberry samples were weighed out in triplicates and extracted at room temperature with acidified solvents. The extracts were transferred to screw-cap centrifuged tubes and centrifuged at 4000 rpm for 10 min. Then, the supernatants were carefully taken and filtered through 0.45 µm injection filter. Final solutions were analysed by HPLC-ESI-MS.
Solvent type, extraction time, and liquid:solid radio (v:w) were examined for quantitative and qualitative analyses of anthocyanins in strawberry. When a parameter was tested, optimum values of other parameters were used. When the optimum parameter was determined, the parameter that yielded the highest amount of anthocyanin was used.
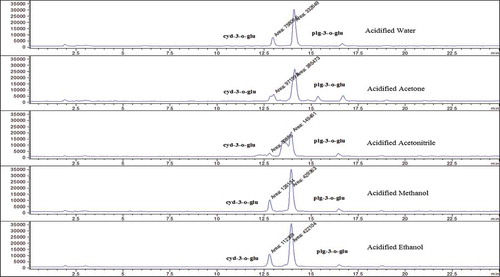
Firstly, solvent type was examined for identification of anthocyanins in strawberry. Different solvents such as water, acetone, acetonitrile, methanol, and ethanol were acidified with 0.1% HCl and added 10 mL of these solutions to each sample. The analytical procedures were performed. The obtained final solutions were analysed by HPLC-ESI-MS, and the chromatograms of anthocyanins were recorded by using different acidified solvents to examine the effect of solvent on the content of anthocyanins and determined the best solvent type.
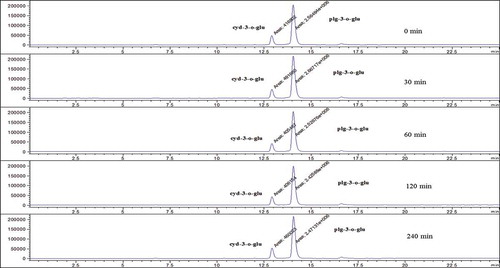
Secondly, different extraction times such as 0, 30, 60, 120, and 240 min were investigated. The analytical procedure was performed. The obtained final solutions were analysed by HPLC-ESI-MS, and the chromatograms of anthocyanins were recorded by using different extraction times to examine the effect of solvent on the content of anthocyanins and determined the best extraction time.
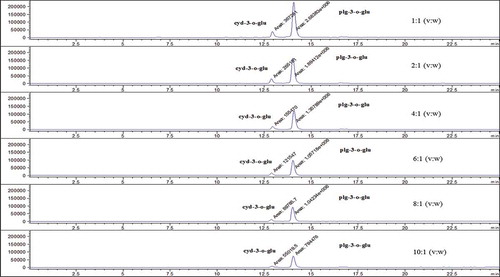
Finally, liquid:solid ratio (v:w) was studied. Different liquid:solid ratios (v:w) of ideal acidified solvent such as 1:1, 2:1, 4:1, 6:1, 8:1, and 10:1 were studied. The analytical procedure was performed. The obtained final solutions were analysed by HPLC-ESI-MS, and the chromatograms of anthocyanins were recorded by using different liquid:solid ratio (v:w) to examine the effect of solvent on the content of anthocyanins and determined the best liquid:solid ratio (v:w).
HPLC-ESI-MS analysis of anthocyanins
Identification and quantification of anthocyanins were performed on an Agilent 1200 LC/Quadrupole 6110 MS system. The analysis of anthocyanins in strawberry extracts was performed by an established optimum procedure as described by Karaaslan and Yaman.[Citation11] The analytical column employed was an Inertsil ODS-3 C18 (4.0 × 150 mm, particle size, 3 µm). The temperature of the column oven was 30°C. Flow rate was 1.0 mL min−1, injection volume was 5 µL for all samples, and a binary gradient elution was performed. presents the applied HPLC-ESI-MS conditions. Gradient elution was performed using 10% formic acid (mobile phase A) and 100% methanol (mobile phase B) as follows: linear gradient from 95% A/5% B to 60% A/40% B, 0–20 min; linear gradient from 60% A/40% B to 0% A/100% B, 20–25 min.
Table 1. HPLC-ESI-MS conditions for anthocyanins.
Results and discussion
The examination of the extraction conditions is one of the important steps in the use of, isolation, and identification of anthocyanins content in fruits.[Citation9] The solvent extraction is a commonly used method for extraction of various compounds found in fruits.[Citation1] Similar to flavonoids, anthocyanins consist of aromatic rings having polar substituent groups (hydroxyl, carboxyl, and methoxyl) and glucosil residues, all of which causes the molecule to be polar.[Citation12–Citation14] Aglycones are extracted with alcohols or alcohol–water mixtures since flavonoid glycosides are more polar. Anthocyanins are extracted with cold acidified solvents such as polar organic solvents, water under mild conditions.[Citation15] Methanol is a commonly used organic solvent for the extraction of these compounds, but acetone, ethanol, and acetonitrile can be also used.[Citation2] From these organic solvents, methanol is the most efficient for extraction. 3-monoside anthocyanins in the glycoside bonds are destroyed in strong acidic media and so it must be avoided during extraction.[Citation16] The recovery of anthocyanins should be investigated in extraction conditions. Extraction conditions such as solvent type, solvent concentration, liquid-to-solid ratio, temperature, and time are important parameters should be optimized.[Citation1,Citation17,Citation18]
In this study, extraction conditions such as solvent type, extraction time, and solvent:solid ratio (v:w) were examined for the determination of anthocyanin contents in strawberry. When a parameter was tested, optimum values of other parameters were used. Firstly, solvent type, an important factor in extraction procedure, was investigated. Strawberry samples were extracted with acidified water, acidified acetone, acidified acetonitrile, acidified methanol, and acidified ethanol to examine the effect of solvent on the content of anthocyanins. In , typical chromatograms obtained by different acidified solvents were given. According to the results, anthocyanin contents were found as maximum in acidified methanol. Therefore, acidified methanol was chosen as extraction solvent. Secondly, extraction time, another important factor in extraction procedure, was examined. For this purpose, strawberry samples were mixed at room temperature for 0, 30, 60, 120, and 240 min. In , typical chromatograms obtained by different extraction time were given. Anthocyanin contents were found as maximum at 30 min and 30 min was chosen as the optimum extraction time. Finally, liquid:solid ratio (v:w), another important factor in extraction procedure, was investigated. The samples extracted 30 min at room temperature with acidified methanol for determination the effect of liquid:solid ratio (v:w) such as 1:1, 2:1, 4:1, 6:1, 8:1, and 10:1 on the content of anthocyanins and chromatograms are given in . From the obtained results, anthocyanin contents were found as maximum in 1:1 liquid:solid ratio (v:w). Therefore, it was selected as the optimum ratio for liquid:solvent (v:w). Consequently, the optimum extraction conditions of solvent type, extraction time, and liquid:solid ratio (v:w) for strawberry fruit were found as acidified methanol, 30 min and 1:1 (v:w), respectively. All chromotograms presented in this study indicated that anthocyanin content in strawberry changes with changing extraction conditions.
Figure 4. The obtained typical chromotograms using different liquid:solid ratio (v:w) for strawberry fruit.
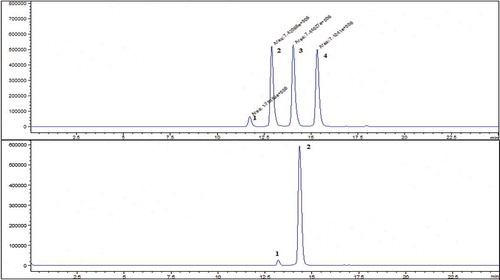
A typical chromatogram that belongs to standard solutions of anthocyanins was given in . The results showed that this method was adequate and appropriate for the anthocyanin analysis in strawberry. Example of a typical chromatogram obtained under optimum extraction conditions and optimum HPLC-ESI-MS parameters of strawberry extracts was given in . Each data is the mean value of the three parallel portions of the same sample. The content of anthocyanins in strawberry was determined by the equation of calibration curve, and it shows that the content of delphinidin-3-o-glucoside, malvidin-3-o-glucoside was <d.l. (detection limit), cyanidin-3-o-glucoside varied from 3.4 ± 0.4 mg kg−1 to 5.0 ± 0.7 mg kg−1 (average 4.2 ± 0.5 mg kg−1), and pelargonidin-3-o-glucoside varied from 39 ± 6.0 mg kg−1 to 64.0 ± 9.0 mg kg−1 (average 50 ± 7.5 mg kg−1) for strawberry on the basis of wet weight.
Figure 5. (a) The obtained typical chromatogram of standard solutions of anthocyanins at optimum conditions Peaks (1) dlp-o-glu, (2) cyd-3-o-glu, (3) plg-3-o-glu, and (4) mlv-3-o-glu. (b) A typical chromatogram for strawberry fruit at optimum conditions. Peaks: (1) cyd-3-o-glu and (2) plg-3-o-glu.
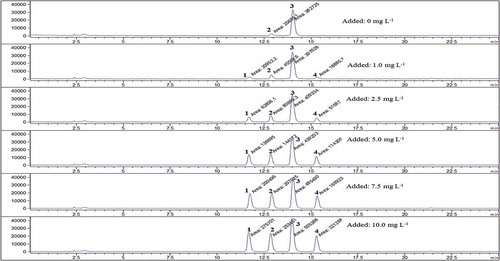
The recovery of the method was investigated by standard addition method for strawberry.[Citation19] For this aim, a proper amount of stock delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside solutions was added to each strawberry extract prior to HPLC-ESI-MS analysis. Then, their chromatograms were obtained at the optimum conditions (). Validation of the method was achieved by determination of the recovery during standard addition. Furthermore, the slope of calibration curves was compared with the slopes obtained by the standard addition method. Since the slopes of calibration curve and standard addition method were identical, it was concluded that the calibration curve could be used for the quantitative analysis. Furthermore, the recoveries at different anthocyanins levels were calculated from the results of standard addition method. The recoveries were found to be at least 90%.
Figure 6. Chromatogram of anthocyanin compounds in strawberry extract by applying standard addition method: peaks: (1) dlp-3-o-glu, (2) cyd-3-o-glu, (3) plg-3-o-glu, and (4) mlv-3-o-glu.
Positive mode of HPLC-ESI-MS was performed to identify the anthocyanins in glycoside form. The obtained mass spectrums under optimum conditions for standards solutions of anthocyanins such as delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside were evaluated. The results were observed as a main peak, isomer of a main peak, and fragment ions of the main peak. Glucoside and aglycone forms of anthocyanins were attributed to the characteristic peaks. Anthocyanin compounds in fruits are usually glucoside form. Main peak, fragment ions, and retention time of the studied anthocyanin compounds are given in . In for delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside shows [M]+ at m/z 465.0, isomer of rise of a hydrogen [M + H]+ at m/z 466.1, and fragment ion of loss of a glucose [M-162]+ at m/z 303.0, [M]+ at m/z 449.0, isomer of rise of a hydrogen [M + H]+ at m/z 450.0, and fragment ion of loss of a glucose [M-162]+ at m/z 287.1, [M]+ at m/z 433.1, isomer of rise of a hydrogen [M + H]+ at m/z 434.2, and fragment ion of loss of a glucose [M-162]+ at m/z 271.0 and [M]+ at m/z 493.1, isomer of rise of a hydrogen [M + H]+ at m/z 494.1, and fragment ion of loss of a glucose [M-162]+ at m/z 331.0, respectively.
Table 2. Identification of anthocyanins by HPLC-ESI-MS.
Kelebek and Selli[Citation20] evaluated the phenolic compositions and antioxidant capacities of strawberry cultivars (Camarosa, Seyhun, Osmanli) which were grown in Turkey. Organic acids and phenolic compounds in strawberry cultivars were examined via HPLC coupled with diode array and coulometric array detectors. They were determined to be cyanidin-3-glucoside, cyanidin-3-rutinoside, pelargonidin-3-glucoside, pelargonidin-3-rutinoside, pelargonidin-3-malonyl-glu, and pelargonidin-3-acetyl-glu for three strawberry cultivars from anthocyanin compounds. The obtained results for cyanidin-3-glucoside and pelargonidin-3-glucoside contents were found ranging from 1.13 ± 0.02 mg/100 g to 4.37 ± 0.05 mg/100 g of fresh weight (fw) and 4.29 ± 0.10 mg/100 g to 36.58 ± 0.60 mg/100 g of fw. In our study, cyanidin-3-o-glucoside and pelargonidin-3-o-glucoside for strawberry were found as average 4.2 ± 0.5 mg kg−1 and 50 ± 7.5 mg kg−1, respectively. Donno et al.[Citation21] determined the antioxidant profiles in currant and strawberries by HPLC-DAD/MS. For this aim, they analysed five strawberry cultivars. They identified four major anthocyanins: cyanidin-3-glucoside, pelargonidin-3-glucoside, pelargonidin-3-rutinoside, and pelargonidin-3-acetylglucoside in five strawberry selections. Cyanidin-3-glucoside, pelargonidin-3-glucoside, pelargonidin-3-rutinoside, and pelargonidin-3-acetylglucoside were found ranging from 0.65 ± 0.04 mg/100 g to 1.47 ± 0.09 mg/100 g of fw, 10.40 ± 0.07 mg/100 g to 24.39 ± 1.08 mg/100 g of fw, 0.42 ± 0.03 mg/100 g to 2.35 ± 0.21 mg/100 g of fw, and not detection (nd) to 6.40 ± 0.28 mg/100 g of fw, respectively. Pelargonidin-3-glucoside was the predominant compound in the strawberries, and this was followed by pelargonidin-3-acetylglucoside, pelargonidin-3-rutinoside, and cyanidin-3-glucoside. In our study, dominant anthocyanin for strawberry was pelargonidin-3-o-glucoside. Kawanobu et al.[Citation22] studied the identification and distribution of anthocyanins in strawberry cultivars. From the obtained results, major anthocyanins cyanidin-3-glucoside, pelargonidin-3-glucoside, and pelargonidin-3-malonylglucoside were identified. Pliszka et al.[Citation23] investigated the effect of extraction on the content of anthocyanins and bioelements in berry fruit such as chokeberry, black currant, strawberry, and bilberry. They compared using two methods: extraction with hydrochloric acid (method A) and extraction with water (method B) to anthocyanins and bioelements contents in fruit. The obtained results were determined to be 85.335 mg/100g for method A and 37.315 mg/100 g for method B. Boeing et al.[Citation24] studied the solvent effect on the extraction of phenolic compounds and antioxidant capacities of berries. For this aim, they used different rates of three different organic solvents (methanol, ethanol, and acetone) and distilled water for extraction. The best anthocyanin content was found in methanol/water/acetic acid (70:29.5:0.5, v/v/v). In the literature, fruits, vegetables, and other foodstuffs are used in different solvent combinations for extraction. For extracting phenolic compounds, the most widely used solvents are water, ethanol, methanol, acetone, and their water mixtures, with or without acid.[Citation25–Citation28] In our study, anthocyanin contents of strawberry fruit were investigated for five different solvent types. The best anthocyanin content was found in acidified methanol. Fernandes et al.[Citation29] carried out phenolic composition and antioxidant properties in strawberry samples from organic farming and integrated pest management. In this study, HPLC-DAD/MS was used to analyse anthocyanin compounds in strawberry extracts. They identified a total of three major anthocyanin compounds: cyanidin-3-glucoside, pelargonidin-3-glucoside, and pelargonidin-3-rutinoside and they were provided mass data. Cyanidin-3-glucoside, pelargonidin-3-glucoside, and pelargonidin-3-rutinoside were identified [M]+ at m/z 449 with a major ion fragment at m/z 287 (loss of hexosyl residue), [M]+ at m/z 433 with a major ion fragment at m/z 271 (loss of a hexosyl residue), and [M]+ at m/z 579 with two major fragments at m/z 433 and m/z 271, respectively. In our study, cyanidin-3-o-glucoside and pelargonidin-3-o-glucoside for strawberry were identified [M]+ at m/z 449.0, [M-162] + at m/z 287.1 (fragment ion of loss of a glucose), and [M]+ at m/z 433.1 and [M-162] + at m/z 271.0 (fragment ion of loss of a glucose), respectively (). Consequently, when our study was compared with other studies in the literature, anthocyanin contents in strawberry were found in the range of reported values. In addition, the compounds of the defined anthocyanins are compatible with literature. In our study, the effects of extraction conditions for the determination of anthocyanins contents in strawberry were investigated. The similar studies related to this fruit is limited in literature.
Conclusion
In the current study, the effect of extraction conditions was investigated in order to examine delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside in strawberry. Anthocyanin content was determined by HPLC-ESI-MS. The applied HPLC-ESI-MS conditions were 0.5 mL min−1 for flow rate of mobile phase, 110 V for fragmentor potential, 5 µL for injection volume, and 30°C for column temperature for these anthocyanin compounds. In order to determine the anthocyanin content in strawberry, three different extraction conditions including solvent type, extraction time, and liquid:solid ratio (v:w) were investigated. According to the results, the best extraction conditions were found to be acidified methanol as solvent, 30 min as extraction time, and 1:1 (v:w) as liquid:solid ratio for these fruit. The best extraction conditions were successfully applied to determine quantitative and qualitative analyses of delphinidin-3-o-glucoside, cyanidin-3-o-glucoside, pelargonidin-3-o-glucoside, and malvidin-3-o-glucoside in strawberry.
Additional information
Funding
References
- Castañeda-Ovando, A.; Pacheco-Hernández, M. D. L.; Páez-Hernández, M. E.; Rodríguez, J. A.; Galán-Vidal, C. A. Chemical Studies of Anthocyanins: A Review. Food Chemistry 2009, 113 (4), 859–871.
- Navas, M. J.; Jiménez-Moreno, A. M.; Bueno, J. M.; Sáez-Plaza, P.; Asuero, A. G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part IV: Extraction of Anthocyanins. Critical Reviews in Analytical Chemistry 2012, 42 (4), 313–342.
- Huang, H.-W.; Hsu, C.-P.; Yang, B. B.; Wang, C.-Y. Advances in the Extraction of Natural Ingredients by High Pressure Extraction Technology. Trends in Food Science & Technology 2013, 33 (1), 54–62.
- Faragher, J. D.;. Temperature Regulation of Anthocyanin Synthesis in Apple Skin. Journal of Experimental Botany 1983, 34 (10), 1291–1298.
- Arakawa, O.; Hori, Y.; Ogata, R. Relative Effective- Ness and Interaction of ultraviolet-B, Red and Blue Light in Anthocyanin Synthesis of Apple Fruit. Physiologia Plantarum 1985, 64 (3), 323–327.
- Robards, K.; Antolovich, M. Analytical Chemistry of Fruit bioflavonoids-A Review. Analyst 1997, 122 (2), 11R–34R.
- Desjardins, Y. Human Health Effects of Strawberry: A Review of Current Research. ISHS Acta Horticulturae 1049: 2014, 827–838.
- Basu, A.; Nguyen, A.; Betts, N. M.; Lyons, T. J. Strawberry as a Functional Food: Anevidence-Based Review. Critical Reviews in Food Science and Nutrition 2014, 54 (6), 790–806.
- Andersen, O. M.; Markham, K. R.; Eds. Flavonoids: Chemistry, Biochemistry, and Applications; Taylor and Francis: Boca Raton, Fla. 2006.
- Zou, T.-B.; Wang, M.; Gan, R.-Y.; Ling, W.-H. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Mulberry, Using Response Surface Methodology. International Journal of Molecular Sciences 2011, 12 (5), 3006–3017.
- Karaaslan, N. M.; Yaman, M. Determination of Anthocyanins in Cherry and Cranberry by High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. European Food Research and Technology 2016, 242 (1), 127–135.
- Bueno, J. M.; Ramos-Escudero, F.; Saez-Plaza, P.; Munoz, A. M.; Navas, M. J.; Asuero, A. G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part I: General Considerations Concerning Polyphenols and Flavonoids. Critical Reviews in Analytical Chemistry 2012, 42 (2), 102–125.
- Bueno, J. M.; Saez-Plaza, P.; Ramos-Escudero, F.; Jimenez, A. M.; Fett, R.; Asuero, A. G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color and Intake of Anthocyanins. Critical Reviews in Analytical Chemistry 2012, 42 (2), 126–151.
- Delgado-Vargas, F.; Jimenez, A. R.; Paredes-Lopez, D. Natural Pigments: Carotenoids, Anthocyanins and betalains-Characteristics, Biosynthesis, Processing and Stability. Critical Reviews in Food Science and Nutrition 2000, 40 (3), 173–289.
- Giusti, M. M.; Jing, P. Analysis of Anthocyanins, In Food Colorants: Chemical and Functional Properties, Socaciu, C.; Ed.; Taylor & Francis: Boca Raton, Fla, 2008, Ch. 6.3, 479–506.
- Kapasakalidis, P. G.; Rastall, R. A.; Gordon, M. H. Extraction of Polyphenols from Processed Black Currant (Ribes Nigrum L.). Residues. Journal of Agricultural and Food Chemistry 2006, 54 (11), 4016–4021.
- Seyedreihani, S. F.; Tan, T.-C.; Alkarkhi, A. F. M.; Easa, A. M. Total Phenolic Content and Antioxidant Activity of Ulam Raja (Cosmos caudatus) and Quantification of Its Selected Marker Compounds: Effect of Extraction. International Journal of Food Properties 2017, 22 (2), 260–270.
- Balyan, U.; Sarkar, B. Aqueous Extraction Kinetics of Phenolic Compounds from Jamun (Syzygium cumini L.) Seeds. International Journal of Food Properties 2017, 20 (2), 372–389.
- Kaplan, O.; Kaya, G.; Ozcan, C.; Ince, M.; Yaman, M. Acrylamide Concentrations in Grilled Foodstuffs of Turkish Kitchen by High Performance Liquid Chromatography-Mass Spectrometry. Microchemical Journal 2009, 93, 173–179.
- Kelebek, H.; Selli, S. Characterization of Phenolic Compounds in Strawberry Fruits by RP-HPLC-DAD and Investigation of Their Antioxidant Capacity. Journal of Liquid Chromatography & Related Technologies 2011, 34 (20), 2495–2504.
- Donno, D.; Cavanna, M.; Beccaro, G. L.; Mellano, M. G.; Marinoni, D. T.; Cerutti, A. K.; Bounous, G. Currants and Strawberries as Bioactive Compound Sources: Determination of Antioxidant Profiles with HPLC-DAD/MS. Journal of Applied Botany and Food Quality 2013, 86, 1–10.
- Kawanobu, S.; Yamaguchi, M.-A.; Zushi, K.; Kondo, K.; Matsuzoe, N. Identification and Distribution of Anthocyanins in Strawberry Cultivars. Journal of Food, Agriculture & Environment 2011, 9 (1), 140–141.
- Pliszka, B.; Huszcza‐Ciołkowska, G.; Wierzbicka, E. Effects of Extraction Conditions on the Content of Anthocyanins and Bioelements in Berry Fruit Extracts. Communications in Soil Science and Plant Analysis 2008, 39 (5–6), 753–762.
- Boeing, J. S.; Barizão, É. E. O.; Silva, B. C. E.; Montanher, P. F.; Almeida, V. D.; Visentainer, J. V. Evaluation of Solvent Effect on the Extraction of Phenolic Compounds and Antioxidant Capacities from the Berries: Application of Principal Component Analysis. Chemistry Central Journal 2014, 8, 48.
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. Journal of Pharmaceutical and Biomedical Analysis 2006, 41 (5), 1523–1542.
- Rababah, T. M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of Extraction Conditions of Total Phenolics, Antioxidant Activities, and Anthocyanin of Oregano, Thyme, Terebinth, and Pomegranate. Journal of Food Science 2010, 75 (7), C626–C632.
- Bunea, C.-I.; Pop, N.; Babes, A. C.; Matea, C.; Dulf, F. V.; Bunea, A. Carotenoids, Total Polyphenols and Antioxidant Activity of Grapes (Vitis vinifera) Cultivated in Organic and Conventional Systems. Chemistry Central Journal 2012, 6, 66.
- Michiels, J. A.; Kevers, C.; Pincemail, J.; Defraigne, J. O.; Dommes, J. Extraction Conditions Can Greatly Influence Antioxidant Capacity Assays in Plant Food Matrices. Food Chemistry 2012, 130 (4), 986–993.
- Fernandes, V. C.; Domingues, V. F.; De Freitas, V.; Delerue-Matos, C.; Mateus, N. Strawberries from Integrated Pest Management and Organic Farming: Phenolic Composition and Antioxidant Properties. Food Chemistry 2012, 134 (4), 1926–1931.