ABSTRACT
Green seeds are considered undesirable impurities in rapeseed mass for edible oil production. The rapeseed maturity degree is mostly determined by visual evaluation of the embryo colour. In this study, digital image analysis (DIA) was used for characterisation of rapeseed samples harvested at different maturity stages. The seed size, surface and cross-section colour in RGB, HSI and L*a*b* colour spaces as well as share of green seeds and content of pigments were determined. The statistically significant correlation coefficients calculated between the chlorophyll pigment content and cross-section colour attributes indicate the potential use of DAI to determine the rapeseed maturity stage.
Introduction
The content of chlorophyll pigments in rapeseeds is determined by the maturity degree, shaped mainly by the pod topography, growing conditions and harvesting technology. The pod topography is associated with plant architecture, with the main momentum from numerous branches (up to 25 pcs.) and side shoots (>9 pcs.). Since the time of the branches growing on the main stem and the side shoots is unequal, the organising of pods and seed ripening also occur gradually. The number, length and the abundance of pods, as well as the packing (from 15 to 40 seeds) and seed maturation are heavily influenced by cultivation and environmental factors, including: sowing date, fertilisation, water and thermal conditions, insolation, harvest date, etc.[Citation1] These factors shape the seed yield size and quality. One of the factors indicating deteriorating rapeseed quality is the presence of immature seeds. The literature on the problem of immature seeds with high chlorophyll content largely concerns the influence of frost occurring during seed maturation. It was found that lethal frosts (≤−5°C) cause a rapid loss of seeds and pod moisture, inhibiting the activity of chlorophyllase and the natural degradation of chlorophylls.[Citation2–Citation5] Furthermore, Canola Watch[Citation6] stated that the chlorophyll content in seeds is also affected by swathing conditions. On hot days, swathed plants may dry down quickly and die when swathing occurs, due to an insufficient time for enzymes to clear chlorophyll pigments before desiccation and the green colour might then be “locked”. The problem of a high content of chlorophylls in rapeseeds is the most noticeable in the northern areas of Canada,[Citation7] but it is also not a stranger to European countries, especially the United Kingdom, where oils from rapeseeds were characterised by a 50% higher content of chlorophylls than in samples from France, Germany and Denmark.[Citation8] Tanaka and Tanaka[Citation9,Citation10] found that chlorophylls in rapeseeds may lead to cell death and play a role in communication between the chloroplasts and nuclei and acclimation to light intensity and senescence.
During the thermal extraction process (roasting, pressing and extraction) and oil refining (degumming and alkaline deacidification), chlorophylls decompose to derivatives, mainly pheophytins and pyropheophytins.[Citation7,Citation11] The content of chlorophyll pigments in raw canola oil is in the range of 2–50 ppm.[Citation12–Citation15] These pigments are considered to be highly unfavourable compounds in oils which reduce durability. In the presence of light, they generate aggressive singlet oxygen and initiate photosensitised oxidation and autoxidation.[Citation16–Citation18] Moreover, chlorophyll pigments are poisonous to hydrogenation and transesterification catalysts and contribute to an undesirable olive-brown oil colour.[Citation19,Citation20] On the other hand, seeds and rapeseed oil also contain carotenoids, which contribute to the improvement of raw material quality. During harvesting, these pigments absorb light and the energy which is then transferred to the chlorophylls. Furthermore, carotenoids play a major role as photoprotective pigments by quenching triplet chlorophyll and singlet oxygen, which results in a reduction of membrane damage.[Citation21–Citation23] Yeum and Russell[Citation24] found that an important function of carotenoids also involves attracting pollinators and seed dispersal.
A practical indicator of the chlorophyll content in rapeseeds is the share of green seeds in the bulk mass. Prijic et al.[Citation25] found that high temperatures and drought stress during seed-filling are the main reason for forming green seeds. Daun and Symons[Citation26] indicated that “green” seeds are seeds that are characterised by a 200–400 mg/kg content of chlorophylls. Elias and Copeland,[Citation27] examining the physiological and collective maturity of six winter and spring varieties of canola and found that this species of oilseeds reaches physiological maturity when pods change colour from green to yellow-green or light brown. In the case of seeds, the colour changes, depending on the variety, from brown to dark brown and black. Ghasemi-Golezani et al.[Citation28] found that three winter varieties of rape (Modena, Opera and SLM046) obtained maximum seed quality at 10 to 22 days after mass maturity, while worse seed quality at the early development stage was caused by immaturity. On the other hand, low seed quality at later stages was due to seed aging.[Citation29] According to the Canadian Grain Commission Grain Research Laboratory (GRL), a limited content of green seeds is defined as less than 2%.[Citation26] This corresponds with the content of chlorophylls in the seeds at levels up to 25 mg/kg of seeds (adopted as the technological maturity level) and with a rate of chlorophylls in the oil content of up to 30 mg/kg.[Citation12]
The content of green seeds is determined usually by visual evaluation of the embryo seed colour.[Citation30,Citation31] This assessment is very time-consuming and subjective. Numerous studies are being conducted currently on the possibility of using image analysis techniques for maturity and quality estimation of fruits, vegetables, as well as seeds.[Citation32–Citation37] In the present study, digital image analysis (DIA) was applied to evaluate the rapeseed maturity degree. Using this technique, the colour of seed surfaces and cross-sections and morphological size were determined for rapeseed samples harvested at different maturity stages. The main purpose of the study was to find if the instrumentally determined colour attributes correlate with content of pigments. This information may be useful in industry for fast prediction of maturity stage of rapeseed bulk mass.
Materials and methods
Rapeseed samples
The research materials were seeds of two winter rape varieties: the hybrid restored variety Kronos F1 and the Contact variety, harvested at different maturity stages (number of days after flowering, DAF): early-green maturity (25 DAF), green maturity (35 DAF), technical maturity (44 DAF), and full maturity (54 DAF). A field trial was performed in 2011 at the Bałcyny Experimental Station (North-eastern Poland, N = 53°35ʹ49ʹ’, E = 19°51ʹ20.3ʹ’). In the study, the optimal date of seed sowing and a density of 120 seeds per m2 were applied. Soil experimental fields were classified as: typical soil lessives, slightly loamy formed from clay, medium class IIIa with good wheat complex. The experiment was established as one factorial in four replications. The plot size for harvest was 15.8 m2. In the autumn, the following fertilisations were applied per 1 hectare: 30 kg N (in form of ammonium sulphate), 35 kg P (in form of triple superphosphate) and 100 kg K (in the form of 60% potassium salt) and in the spring: 200 kg N (120 kg at the beginning of the vegetation – BBCH 20–22, 80 kg at the beginning of bud development – BBCH 50–52) and 60 kg S (in the form of ammonium sulfate). The plants were protected from weeds using metazachlor and quinmerac (Butisan Star 416 SC after sowing) and haloxyfop-R (Perenal 104 EC at the 4 leaf stage, BBCH 14) and cypermethrin using 8.75 g/ha delmathrin (BBCH 12), 300 g/ha chlorpyrifos + 30 g/ha cypermethrin (BBCH 21), 25 g/ha cypermethrin (BBCH 51) and alpha-cypermethrin (BBCH 53).
Determination of the total lipid content in seeds
The lipid content in rapeseeds was determined by extraction in Soxhlet’s apparatus type FoodAlyt R 60 (Omnilab, Germany). First, the samples of rapeseeds were ground in a laboratory mill (WZ-1 type, Spomasz, Poland). The lipids were extracted from the seeds with hexane for 16 h. The extract was distilled on a vacuum evaporator (type R-210, Büchi Labortechnik, Switzerland) and weighed. The lipid content in seeds was determined in triplicate and was expressed in percentage.
Green seed percentage in bulk mass
The percentage of green (immature) rapeseeds was determined visually based on embryo colour screening. An acrylic paddle with 100 depressions was used with masking tape and wallpaper roller to crush the seeds. A 10 g sample of seeds was poured over paddle until each depression was filled with seed. A piece of masking tape was placed evenly over the top of the paddle. After tape removing all seeds were crushed by wallpaper roller. Then visual inspection of distinctly green seeds was performed. The analysis was determined in five replicates.
Content of pigments in seeds
The content of chlorophylls was determined according to the American Oil Chemists’ Society Official Method AK 2–92 with some modifications.[Citation38] The chlorophylls were extracted with heptane and ethanol mixture (3:1, v:v), and the absorbance of the extracts was measured at the 630, 665, and 700 nm wavelengths using a UNICAM UV/Vis UV2 spectrophotometer (ATI Unicam, Cambridge, United Kingdom). The content of carotenoids was determined according to the method described by Franke et al.[Citation39] The carotenoids were extracted with a petroleum ether and acetone mixture (1:1, v:v) and the absorbance was measured at 445 nm using a UNICAM UV/Vis UV2. The pigment determinations were conducted in triplicate and the content was expressed in mg per 1 kg of seeds.
1,000 Seed weight
The weight of 1,000 seeds was determined using an LN-S-50 Unitra Cemi (Szczytno, Poland) seed counter in five replicates and was expressed in g.
Digital image analysis
The colour and geometrical features of seeds were measured with a Digital Image Analysis (DIA) set according to the method described by Tańska et al.[Citation40] The images of 300 single seeds (without visible symptoms of mechanical damage, selected under a magnifying glass) for each sample were acquired by a high resolution, low-noise CCD Nikon DXM 1200 colour camera (Nikon Inc., Melville, USA), saved as LIM files and analysed by LUCIA G v. 4.8 software (Laboratory Imaging, Prague, Czech Republic). The frame grabber was at a resolution of 1,280 × 1,024 pixels. The light source was provided by 4 BOB OM optical lamps with 4,100 W (60 kLx) halogen bulbs (OSRAM firm, Poland), with a colour temperature of 3,000 K (warm white). The lamps were attached at a 45° angle from above the object on a par with the camera. Before the measurements, the testing set was calibrated in accordance with the international whiteness standard using a calibration plate. The seeds were positioned in a horizontal orientation, i.e. the bend of the embryonic axis was parallel to the horizontal (seeds usually attain this orientation after falling onto a surface) and measured from a distance (lens-to-object) of 13 cm.
Four geometrical features of the rapeseeds were measured: length (maximum linear dimension of seed calculated from projected area, expressed in mm), width (minimum linear dimension of seed calculated from projected area, expressed in mm), equivalent diameter (average dimension of seed calculated from projected area, expressed in mm) and circularity (calculated from the formula = (4*π*area)/(perimeter2), equals to 1 only for circle, parameter without unit). These features are typical parameters used for characterisation of size and shape of rapeseeds. The values of these features were calculated automatically by the LUCIA G ver. 4.8 software. The results of rapeseed surface and cross-section colour were expressed in three colour spaces: RGB model (red, green, blue) in which R, G and B components were in the range 0–255, HSI model (hue, saturation, intensity) in which the H component was in the range 0–360°, S and I components were in the range 0–100%, CIE L*a*b* model (lightness, greenness-redness, blueness-yellowness) in which the L* component was in the range 0–100%, a* and b* components were in the range from −120 to +120.[Citation41]
Statistical analysis
The results of all determinations were calculated as means ± SD via Microsoft Office Excel 2016 and statistically analysed using the Statistica 12.5 PL program (2015, StatSoft, Cracow, Poland). In order to indicate the significance of differences between samples for each analysed parameter, a one-way analysis of variance (ANOVA) with Tukey’s test with a p ≤ 0.05 significance level was used. Shapiro-Wilk and Levene tests were used to check normality and homoscedasticity assumptions, respectively. Moreover, the Pearson correlation coefficients between individual discriminates were calculated (p ≤ 0.05).
Results and discussion
The content of lipids and pigments in rapeseeds and green seed percentage in the bulk mass of rapeseeds
The seeds of analysed varieties collected at different stages of maturity were characterised by the statistically different lipid content and share of green seeds in the bulk rapeseed mass (p ≤ 0.05) (). In the early-green stage of maturity (25 DAF), the lipid content was the lowest and the share of green seeds was the highest. In the green stage of maturity (35 DAF), the share of green seeds was at the same level, but the content of lipids increased significantly (p ≤ 0.05). The highest (comparable in both varieties) lipid content was found for seeds in the technical and full maturity stages (). Moreover, these samples were characterised by a lack of green seeds.
Table 1. Basic characteristic of rapeseeds of Kronos and Contact varieties.
The chlorophyll content in seeds decreased during maturation, at a rate depending on the variety (). Generally, seeds of the early-green stage were characterised by the highest chlorophyll content compared to other stages of maturity for both varieties. However, the amount of these pigments in the Contact sample was lower (124.6 mg/kg) than for the Kronos sample (136.7 mg/kg). For the samples collected in 35 DAF, the chlorophyll content decreased for the Kronos variety, while for the Contact it was maintained at the early-green maturity stage. One the other hand, samples in the last two stages of maturity (44 and 54 DAF) were characterised by a small amount of chlorophylls (less than 3 mg/kg), which was also found by Smolikova et al.[Citation42], who presented the role of chlorophylls in seed tolerance. The cited authors found that in the green maturity stage, the highest content of chlorophylls, determined as a sum of chlorophyll a and chlorophyll b, was observed in second order shoots and the lowest was in the main shoot. For the wax maturity stages (10 DAF under accelerated conditions in 22°C), sorting by seed-coat colour (pale-brown, brown and black), it was found that the pale-brown sample was characterised by a 2.5-fold higher content of these pigments than the black one. Smolikova et al.[Citation42] comparing the content of chlorophylls between analysed stages of rapeseed maturity, found that in technical maturity, the content of chlorophylls in seeds was the lowest. Moreover, those authors observed that the content of these compounds in the embryo axis was 5-fold higher than in the seed coat. Onyilagha et al.,[Citation38] analysing the influence of seed chlorophyll on vigour in oilseed rape, observed that the conducted germination test had suggested that under favourable environmental conditions, the content of chlorophylls in seeds might have no significant effect on germination. However, under any environmental stress, the germination of rapeseeds characterised by high amounts of chlorophylls might be impaired. On the other hand, Schoefs,[Citation43] analysing the pigment composition and location in honey locust (Gleditsia triacanthos) seeds before and after desiccation, found that the amount of total chlorophylls did not vary throughout the period of maturity and that the decrease in chlorophyll a/chlorophyll b ratio could be explained by stoichiometric transformation of chlorophyll b to chlorophyll a during seed maturation. Din et al.[Citation44] found that flowering canola varieties exposed to drought stress were characterised by a reduced total content of chlorophyll a and b. Ladjal et al.[Citation45] observed that the drought reduction in pigments and disorganisation of thylakoid membranes resulted from the loss of chlorophylls.
The carotenoid content in rapeseeds was statistically different (p ≤ 0.05) at an individual stage of maturity (). Seeds in 25 DAF, for both varieties, were characterised by a high content of these pigments, while in the next stage of maturity (35 DAF) a 1.5-fold lower content of these compounds was found for a sample of the Kronos variety (45.3 mg/kg) and 2-fold lower for the Contact variety (36.9 mg/kg). For technical maturity, an approximately 11-fold decrease in the carotenoid content in the samples of both analysed varieties was noted. On the other hand, samples presenting the full-maturity stage were characterised by a slight increase in these pigments. Smolikova et al.[Citation42] analysed content of carotenes and xanthophylls in rapeseeds and found a decrease in their contents during seed ripening. A sample of the green maturity stage was characterised by the highest amounts of carotenoids, especially in second-order shoots, while a low level of those compounds was observed in technical maturity stage. It is important to note that at the technical maturity stage, carotenoids were not detected in the seed coat.
An analysis of the carotenoid/chlorophyll ratio showed that the highest value of this discriminant was observed for samples of the full maturity stage (Kronos – 4.43 and Contact – 2.29) while it was the lowest for the green maturity samples (Kronos – 0.49 and Contact – 0.30). Generally, it was also found that chlorophyll content was in inverse correlation with carotenoid content, which was also presented in Smolikova et al.[Citation42] Mokronosov et al.,[Citation46] Polesskaya,[Citation47] and Solovchenko and Merzlyak[Citation48] found a relatively high proportion of these seed pigments to be an energy indicator of autotrophic organs characterised by increased tolerance and could be used to determine the stress tolerance of photosynthesising tissues.
Size and shape of rapeseeds
The 1,000-seed weight was statistically differentiated (p ≤ 0.05) by the maturity stages (). Samples collected in 25 DAF, for both varieties, were characterised by the lowest values of this discriminant, while for these analysed in 35 DAF, a noticeable increase in weight was observed. Seeds of Kronos and Contact varieties (in technical and full maturity stages) reached the highest and comparable lipid content, while the highest 1,000 seed weight was reached by the studied varieties in different maturity stages (Contact in technical stage, Kronos in full stage). The statistical analysis showed an important correlation between the 1,000-seed weight and the chlorophyll content in seeds. It was observed that with an increasing 1,000-seed weight, the content of chlorophyll pigments decreased, a finding which is in agreement with Onyilagha et al.[Citation38] and Jalink et al.[Citation49]
Table 2. Geometrical characteristics of rapeseeds in different stages of maturity.
The seed dimensions (estimated by the length, width and the equivalent diameter) gradually increased from the green stage of seed maturity to the technical stage, to reach a similar level in the full maturity stage (). No detailed research concerning the physical properties of rapeseeds during its development has been reported. Çalışır et al.[Citation50] and Unal et al.[Citation51], for example, analysed the physical properties of different varieties of rapeseed, taking into account their future utilisation and found that, among others, the geometrical features of rapeseeds are necessary for the design of devices for harvesting, processing, transporting, sorting, separating and packing. On the other hand, Tańska et al.[Citation40] presented digital image analysis that can be used to determine different physical characteristics of rapeseeds. Furthermore, Razavi et al.[Citation52] and Duc et al.[Citation53] analysing the effect of different compounds of rapeseed on geometrical discriminants and found a correlation between moisture and the individual physical properties of seeds.
The circularity (shape factor) of rapeseeds slightly changed during maturation. For both varieties of rapeseed, a slight increase of circularity was observed for seeds at the green maturity stage. The values of this discriminant for immature (25 DAF) and fully-matured seeds (54 DAF) were statistically equal (). It is believed that the cause of this tendency is related to the fact that seeds increased their volume and fill the whole pods, coming into contact with each other.
Surface and cross-section colour of rapeseeds
RGB colour space
The surface colour of seeds in the RGB model showed some differentiation of all attributes for samples of both rape varieties characterised by different maturity stages. However, this diversification was not always correlated with a clear relationship to maturity state. Mean values of R attribute were lower for seeds in 25 DAF (early-green stage; Kronos = Contact = 72), while they were higher for the sample in 35 DAF (green stage; Kronos = 77.9; Contact = 80.8) and the lowest and comparable for seeds in 44 and 54 DAF (technical and full stages; Kronos = 62.0 and 63.0, Contact = 67.0 and 66.9, respectively) (). shows that the highest and narrowest variations of R and G attributes were observed for the Contact sample in 54 DAF. For the B attribute, the highest variation was noted for the Contact sample in 35 DAF. Moreover, the most offset values to the zero point of coordinates were found for samples of both varieties in 44 and 54 DAF. One the other hand, the most shifted values to the zero point of coordinates, for the first two attributes, were observed for the Contact samples in 44 and 54 DAF, while for B attributes it was for samples of the same variety, however it was in 25 and 35 DAF.
Table 3. The colour of the surface and cross-section of rapeseeds in different stage of maturity expressed in the RGB model.
Figure 1. Histograms of the RGB colour space attributes. Maturity stages (days after flowering): early-green (25 DAF), green (35 DAF), technical (44 DAF) and full (54 DAF).
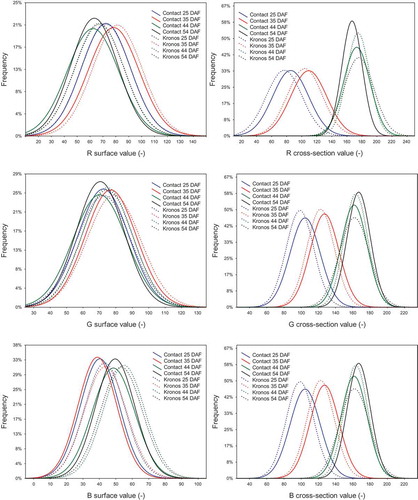
The colour of seed cross-sections in the RGB model very clearly differentiated maturity stages, showing different values of each attribute. Seeds of two rapeseed varieties in the early-green maturity stage were characterised by the lowest mean values of R, G and B attributes, while the highest were in the full maturity stage (). For example, the mean values of R attribute for seed cross-sections of the Kronos variety at consecutive stages of maturity were, as follows: 85.0; 108.8; 172.3 and 182.0 (). It was also observed the highest and narrowest attribute variations of the model for the Contact sample in 54 DAF. Moreover, each attribute reached the most shifted values to the zero point of coordinates for samples of both varieties in 25 DAF, while the most offset for the Contact sample in 54 DAF was for G and B attributes, but for the Kronos sample in 44 DAF it was for the R attribute ().
Generally, the colour of the seed surface in the RGB model distinguished immature seeds (early-green and green maturity stages) from mature ones (technical and full maturity stages). However, it did not differentiate the seeds of the early-green maturity stage from the seeds of the green maturity stage, or the seeds of technical maturity from the seeds in the full maturity stage. For these reasons, the measurement of seed surface colour in the RGB model cannot be considered a good method for assessing rapeseed maturity. Also Tańska et al.,[Citation41] who examined the surface colour of different seed fractions (fraction I – > 2.0 mm, fraction II – 1.6–2.0 mm and fraction III – <1.6 mm), reported that samples were differentiated only by the R attribute. The highest value of this attribute was noted for the smallest seeds, while the lowest values were for the largest ones. The values of other colour attributes, G and B, were almost at the same level.
HSI colour space
When using the HSI model to determine the seed surface colour, only H and S attributes distinguished seeds with different maturity stages. However, the configuration of these attribute values allowed for better seed distinction in the early-green or green maturity stages from the technical and full maturity stages than RGB colour space. Mean H values for seeds of both varieties were significantly lower in the early-green and green maturity stages (39.5–42.8°) than in the technical and full maturity stages (56.4–58.4°). Also mean S values were distinctly higher in the green maturity stages; 36.0–40.3% for the Contact variety and 39.7–44.9% for the Kronos variety (). In turn, mean I values were in a similar range, independent of maturity stage, for both varieties (from 23.5% to 26.4%). Moreover, it was found that H values determined for surface of seeds in maturity stages (technical and full) were in very wide range, from 10° to 100° (). Relatively low variation of the attribute characterised seeds in early-green stage (35 DAF), both Kronos and Contact varieties. The opposite situation was observed for S attribute. However, values of I attribute fluctuated in the same range, independent of maturity stage and variety.
Table 4. Colour of surface and cross-section seeds characterised by different maturity degree expressed as HSI attributes.
Figure 2. Histograms of the HSI colour space attributes. Maturity stages (days after flowering): early-green (25 DAF), green (35 DAF), technical (44 DAF) and full (54 DAF).
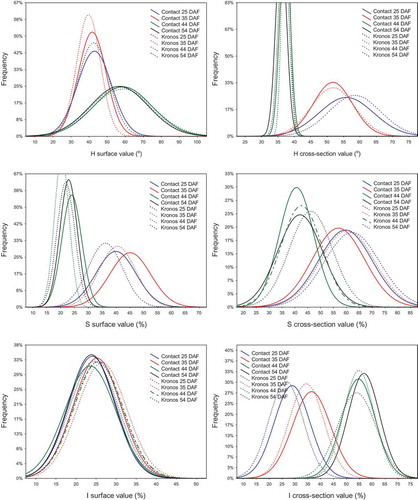
An analysis of seed cross-sections in the HSI model allowed for the distinction of seed maturity stages. Generally, higher mean values of H and S and lower mean values of I were noted for samples with a higher degree of maturity (). It was also observed that the H values for cross-sections of all seeds in the technical and full maturity stages were very similar and close to the zero coordinate point, while immature samples were more differentiated. The variation of S attribute was comparable for all samples, but the Kronos seeds in 54 DAF reached more offset values from the zero coordinate point than the Contact sample in the same stage of maturity. For I attribute was found opposite relationships. The Kronos sample in 25 DAF was the most shifted to the coordinate zero point, while the Contact samples in 54 DAF was the most offset ().
The results indicated that HSI colour space is useless for estimating the degree of seed maturity when surface colour measurements are carried out. However, Tańska et al.,[Citation41] used the model to analyse the surface colour of different rapeseed fractions and found that it could be used to distinguish small seeds from medium and large seeds, because S and I attributes were significant higher for small seeds, while H attribute was significantly lower.
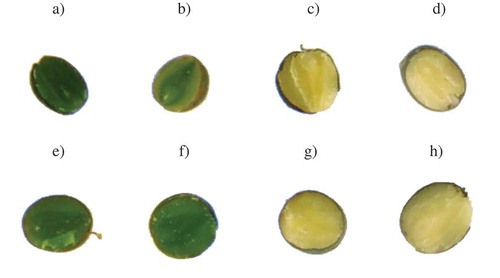
Values of the H and S attributes determined for the area of cross-sections corresponded well with the photograph embryo colour (). However, photographs of seed cross-sections characterised by varied maturity degree showed statistically significant differences (p ≤ 0.05) in the colour of external and internal cotyledons and radicle. In the early-green stage of maturity, the colour of external cotyledons was deeply green, while internal cotyledons were dark green and the radicle was light green. Moreover, in the next stages of development, the external cotyledons were greenish-yellow, interior ones were green while the radicle was yellow-green. In the technical stage of maturity, the external cotyledons and radicle were yellow, while the internal cotyledons were dark yellow. Moreover, all morphological embryo elements in the last maturity stages were light yellow. Borisjuk et al.[Citation54] explained that changes in the seed colour of morphological elements are associated with lipid decomposition during seed maturation and they showed that in the early stage of seed development, lipids were observed in the embryo, where their main transformations occurred. With the seed development, an increase in embryo lipid level was observed, while the outer cotyledon was characterised by a higher lipid concentration than an inner concentration. Borisjuk et al.[Citation54] also found that the most noticeable changes in lipids were observed for the outer tissue facing the endosperm and testa. As the development continued, the embryo was characterised by a substantial increase in lipid concentration. However, the earlier differences in the levels of these compounds for the outer and inner cotyledon had disappeared.
Figure 3. Images of rapeseed cross-sections: A) Contact 25 DAF, b) Contact 35 DAF, c) Contact 44 DAF, d) Contact 55 DAF, e) Kronos 25 DAF, f) Kronos 35 DAF, g) Kronos 44 DAF, h) Kronos 54 DAF. Maturity stages (days after flowering): early-green (25 DAF), green (35 DAF), technical (44 DAF) and full (54 DAF).
CIEL*a*b* colour space
In the CIEL*a*b* model, the suitability of individual attributes to discriminate the seed maturity during seed surface measurement of colour was found to be unequal. Since the mean values of L* and a* attributes, for seeds of both varieties, regardless of their maturity degree, were very similar, they unsuitable for distinguishing immature seeds from mature seeds. The mean values of b* for seeds in 25 DAF and 35 DAF were significantly higher (Kronos = 18.6–21.9 and Contact = 16.7–19.6), than values estimated for samples in 44 DAF and 54 DAF (Kronos = 11.1–10.6 and Contact = 9.5–10.4) (). It was also found that the variations of L* values for all seed surface samples were almost similar (). In turn, variation of a* values was the lowest for the Kronos samples in 44 and 54 DAF and was the highest for the Contact sample in 25 DAF. For b* attribute, it was found that the widest value ranges characterised samples of both varieties in 25 DAF and 35 DAF, while mature seeds were similar in this case.
Table 5. Surface and cross-section colour of rapeseeds characterised by different maturity degree expressed in CIEL*a*b* model attributes.
Figure 4. Histograms of the CIEL*a*b* colour space attributes. Maturity stages (days after flowering): early-green (25 DAF), green (35 DAF), technical (44 DAF) and full (54 DAF).
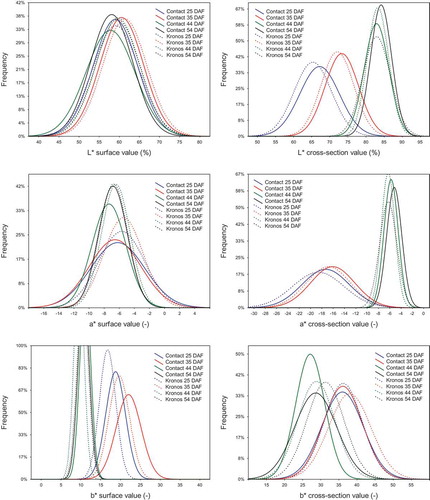
The colour of cross-sections in the CIEL*a*b* model differentiated immature seeds from mature seeds, because each of these components were characterised by statistically different values (p ≤ 0.05) for these seed groups (). The mean values of L* for both varieties were the lowest for seeds in 25 DAF, average for those in 35 DAF and were the highest and comparable to mature seeds in 44 DAF and 54 DAF. This shows that the L* attribute of the seed cross-section colour accurately distinguished seeds in the early-green maturity stage from green seeds, which is a valuable feature of this parameter because seeds in the early stage of maturity are characterised by a higher content of chlorophyll pigments, and results in a darker embryo colour (). The mean values of a* and b* attributes were higher and comparable for seeds of early-green and green stages of maturity, while lower and comparable for seeds at the technical and full development stages (). These attributes, although imprecise in distinguishing the green seed maturity degree, could be considered as less useful in estimating the maturity degree of seeds. Analysing the attributes determined for cross-sections of the seed samples, it was observed a completely different distribution of L* and a* values compare to those determined for seed surfaces. The differences were also noted for the b* attribute. Although for the first attribute (L*), it was found that the lowest and widest histogram was observed for the Contact sample in 25 DAF, while the highest and narrowest for a sample of the same variety in 54 DAF. The most shifted values of L* and a* attributes to the zero coordinate point were found for the Kronos and Contact samples in 25 DAF, while the most offset were found for the Contact sample in 54 DAF. The most distinctive variation of b* attribute was found for the Contact sample in 44 DAF and other samples were characterised by a similar distribution of this attribute ().
Based on the results expressed in attributes of the CIEL*a*b* colour space, we concluded that the cross-section colour measurement can be considered as a tool for estimation of rapeseed maturity stage. Although it was found that b* distinguished immature seeds from mature seeds, but it did not distinguish seeds in the early-green maturity stage from the green stage or technical maturity from full maturity. Therefore, the usefulness of b* for estimating seed maturity degree using surface colour measurement could be regarded as limited. Sinnecker et al.[Citation55] used CIEL*a*b* colour space for predicting green pigment in ripening soybean seeds. They reported that, from an industrial point of view, a* attribute is easily measured and could be a useful tool for quality control in classifying soybean seeds.
Correlations between colour attributes and pigment content in rapeseeds
It was found that the content of chlorophyll pigments was highly correlated with the values of all attributes of the RGB, HSI and CIEL*a*b* colour spaces, which were determined for cross-sections of rapeseeds (). Most of the calculated correlations for seed surface, between attributes and content of chlorophyll pigments were weaker and were only significant for R, B, H, a* and b* attributes. The content of carotenoid pigments was significantly correlated with the values of all attributes of the cross-section colour and only with B, H, S and b* attributes of the seed surface colour ().
Table 6. Correlation coefficients calculated for the relationship between the color attributes and content of pigments in rapeseeds.
Relationships between colour attributes and content of pigments in seeds were considered only by some researches. Tańska et al.,[Citation40] examining colour of rapeseed fractions differentiated in size using digital image analysis, found that an increase in the green intensity (lower negative values of a* attribute) was accompanied by a decrease in chlorophyll content, while a higher value of the b* attribute (increase yellow intensity) was indicated for samples containing a higher carotenoid content. Sinnecker et al.[Citation55] studied Brazilian soybean varieties harvested at different maturity stages. They showed high and significant linear correlations (r = −0.815 and r = −0.878) between total chlorophyll content and two attributes of CIEL*a*b* model (L* and a*, respectively). In turn, Rodríguez-Pulido et al.[Citation56] used the computer vision to determine the phenolic maturity stage of grape seeds. The phenomenon of seed coat browning they explained as a decrease of the quantitative colorimetric parameters such as L* (lightness) and C*ab (chroma). They found high correlation coefficients between phenolic monomers and lightness (r = 0.96) and galloylated compounds and chroma (r = 0.90).
Conclusion
This study showed gradually increase of rapeseed size (expressed as 1,000-seed weight, length, width and equivalent diameter) from the green stage to the technical stage of seed maturity. In turn, shape of rapeseeds only slightly changed during maturation. For both varieties of rapeseed, a slight decrease of circularity was observed for seeds at the technical and full maturity stages. However, seed size and shape are strongly associated with genetic factor, soil, technology and climate conditions. Therefore, use these parameters to estimate the maturity stage seems to be difficult. The seed surface colour determined by RGB, HSI and CIEL*a*b* models provide only limited information on the rapeseed maturity degree and the chlorophyll content. This is evidenced by the mostly insignificant or low values of correlation coefficients calculated between the colour attributes and the chlorophyll content. Among all attributes, only the H attribute of the seed surface colour differentiated seeds with different maturity stages. In turn, the seed cross-section colour, expressed by all attributes of the tested models, indicated the content of chlorophyll and carotenoid pigments very well. The results indicated the potential use of the digital image analysis technique to determine chlorophyll pigment content and the corresponding rapeseed maturity degree, but only for bulk crushed seed mass. Moreover, the measurement of seed surface colour using the described technique can be successfully used to determine the onset of rapeseed technical maturity and the state in which the rapeseed achieves technological suitability for edible oil production. The results presented above are the first stage of studies on the possibility of applying digital image analysis to rapeseed maturity estimation. In future research we plan that other cultivars and more maturity degrees will be investigated to increase the number of samples. The research will be performed in order to establish the discriminant power of colour attributes as well as to propose classification algorithm on the base of extracted attributes.
References
- Diepenbrock, W. Yield Analysis of Winter Oilseed Rape (Brassica Napus L.): A Review. Field Crops Res. 2000, 67, 35–49. doi:10.1016/S0378-4290(00)00082-4.
- Johnson-Flanagan, A. M.; Spencer, M. S. Chlorophyllase and Peroxidase Activity during Degreening of Maturing Canola (Brassica Napus) and Mustard (Brassica Juncea). Physiol. Plant 1996, 97, 353–359. doi:10.1034/j.1399-3054.1996.970220.x.
- Green, B. R.; Singh, S.; Babic, I.; Bladen, C.; Johnson-Flannagan, A. M. Relationship of Chlorophyll, Seed Moisture and ABA Levels in the Maturing Brassica Napus Seed and Effect of a Mild Freezing Stress. Physiol. Plant 1998, 104, 125–133. doi:10.1034/j.1399-3054.1998.1040116.x.
- Bonham-Smith, P. C.; Gilmer, S.; Zhou, R.; Galka, M.; Abrams, S. R. Non-Lethal Freezing Effects on Seed Degreening in Brassica Napus. Planta 2006, 224, 145–154. doi:10.1007/s00425-005-0203-y.
- Chung, D. W.; Pružinská, A.; Hörtensteiner, S.; Ort, D. R. The Role of Pheophorbide α Oxygenase Expression and Activity in the Canola Green Seed Problem. Plant Physiol. 2006, 142, 88–97. doi:10.1104/pp.106.084483.
- Canola Watch. Swathing When Hot Can Lock in Green. 2011, 22. http://www.canolawatch.org/2011/08/31/swathing-when-hot-can-lock-in-green/ (accessed on February 2017).
- Diosady, L. L. Chlorophyll Removal from Edible Oils. Int. J. Appl. Sci. Eng. 2005, 3, 81–88.
- Bingham, I. J.; Kinghtley, S. P. J.; Walker, K. Identifying the Factors Determining the Chlorophyll Content of UK Rapeseed. HGCA Project Raport No. OS61 2003, 1–40.
- Tanaka, A.; Tanaka, R. Chlorophyll Metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. doi:10.1016/j.pbi.2006.03.011.
- Tanaka, R.; Tanaka, A. Tetrapyrrole Biosynthesis in Higher Plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. doi:10.1146/annurev.arplant.57.032905.105448.
- Daun, J. K. Spectrophotometric Analysis of Chlorophyll Pigments in Canola and Rapeseed Oils. Lipid Technol. 2012, 24, 134–136. doi:10.1002/lite.v24.6.
- Daun, J. K. How Green Is Green? Long-Term Relationships between Green Seeds and Chlorophyll in Canola Grading. J. Am. Oil Chemists’ Soc. 2003, 80, 119–122. doi:10.1007/s11746-003-0662-8.
- Canadian Grain Commission. Quality of Western Canadian Canola. 2015. Grain Research Laboratory, Canadian Grain Commission, Winnipeg, Canada. https://www.grainscanada.gc.ca/canola/harvest-recolte/2015/hqc15-qrc15-en.pdf (accessed on June 2017).
- Khattab, R.; Goldenberg, E.; Lin, L.; Thiyam, U. Quantitative Analysis and Free-Radical-Scavenging Activity of Chlorophyll, Phytic Acid, and Condensed Tannins in Canola. Food Chem. 2010, 122, 1266–1272. doi:10.1016/j.foodchem.2010.03.081.
- Tańska, M.; Rotkiewicz, D.; Ambrosewicz-Walacik, M. Effect of Industrial of Heat Condition Treatment of Rape, Mustard, Flax and Cameline Seeds on the Quality of Oils Intended for Biodiesel Production. Polish J. Nat. Sci. 2013, 28, 449–462.
- Clerkx, E. J.; Vries, H. B.; Ruys, G. J.; Groot, S. P.; Koorneef, M. Characterization of Green Seed, an Enhancer of Abi3-1 in Arabidopsis that Affects Seed Longevity. Plant Physiol. 2003, 142, 88–97.
- Lanfer-Marquez, U. M.; Barros, R. M. C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Food Res. Int. 2005, 38, 885–895. doi:10.1016/j.foodres.2005.02.012.
- Choe, E.; Min, D. B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. doi:10.1111/crfs.2006.5.issue-4.
- Canjura, F. L.; Schwartz, S. J.; Nunes, R. V. Degradation Kinetics of Chlorophylls and Chlorophyllides. J. Agric. Food Sci. 1991, 56, 1639–1643.
- Endo, Y.; Thorsteinson, C. T.; Daun, J. K. Characterization of Chlorophyll Pigments Present in Canola Seed, Meal and Oil. J. Am. Oil Chemists’ Soc. 1992, 69, 564–568. doi:10.1007/BF02636109.
- Cuttriss, A. J.; Pogson, B. J. Carotenoids. In Plant Pigments and Their Manipulation; ed, Davies, K. M.; CRC Press: Boca Raton, FL, USA, 2004; 57–91.
- Howitt, C. A.; Pogson, B. J. Carotenoid Accumulation and Function in Seeds and Non-Green Tissues. Plant Cell Environ. 2006, 29, 435–445. doi:10.1111/pce.2006.29.issue-3.
- Pogson, B. J.; Rissler, H. M.; Frank, H. A. The Roles of Carotenoids in Energy Quenching. In Photosystem II: the Water/Plastoquinone Oxidoreductase in Photosynthesis (eds. Wydrzynski, T., Satoh, K.), Springer, Dordrecht, The Netherlands, 2006, 515–537.
- Yeum, K. J.; Russell, R. M. Carotenoid Bioavailability and Bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504. doi:10.1146/annurev.nutr.22.010402.102834.
- Prijic, L.; Jovanovic, M.; Glamoclija, D. Germination and Vigor of Wrinkled and Greenish Soybean Seed. Seed Sci. Technol. 1998, 26, 377–383.
- Daun, J. K.; Symons, S. How Green Is Green? Sampling and Perception in Assessing Green Seeds and Chlorophyll in Canola. J. Am. Oil Chemists’ Soc. 2000, 77, 1209–1214. doi:10.1007/s11746-000-0188-0.
- Elias, S. G.; Copeland, L. O. Physiological and Harvest Maturity of Canola in Relation to Seed Quality. Agron. J. 2001, 93, 1054–1058. doi:10.2134/agronj2001.9351054x.
- Ghasemi-Golezani, K.; Sheikhzadeh-Mosaddegh, P.; Shakiba, M. R.; Mohamadi, A.; Naspollahzadeh, S. Development of Seed Physiological Quality in Winter Oilseed Rape (Brassica Napus L.). Cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2011, 39, 208–212.
- Ghassemi-Golezani, K.; Mazloomi-Oskooyi, R. Effect of Water Supply on Seed Quality Development in Common Bean (Phaseolus Vulgaris Var.). Int. J. Plant Prod. 2008, 2, 117–124.
- Gusta, L. V.; Johnson, E. N.; Nesbitt, N. T.; Kirkland, K. J. Effect of Seeding Date on Canola Seed Quality and Seed Vigor. Can. J. Plant Sci. 2004, 84, 463–471. doi:10.4141/P03-100.
- Harker, K. N.; O’Donovan, J. T.; Blackshaw, R. E.; Johnson, E. N.; Lafond, P. G.; May, W. E. Seeding Depth and Seeding Speed Effects on No-Till Canola Emergence, Maturity, Yield and Seed Quality. Can. J. Plant Sci. 2012, 92, 795–802. doi:10.4141/cjps2011-189.
- Obulesu, M.; Bhattacharya, S. Color Changes of Tamarind (Tamarindus Indica L.) Pulp during Fruit Development, Ripening, and Storage. International. J. Food Properties 2011, 14, 538–549. doi:10.1080/10942910903262129.
- Nizamlioglu, N. M.; Nas, S. Kinetic of Color Changes in Almond (Akbadem Variety) during Roasting and Storage. Int. J. Food Properties 2016, 19, 2363–2376. doi:10.1080/10942912.2015.1086786.
- Pieniazek, F.; Messina, V. Texture and Color Analysis of Freeze-Dried Potato (Cv. Spunta) Using Instrumental and Image Analysis Techniques. Int. J. Food Properties 2016, 20, 1422–1431. doi:10.1080/10942912.2016.1211143.
- Segura, L. I.; Salvadori, V. O.; Goñ, S. M. Characterisation of Liquid Food Colour from Digital Images. Int. J. Food Properties 2017, 1–11. latest articles. doi:10.1080/10942912.2017.1299758.
- Nambi, V. E.; Thangavel, K.; Shahir, S.; Chandrasekar, V. Color Kinetics during Ripening of Indian Mangoes. Int. J. Food Properties 2016, 19, 2147–2155. doi:10.1080/10942912.2015.1089281.
- Cáez Ramírez, G.; Téllez-Medina, D. I.; García-Armenta, E.; Gutiérrez-López, G. F. Digital Image Analysis and Fractal Metrics as Potential Tools to Monitor Colour Changes in Fresh-Cut Papaya (Carica Papaya L.). Int. J. Food Properties 2017, 1–13. latest articles. doi:10.1080/10942912.2017.1293090.
- Onyilagha, J. C.; Elliott, B. H.; Buckner, E.; Okiror, S.; Raney, P. J. Seed Chlorophyll Influences Vigor in Oilseed Rape (Brassica Napus L. Var AC ExceL.). J. Agric. Sci. 2011, 3, 73–79. doi:10.5539/jas.v3n2p73.
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of Carotenoids and Vitamin E in Selected Oilseeds, Press Cakes and Oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. doi:10.1002/ejlt.v112:10.
- Tańska, M.; Rotkiewicz, D.; Kozirok, K.; Konopka, I. Measurement of the Geometrical Features and Surface Color of Rapeseeds Using Digital Image Analysis. Food Res. Int. 2005, 38, 741–750. doi:10.1016/j.foodres.2005.01.008.
- Tańska, M.; Konopka, I.; Korzeniewska, E.; Rotkiewicz, D. Color of Rapeseed (Brassica Napus) Surface and Contamination by Fungi during Storage of Dry and Wet Seeds. Int. J. Food Technol. 2011, 46, 2265–2273. doi:10.1111/j.1365-2621.2011.02745.x.
- Smolikova, G. N.; Laman, N. A.; Boriskevich, O. V. Role of Chlorophylls and Carotenoids in Seed Tolerance to Abiotic Stressors. Russ. J. Plant Physiol. 2011, 58, 965–973. doi:10.1134/S1021443711060161.
- Schoefs, B. Pigment Composition and Location in Honey Locust (Gleditsia Triacanthos) Seeds before and after Desiccation. Tree Physiol. 2002, 22, 285–290. doi:10.1093/treephys/22.4.285.
- Din, J.; Khan, S. U.; Ali, I.; Gurmani, A. R. Physiological and Agronomic Response of Canola Varieties to Drought Stress. J. Anim. Plant Sci. 2011, 21, 78–82.
- Ladjal, M.; Epron, D.; Ducrey, M. Effects of Drought Preconditioning on Thermo Tolerance of Photosystem II and Susceptibility of Photosynthesis to Heat Stress in Cedar Seedlings. Tree Physiol. 2000, 20, 1235–1241. doi:10.1093/treephys/20.18.1235.
- Mokronosov, A. T.; Gavrilenko, V. F.; Zhigalova, T. V. Photosynthesis: Physiological, Ecological, and Biochemical Aspects. Fotosintez. Fiziologo ekologicheskie i biokhimicheskie aspekty. In Ermakov, I. P., Ed.; Akademiya: Moscow, 2006.
- Polesskaya, O. G. Plant Cell and Reactive Oxygen Species. Rastitel’naya kletka i aktivnye formy kisloroda. In Ermakov, I. P., Ed.; KDU: Moscow, 2007.
- Solovchenko, A. E.; Merzlyak, M. N. Screening of Visible and UV Radiation as a Photoprotective Mechanism in Plants. Russ. J. Plant Physiol. 2008, 55, 719–737. doi:10.1134/S1021443708060010.
- Jalink, H.; Frandas, A.; Van Der Schoor, R.; Bino, J. B. Chlorophyll Fluorescence of the Testa of Brassica Oleracea Seeds as an Indicator of Seed Maturity and Seed Quality. Scientia Agricola 1998, 55, 88–93. doi:10.1590/S0103-90161998000500016.
- ÇAlışIr, S.; Marakoglu, T.; Ogut, H.; Ozturk, O. Physical Properties of Rapeseed (Brassica Napus Oleifera L.). J. Food Eng. 2005, 69, 61–66. doi:10.1016/j.jfoodeng.2004.07.010.
- Unal, H.; Sincik, M.; Izli, N. Comparison of Some Engineering Properties of Rapeseed Cultivars. Ind. Crops Prod. 2009, 30, 131–136. doi:10.1016/j.indcrop.2009.02.011.
- Razavi, S. M. A.; Yeganehzad, S.; Sadeghi, A. Moisture Dependent Physical Properties of Canola Seeds. J. Agric. Sci. Technol. 2009, 11, 309–322.
- Duc, L. A.; Han, J. W.; Hong, S. J.; Choi, H. S.; Kim, Y. H.; Keum, D. H. Physical Properties of Rapeseed (I). J. Biosystems Eng. 2008, 33, 101–105. doi:10.5307/JBE.2008.33.2.101.
- Borisjuk, L.; Neuberger, T.; Schwender, J.; Heinzel, N.; Sunderhaus, S.; Fuchs, J.; Hay, J. O.; Tschiersch, H.; Braun, H.-P.; Denolf, P.; Lambert, B.; Jakob, P. M.; Rolletschek, H. Seed Architecture Shapes Embryo Metabolism in Oilseed Rape. Plant Cell 2013, 25, 1625–1640.
- Sinnecker, P.; Gomes, M. S. O.; Arêas, J. A. G.; Lanfer-Marquez, U. M. Relationship between Color (Instrumental and Visual) and Chlorophyll Contents in Soybean Seeds during Ripening. J. Agric. Food Chem. 2002, 50, 3961–3966. doi:10.1021/jf0113023.
- Rodríguez-Pulido, F. J.; Ferrer-Gallego, R.; González-Miret, M. L.; Rivas-Gonzalo, J. C.; Escribano-Bailón, M. T.; Heredia, F. J. Preliminary Study to Determine the Phenolic Maturity Stage of Grape Seeds by Computer Vision. Anal. Chim. Acta 2012, 732, 78–82. doi:10.1016/j.aca.2012.01.005.
