ABSTRACT
Microencapsulation is frequently used to enhance the stability of liquid food by transforming it to a free-flowing powder. This study aimed to evaluate the effect of storage for 24 days at 65°C on the overall quality and stability of microencapsulated Nigella sativa oil (MNSO) compared to uncapsulated Nigella sativa oil (NSO). During storage time, the total oil was extracted from the MNSO and was examined every six days along with the uncapsulated NSO. The uncapsulated oil showed many changes to its properties including reductions in oxidative stability, content of bioactive compounds, and antioxidant activity, as well as changes in fatty acid composition. The same parameters were evaluated for MNSO, which showed increased stability and resistance under the same storage conditions. The results confirmed the efficacy of the microencapsulation in protecting the oil.
Introduction
The species Nigella sativa L. belongs to the Ranunculaceae family and is an annual oilseed-producing which is native to the Mediterranean region.[Citation1] Nigella sativa oil (NSO) is a known functional oil has different health benefits due to its high content of essential fatty acids and lipid-soluble bioactive compounds.[Citation2] It is particularly important as it can be used to produce formulations of dietary supplements with significant antioxidant activity.[Citation3] That is mainly due to thymoquinone (TQ) which is the major volatile biologically active constituent of NSO that has antioxidant and anti-inflammatory activities.[Citation3,Citation4] The oxidation of oils is affected by external and internal factors such as temperature, light, exposure to oxygen, fatty acid composition, presence of antioxidants, and prooxidants. In addition, the oxidative stability of oils during storage depends heavily on the oil source and the manufacturing process.[Citation5] Recently, the supercritical fluid extraction (SFE) process has been proven to be useful in obtaining NSO with high antioxidant activity and rich TQ content.[Citation6]
According to Ramadan et al.[Citation7], NSO is prone to oxidative degradation due to its high content of polyunsaturated fatty acids, especially linoleic. Also, Ramadan et al.[Citation7] stressed that oxidative degradation is one of the main factors that cause serious damage to the oil’s overall quality, in particular, the nutritional value, storage stability, and sensory properties. Although the potential health benefits of NSO, there are several limitations which have influence on its wide consumption, hot peppery taste and susceptibility to oxidation. Moreover, the poor water solubility due to different polarity of oil (non-polar) and water (polar) which is the main reason of reducing the bioavailability due to decreases the absorption rate in the gastrointestinal tract.[Citation8]
Microencapsulation has been regularly used in the production of powdered edible oil products by blending the core material (functional oil) into the capsule wall material in order to lengthen shelf life. This process can protect oils from oxidation and thereby also enhance the handling of oils.[Citation9] The microencapsulation technique coats the hygroscopic, volatile, or sensitive materials with edible barriers to make a stable powdered form that protects against moisture and oxidation.[Citation10] Proteins and carbohydrates are frequently used as matrices to microencapsulate lipophilic compounds by spray drying.[Citation11] Tonon and Brabet[Citation12] highlighted some of the advantages including low cost, availability, and flexibility of equipment and providing high quality powders. Gupta and Jadhav[Citation13] stressed that it is important to protect PUFA in edible oil against lipid oxidation in order to boost its shelf life, which can be accomplished by applying the efficient technique of microencapsulation. Due to the many advantages of the spray drying method, it is commonly used for microencapsulation in the food sector as well documented in the literature: Terminalia arjuna extracts[Citation14], pequi extracts[Citation15], fish oil,[Citation16] flaxseed oil,[Citation17] menhaden oil,[Citation18] and chili seed oil.[Citation19]
A crucial objective to ensure the usefulness of spray-dried products for food application is to evaluate its stability and functionality during storage.[Citation20] However, very few information is available and few studies only reported the process conditions for the microencapsulation of NSO. Edris et al.[Citation21] studied the microencapsulation of NSO by spray drying using gum Arabic and maltodextrin as encapsulating wall materials for food and nutraceutical applications. Abedi et al.[Citation22] reported production of functional yogurt with microencapsulated NSO using a mixture of modified starch and maltodextrin as wall materials. Mohammed et al.[Citation23] optimized the process conditions of the NSO microencapsulation and suggested to investigate the stability of the MNSO in details.
Furthermore, there is no study on the effects of storage conditions on the MNSO powder. Hence, the main goals of this study were to evaluate the protective effects of microencapsulation on the quality of oils during storage. The oxidative stability, antioxidant activity, fatty acid composition, TQ content, and particle morphology of microencapsulated Nigella sativa oil (MNSO) were examined along with the NSO (uncapsulated oil) to study their changes in the course of storage.
Materials and methods
Chemicals and materials
Nigella sativa L. seed was purchased from a local food ingredient supplier (Selangor, Malaysia). Maltodextrin DE10 was purchased from (VIS Food Tech Ingredient Supplies, Kuala Lumpur, Malaysia). Sodium thiosulfate butanol, chloroform, acetic acid, and potassium iodide were supplied by Merck (Darmstadt, Germany). Sodium caseinate, soy lecithin, 1,1-diphenyl-picryl-hydrazyl, TQ (99.9% purity) and solvents were also purchased from (Sigma-Aldrich Co., St. Louis, MO, USA). All other chemicals used in this study were of analytical grade.
Nigella sativa oil extraction
Nigella sativa oil (NSO) was extracted following the method of Mohammed et al.[Citation24] The oil was extracted using Supercritical Fluid Extraction (SFE) (FeyeCon Development B.V, Netherland). In this study, the dried seeds were completely crushed for 5 min using a 1-liter stainless steel grinder (Waring Commercial blender, Torrington, CT, USA). Supercritical fluid extractions were conducted at pressures of 600 bar and temperatures of 40°C for a duration of 1 h.
Preparation of the emulsion
The emulsion of microencapsulated oil was processed according to the following method:
NSO (core material) was homogenized with maltodextrin DE10 and sodium caseinate (wall material), in a 1:3 core:wall ratio, to obtain 40% of total solids content in the emulsion which was the ideal ratio according to the desired properties reported byprevious study of Mohammed et al.,[Citation23] The protein/carbohydrate ratio was 1:9 (w/w), when soy lecithin was combined in the ratio of 0.1:1 (w/w) of protein as an extra emulsifier to enhance the process of homogenization. This mixture was gradually dissolved in deionized water (65°C) (Millipore, USA) under magnetic agitation to facilitate hydration. The solution was left overnight at 4°C for full hydration. Coarse emulsions were prepared by adding NSO gradually into the solution, which was then homogenized by subjecting the pre-emulsions to a shear homogenizer (Silverson L4R, UK) for 5 min at 14000 rpm until full dispersion. The coarse emulsion was further homogenized in a high-pressure homogenizer (APV, Crawley, UK) at 200 and 180 MPa then dried by spray drying.
Spray drying process
The spray drying process was carried out as described by Mohammed et al.,[Citation23] The emulsion was poured into the mini spray dryer (Büchi Labortechnik AG, Switzerland) which comes with a pneumatic nozzle with a standard tip opening of 0.7 mm in diameter. The emulsion was drawn out using a peristaltic pump with a feed rate of 10 mL/min, while the air flow rate through the chamber was set to the maximum. The emulsion was atomized with a rotary atomizer and atomizer pressure was 450 ± 10 kPa, and the feed temperature was 25.0 ± 0.5°C. The inlet temperature was 160°C and the outlet temperature was 85 ± 2°C. Then, the MNSO was sealed in high-density polyethylene plastic bags and kept inside the freezer until use.
Accelerated storage conditions
Following an earlier process Wanasundara and Shahidi[Citation25], it was decided to use the accelerated Schaal oven test to assess the oxidative stability, fatty acid composition, antioxidant properties of bulk uncapsulated oil and encapsulated oil, moreover the morphology of the microencapsulated oil was observed. The uncapsulated NSO (liquid) and the MNSO (powder) were further evaluated during extended storage for 24 days at 65°C, where one day of storage represented one month of storage at room temperature.[Citation26] In this study, oil was recovered from the coating material following the method used in in next subsection. Uncapsulated NSO was also used as a control sample for oxidative stability. The samples of NSO and MNSO were stored in 250 mL Schott bottles in a drying oven (Protech, Belgium). The chemical analyses were conducted on days 0, 6, 12, 18, and 24 to gauge the changes that took place during accelerated storage.
Microencapsulation efficiency
Microencapsulation efficiency of MNSO under accelerated storage was determined according to the previously reported method by Ahn et al.[Citation9] using the following equation:
where TO is the total oil content and SO is the surface oil content
The total oil of MNSO was controlled according to the method described by Ng et al.[Citation27] with minor modification. The de-emulsifier reagent was prepared for total oil extraction. Briefly, 20 g each of sodium citrate and sodium salicylate were weighed and dissolved individually in deionized water. The total of 36 mL of n-butanol was mixed with these two solutions, and then deionized water was added until the volume reached 180 mL. Following this, 10 mL of distilled water at 50°C was mixed with 5 g of MNSO powder using a conical flask with a stopper. The next stage was the addition of 7.5 mL of the previously prepared de-emulsifier reagent, and after vigorous shaking the mixture was left to stand for 5 min in a water bath at 70°C. Consequently, the mixture was transferred into a 50 mL tube and then subjected to centrifugation at 3000 rpm for 10 min, followed by collection of the total oil from the upper layer of the tube.
Surface oil content of the powders was determined according to the method described by Ahn et al. with some modifications. 200 mL from light petroleum of (bp 60–80°C) was added to 5 g of powders in a sealed glass bottle, with magnetic stirring for 15 min at 25°C in darkness to extract the free oil. The mixture was then filtered with Whatman No. 4 filter paper, and the residue was further washed with 20 mL of light petroleum. The solvent was evaporated at 30°C to avoid the lipid oxidation from the influence of heating.
Peroxide value determination
Changes in oxidation in both the uncapsulated oil of NSO as a control and MNSO produced at the optimized conditions were studied. Both samples were stored in Schott bottles at 65°C for 24 days, and the peroxide value (PV) was determined every six days in darkness as previously described AOAC International.[Citation28] At the end of the experiment, peroxide values in mEq O2/Kg oil was plotted in Y axis against time in days to determine the peroxidation profile of each sample over the entire study period and the curve fitting was perfermoed using both linear and exponential regression analyses using Microsoft excel 2016.
Antioxidant stability of the microcapsule during accelerated storage
The antioxidant activity has been evaluated using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) assay performed according to Blois,[Citation29] with some modifications to determine the radical scavenging activity of MNSO and uncapsulated NSO. In brief, 0.25 mL of samples were mixed with 1.75 mL methanolic DPPH in a 96-well plate. The DPPH solution was prepared fresh by diluting 2.5 mg DPPH in 100 mL methanol. The changes of absorbance was measured after 30 min incubation at 515 nm in darkness and at 25°C. The wavelength of maximum absorbance of DPPH was determined by an ELISA reader (labomed, model UVD-2950, USA). In order to prepare the control, same procedure applied without using the test material (oil),
Estimation of total phenolic content
The total phenolic content (TPC) of MNSO and uncapsulated NSO was determined using the Folin–Ciocalteu reagent (FCR) as previously described[Citation30] with some modifications. The amount 0.5 mL of uncapsulated NSO or extracted oil of MNSO were blended with 0.5 mL of FCR. This blend of the mixture was set aside for 5 min to allow it to react, after which 10 mL of 7% aqueous Na2CO3 sodium carbonate solution were added and mixed. It was then kept for 1 h in total darkness at 25°C. The absorbance was read at 725 nm using an ELISA reader (Power Wave. X340, BioTek instruments, INC., Winooski, VT, USA). The standard calibration curve was made from different concentrations of gallic acid (0–100 mg/mL). The results of the total phenolic content were expressed in gallic acid equivalents (GAE) mg/100 mL dry matter. All experiments were conducted three times and absorbance measurements were obtained in triplicates.
Changes in thymoquinone content upon accelerated storage
The change in thymoquinone (TQ) content in MNSO and uncapsulated NSO during accelerated storage was determined following the method of Ghosheh, Houdi[Citation31] with minor modifications. The total oil extracted from samples. The samples were analyzed using the Agilent 1200 HPLC system (Agilent Technologies, Palo Alto, USA) with a diode array detector (DAD). A Prevail C18 column (250 × 4.6 mm I.D., with 5 µm silica beads, Agilent technologies, USA) was employed with a mobile phase of water: methanol: 2-propanol (50:45:5 v:v:v). The sample was filtered using a 0.45-mm Millipore filter, and 20 µL was the volume of the injection. The TQ was detected at 254 nm at 25°C. A 1.5 mL/min flow rate was used, and identification was achieved by comparison with retention time of standard. The quantified calculations were achieved by constructing standard linear calibration curves.
Fatty acid methyl esters (FAME)
Cocks and van Rede[Citation32] introduced a method for preparing fatty acid methyl esters (FAMEs) to ensure a sufficient volatility when the sample is analyzed. The method applied to 0.2 mL oil samples which were dissolved in n-hexane and subsequently added to 0.2 mL of 2 M potassium hydroxide (KOH). The blended mixtures were vortexed for a few seconds and left to settle for 5 min. The upper layer (FAME solution) was collected and injected into an Agilent 6890N (Little Falls, DE, USA) gas chromatograph equipped with a split-splitless injector using a flame ionization detector. A BPX70 column (0.32 mm internal diameter, 30 m length and 0.25 µm film thickness; SGE International Pty. Ltd., Victoria, Australia) was used in this experiment at a column pressure of 10 psi. The column was initially set at 115°C, and the temperature was programmed to increase to 180°C at a rate of 8°C/min, which was then kept constant until the end of the analysis. The FAME peaks identification were compared with the retention times of standards. The area percentage was determined in triplicates and presented as a mean and standard deviation.
Scanning electron microscopy
To assess the morphology, surface appearance and shape of the spray-dried powders, scanning electron microscopy (SEM) was used. A small amount of MNSO microcapsules were attached with double‐sided adhesive tape to a specimen stub, and coated with gold. The coated microcapsules were examined via SEM (S-3400N model, Hitachi, Tokyo, Japan) at 20.0 kV.
Evaluation of color
The Hunter Lab Colorimeter (Hunter Associates Laboratory, Inc., Virginia, USA) was used to determine the color for the five samples of MNSO The process was done by placing the powder samples into a 3 × 5 in plastic bag before starting the analysis. The analysis was conducted in triplicate, and the values of L, a and b were resolved.
Statistical analysis
The Minitab 16 software was used to analyze all results. Each analysis was measured in triplicate, and the data were expressed as means ± standard deviation (SD). After the one-way analysis of variance was conducted, Tukey’s test was applied for post hoc multiple comparisons of means, and p < 0.05 was considered statistically significant. The correlations derived from the data were then calculated using the Pearson’s correlation coefficients (r).
Results and discussion
Microencapsulation efficiency
Microencapsulation efficiency (MEE) refers to the ability of the wall material to encapsulate the core material within the spherical structure.[Citation33] In order to ensure the powder stability and quality, it should be also kept constant throughout the storage period. The changes in total oil, surface oil and microencapsulation efficiency of MNSO during storage at 65°C for 24 days were given in . In this study, the oil percentage used to prepare the emulsion of NSO to be fed into spray dryer was 25% in dry matter. The total oil contents of MNSO after microencapsulation were in the range of 23–24% due to the loss of oil during spray drying.
Table 1. Total oil (TO), surface oil (SO), and microencapsulation efficiency (MEE) of microencapsulated Nigella sativa oil (MNSO) under accelerated storage.
Table 2. Antioxidant activity (DPPH), total phenolic content (TPC), thymoquinone content (TQ) of uncapsulated (NSO) and microencapsulated (MNSO) under accelerated storage.
Table 3. Relative percentage composition (%) of fatty acids in uncapsulated (NSO) under accelerated storage.
Table 4. Color (Hunter Lab L* a* b*) value MNSO for five different samples.
As expected, total oil contents in MNSO were stable over storage period with slight reduce. This may be due to the protection features of coating materials formed by spray drying which minimized oxygen diffused into the oil core. In addition, surface oil contents in MNSO observed slight decrease with storage period. The same behavior was observed by Bastıoğlu et al.,[Citation34] surface oil contents of microcapsules increased and MEE decreased significantly with storage time in the study of stabilty of microencapsulated extra virgin olive oil. Lipid oxidation could be lower due to a decrease in the diffusivity of O2 into the core. Gharsallaoui et al.[Citation35] reported that lactose in its amorphous state acted as a hydrophilic sealant that significantly limited the diffusion of the hydrophobic core through the wall material, thus leading to high MEE values. According to Ferreira et al.,[Citation36] high MEE values likely exhibits greater protection against lipid oxidation, given that there is less surface oil.
Total phenolic content
The TPC of uncapsulated NSO and the recovered oil of MNSO were assessed over 24 days of accelerated storage (). The TPC in both samples decreased noticeably over the storage period at about the same rate. Polyphenolic compounds are acknowledged to have antioxidant activity, and other components may contribute to antioxidant activity.[Citation37] The results of this study showed that the TPC value at day 0 was 161.96 mg GAE/100 mL, which is higher to a previously reported result (726.67 mg/L).[Citation38] Initial values of NSO and MNSO slightly differed, according to a small decreasing effect of spray-drying. Day 6 showed a significant TPC decrease (p < 0.05) for NSO, while, there was no any significant difference between TPC in days of 0, 6, and 12 for MNSO samples. The total phenolic content of the uncapsulated NSO showed a significant reduction on day 24, with a value of 109.90 mg GAE/100 mL, compared to the first day of the study. In contrast, the TPC of MNSO was stable over the accelerated storage time. This indicates the efficiency of microencapulation in protecting phenolic compounds of NSO during storage.
Evaluation of antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
The antiradical activity of uncapsulated NSO and recovered oil of MNSO to scavenge DPPH was determined under accelerated storage for 24 days as shown in . The activities of the oil samples expressed on the basis of their IC50 values represent the concentration of antioxidant compounds that caused 50% scavenging of DPPH radicals in the specified time period. Both samples showed a decreasing trend in their antioxidant activity during the storage period. On day 0, there were high IC50 values of both NSO and MNSO of 1.64 and 1.46 mg/mL, respectively. Initial values of NSO and MNSO slightly differed according to a small decreasing effect of spray-drying. From day 0 onwards, the NSO sample showed significantly lower IC50 values than those of MNSO. These results are in agreement with earlier studies[Citation6,Citation39] which showed an IC50 of 2.26 mg/mL, which is slightly higher compared to this study. During accelerated storage, there was no significant difference (p > 0.05) in the IC50 values of MNSO samples between days of 0, 6, and 12, versus the IC50 values of NSO which showed significant degradation from the first point. Furthermore, the IC50 values of MNSO demonstrated a slight decrease after day 6 and reached 1.48 mg/mL on day 24. Similarly, Suhag et al.[Citation40] reported that encapsulated honey powder under prolonged storage resulted with initial increase of antioxidants and phenolic contents followed by decreased values through the storage duration. Overall, the antiradical activity of MNSO showed more stability than that of NSO during the accelerated storage period. These results indicate that the wall materials used in the microencapsulation of NSO acts as a barrier to protect the core from oxygen reactions that reduce antioxidant activity.
Fatty acid methyl esters (FAME) compositional analyses
The fatty acid composition of NSO (without encapsulation) and microencapsulated Nigella sativa oil (MNSO) over 24 days of accelerated storage is presented in . The fatty acid composition on day 0 contained a high percentage of unsaturated fatty acid (UFA). The most abundant fatty acids found in the uncapsulated NSO were Linoleic acid (C18:2n-6c) at 59.03% and oleic acid (C18:1n-9c) at 21.67% (). It was also found that small amounts of palmitoleic acid (C16:1) and linolenic acid (C18:3) were present at 0.25% and 0.26%, respectively. Palmitic acid (C16:0) was the predominant saturated fatty acid at 11.49%. These findings are consistent with those of previous studies.[Citation41,Citation42]
After accelerated storage, the total amount of unsaturated fatty acids of uncapsulated NSO dropped (p < 0.05) from 21.94% (day 0) to 20.59% (day 24) for MUFA, and from 59.3% (day 0) to 56.16% (day 24) for PUFA. These changes in MUFA and PUFA are because of breakage of the double bond of unsaturated fatty acids which is due to oil oxidation. The proportion of saturated fatty acids did not show a big change during the period of storage at 65°C despite a decrease in the proportion of unsaturated fatty acids. This is because unsaturated fats are more susceptible to oxidation than saturated ones. The difference in oxidation rate between unsaturated and saturated fats has been reported previously.[Citation43]
On the contrary, as illustrated in , the microencapsulated oil did not show significant differences in any types of fatty acids in MNSO after storage. In addition, the overall percentage of MUFA and PUFA did not show any signs of reduction due to oxidation after being kept in storage. Nevertheless, microencapsulation and the formulation of the wall materials/emulsifiers were effective at preventing an increase in saturated fatty acids (SFA) during accelerated storage. It is well-reported that saturated fats increase the risk of heart disease by increasing LDL cholesterol levels,[Citation44] and the American Heart Association recommends the consumption limit to less than 7% of daily calories.
Oxidative stability
In determining oxidative stability, the peroxide value (PV) is the most widely used standard method. It is often used to measure the amount of hydroperoxide, which represents the primary stage of oil deterioration and can be used as an oxidative index for the monitoring of the early stage of lipid oxidation in food quality control.[Citation45] illustrates the development of PV in uncapsulated oil and encapsulated oil every six days for 24 days storage at 65°C. At the beginning of storage, bulk oil showed 3.43 ± 0.02 mEq O2/kg oil and was not significantly different (p ˃ 0.05) from encapsulated oil (3.53 ± 0.07 mEq O2/kg oil). This result is in agreement with previously reported data,[Citation24] which showed 3.4 mEq O2/kg oil for NSO obtained using supercritical fluid extraction SFE. Consequently, a rapid and significant (p < 0.05) increase was noticeable in the PV of uncapsulated NSO: 6.43 ± 0.15 mEq O2/kg oil (day 6), 18.46 ± 0.11 mEq O2/kg oil (day 12), 37.62 ± 0.25 mEq O2/kg oil (day 18), and 78.57 ± 0.22 mEq O2/kg oil (day 24), respectively.
Figure 1. Peroxide value of microencapsulated Nigella sativa oil (MNSO) and Nigella sativa oil (NSO) upon accelerted storage at 65°C. The most optimum curve fitness was obtained with the exponential regression analysis [y = 3.379e°0.1326x R2 = 0.994 and y = 3.0197x - 7.3027 R2 = 0.864 for each of the exponential and linear regressions respectively] for the encapsulated oil, and [y = 3.054e°0.035x R2 = 0.815 and y = 0.193 x + 2.642 R2 = 0.749 for each of the exponential and linear regressions respectively] for the uncapsulated oil.
![Figure 1. Peroxide value of microencapsulated Nigella sativa oil (MNSO) and Nigella sativa oil (NSO) upon accelerted storage at 65°C. The most optimum curve fitness was obtained with the exponential regression analysis [y = 3.379e°0.1326x R2 = 0.994 and y = 3.0197x - 7.3027 R2 = 0.864 for each of the exponential and linear regressions respectively] for the encapsulated oil, and [y = 3.054e°0.035x R2 = 0.815 and y = 0.193 x + 2.642 R2 = 0.749 for each of the exponential and linear regressions respectively] for the uncapsulated oil.](/cms/asset/2489885e-ffdc-4df5-ae96-61443200e97c/ljfp_a_1371189_f0001_oc.jpg)
These results were in accordance with the findings reported by Ramadan et al.,[Citation46] They evaluated the oxidative stability of NSO using PV test for 21 days storage at 60°C and reported an increase in PV values up to 64.5 mEq O2/kg oil by increasing the storage time. Moreover, the same trend was observed by Kiralan et al.,[Citation47] who reported that NSO showed increasing in PV up to (117.78 meq O2/kg oil) after storage up to 16 days at 60°C.
In fact, NSO is rich in MUFA and contains functional compounds which are susceptible to oxidative degradation,[Citation3] thus, its may be the reason for the degradation in oxidative stability of the uncapsulated oil during storage. On the other hand, the encapsulated oil showed stability throughout storage with no significant differences (p ˃ 0.05) up to 12 days in PV: 3.71 ± 0.02 mEq O2/kg oil (day 6) and 4.61 ± 0.25 mEq O2/kg oil (day 12). Thereafter, PV values increased slightly (p ˂ 0.05): 6.06 ± 0.10 mEq O2/kg oil (day 18) and 8.66 ± 0.16 mEq O2/kg oil (day 24). On day 24, PV results for both samples were significantly different. These results are in agreement with those reported by Calvo et al.,[Citation48] who found that a blend of maltodextrin, carboxymethylcellulose and lecithin was an effective barrier against walnut oil oxidative degradation. According to Takeungwongtrakul,[Citation11] the use of suitable formulation of wall materials in combination with antioxidants resulted in a stable microencapsulated shrimp oil against oxidation. In addition, Hamilton et al.[Citation49] reported that refined fish oil with δ-tocopherol, ascorbyl palmitate, and lecithin showed no significant peroxidation at 20°C over a period of 6 months. The lecithin was found to give small improvements in overall oxidative stability. Wang[Citation50] also studied the effects of soy lecithin and egg lecithin and observed that soy lecithin showed higher oxidative stability compared to egg lecithin under the same experimental conditions. These results incompatible with the findings of Palacios,[Citation51] they also reported that soy lecithin and egg lecithin had high oxidative properties evaluated by peroxide value (PV) test when used as an emulsifier for the oil/water emulsion, soybean lecithin clearly performed much better than egg-yolk lecithin in creating a stable oil/water emulsion. A similar trend was reported recently by Wang[Citation19] during microencapsulation of chili seed oil using spray drying. The encapsulation using the optimum composite wall material with a ratio of starch sodium octenylsuccinate and maltodextrin of 4:1; minimize the oxidation of the oil. In other study, the selection of wall material combinations was the main key to the success of the product oxidative stability during storage as each compound will have its important role and above of all, is the encapsulation process that attaches the oil to the protective walls of the materials.[Citation10]
Oxidation of oil creates a variety of compounds including free radicals and hydroperoxides. According to the Codex Alimentarius Commission,[Citation52] the permitted value of PV in oil products should not exceed 10 mEq O2/kg oil. showed better fitness in the exponential regression with [R2 exponential = 0.994 and R2 linear = 0.864] (). Accordingly; it is better to use the exponential equation to predict PV at different intervals . The time required to raise the PV value to 10 mEq O2/kg oil, as calculated by the exponential regression analysis, was 8 and 33 days for the uncapsulated and encapsulated oils, respectively.
Quantification of thymoquinone content upon accelerated storage
The content of TQ in uncapsulated NSO and the extracted oil of MNSO powder was quantified every six days for 24 days under accelerated storage at 65°C using HPLC analysis (). The TQ is a major phytochemical compound of NSO, and contributes to the overall stability and potential health benefits of the oil.[Citation53] In this study, the fresh uncapsulated NSO and MNSO (day 0) showed a high content of TQ of 6.90 and 6.59 mg/mL, respectively (). This result is in agreement with previous study of Ismail, Al-Naqeep[Citation54] who reported that NSO extracted with SFE was rich in TQ, with a percentage of about four times higher compared to the conventional extraction methods. Under accelerated storage, uncapsulated NSO exhibited a rapid and significant decrease (p ˂ 0.05) to 5.66 mg/mL (day 6). On the same day, MNSO was not significantly different (p ˃ 0.05) and remained stable at 6.59 mg/mL. This also suggests that there is high oxidative stability of MNSO during accelerated storage in spite of its high content of susceptible bioactive compounds like TQ and fatty acids. However, the quantity of TQ in NSO decreased significantly (p ˂ 0.05) with storage time: 5.06 mg/mL (day 12), 4.73 mg/mL (day 18) and 3.83 mg/mL (day 24).
In the mid-storage period, MNSO showed a slight decrease in TQ content. The content of TQ reached to 5.90 mg/mL on day 24 which is almost double compared to that of uncapsulated NSO. The results indicates importance of preventing TQ of NSO using microencapsulation process. Similarly, Ng, Wong[Citation27] reported the efficacy of kenaf seed oil microencapsulation using the same type of wall material as well as spray drying to limit the loss of bioactive compounds. Kha[Citation55] also observed high stability of the carotenoids in encapsulated gac oil during the storage was achieved with encapsulation using spray drying.
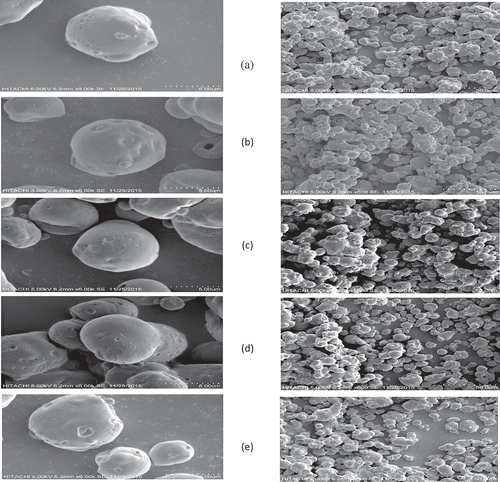
Morphology of microcapsules upon accelerated storage by SEM
illustrates the surface characteristics, shape and size of the MNSO under accelerated storage conditions using SEM. In this study, the morphology of the oil coated by maltodextrin DE10 and sodium caseinate showed a spherical shape with various sizes in diameter. The microcapsules obtained by the spray drying technique resulted in spherical and smooth surfaces with very few wrinkled capsules (). In spite of the non-uniform size of the MNSO particles and that they seemed to be agglomerated, it is appear that the wall materials covering the oil with no observed cracks on the suface. In fact, the spray drying method commonly produces particles of different sizes.[Citation17] No apparent cracks, sharp edges or fissures were observed on the surfaces of the MNSO during the 24 days (). In addition, shows the morphology of MNSO powder on day 24, demonstrating that it had a smooth surface and was free from shrinkage. This proves the efficacy of the wall material used in NSO microencapsulation after 24 days of accelerated storage. In a recent study by Shamaei[Citation56] who studied the microencapsulation of walnut oil using spray drying. The morphology observations showed almost no cracks or fissures on the surface of microcapsules produced. Sanchez and Baeza[Citation57] asserted that maltodextrin is the best thermal defender, is a powerful barrier against oxidation of core materials and is protective against undesired physical and chemical changes.
Color determination
Color determination was performed by the Hunter Lab colorimeter method and was determined by its color coordinates: L* indicates the variance between light (L* = 100) and dark (L* = 0), a* indicates the variance between green (–a*) and red (+a*), and b* indicates the variance between blue (−b*) and yellow (+b*). It is important to monitor the brightness and the yellowness of the MNSO samples. Color plays a very important role in the acceptance of food powders. Basically, NSO is yellowish brown oil, while the wall material mixture of maltodextrin and sodium caseinate is white. Microencapsulation produced a white powder compared to the oil. The color of the powder can be affected by many factors such as the type of wall materials, variety of microencapsulation techniques and the processing conditions. shows that the highest value of brightness was achieved by powder on day 0 (p < 0.05), while the lowest value was noted on day 24. This result was in line with the findings of Binsi,[Citation58] who observed a minimum reduction in L* values during storage of microencapsulated fish oil which has been spray dried with gum Arabic and sage extract, throughout the accelerated storage study at 60°C. During storage, the MNSO powder did not change significantly from day 6 until the end of the accelerated storage period which satisfies consumers desire. In comparison, the lowest value for yellowness was on day 0, while the highest value was on day 24.
Conclusion
The stability of Nigella sativa oil (NSO) without encapsulation was found to significantly decrease (p ˂ 0.05) over the period of accelerated storage (24 days). The findings of the present study indicated that the degradation in oxidation stability, antioxidant activity, phenolic content and the amount of TQ in the NSO were due to storage without encapsulation. In addition, results from this study clearly showed that the oxidative stability of microencapsulated oil was mainly due to the encapsulation compared to the uncapsulated oil which easily oxidized under accelerated storage. It was observed that the MUFA and PUFA in NSO showed a significant but slight change (p < 0.05) when stored for 24 days. The cause of the lipid oxidation was likely the breakdown in the double bond of MUFA and PUFA. The morphology of the powder illustrated that the walls successfully coated the oil particles, which protects the oil against oxidation. The microencapsulation technique can effectively prolong the shelf life of NSO and enhance the resistance against oxidation while retaining the content of bioactive compounds.
Acknowledgments
This work was supported by Universiti Putra Malaysia grant (GP-IPS/2014/9438743). The authors declare no conflict of interest of any kind.
References
- Khazdair, M. R.; The Protective Effects of Nigella Sativa and Its Constituents on Induced Neurotoxicity. J. Toxicol. 2015, 1–7.
- Kiralan, M.; et al. Physicochemical Properties and Stability of Black Cumin (Nigella Sativa) Seed Oil as Affected by Different Extraction Methods. Ind. Crops Prod. 2014, 57, 52–58.
- Ramadan, M. F.; Kroh, L. W.; Mörsel, J.-T. Radical Scavenging Activity of Black Cumin (Nigella Sativa L.), Coriander (Coriandrum Sativum L.), And Niger (Guizotia Abyssinica Cass.) Crude Seed Oils and Oil Fractions. J. Agric. Food Chem. 2003, 51 (24), 6961–6969.
- Woo, C. C.; et al. Thymoquinone: Potential Cure for Inflammatory Disorders and Cancer. Biochem. Pharmacol. 2012, 83 (4), 443–451.
- Kim, T.-S.; et al. The Effect of Storage Duration on Bio-Oil Properties. J. Anal. Appl. Pyrolysis 2012, 95, 118–125.
- Solati, Z.; Baharin, B. S.; Bagheri, H. Antioxidant Property, Thymoquinone Content and Chemical Characteristics of Different Extracts from Nigella Sativa L. Seeds. J. Am. Oil Chemists’ Soc. 2014, 91 (2), 295–300.
- Ramadan, M. F.; Wahdan, K. M. M. Blending of Corn Oil with Black Cumin (Nigella Sativa) and Coriander (Coriandrum Sativum) Seed Oils: Impact on Functionality, Stability and Radical Scavenging Activity. Food Chem. 2012, 132 (2), 873–879.
- McClements, D. J.; Edible Nanoemulsions: Fabrication, Properties, and Functional Performance. Soft Matter 2011, 7 (6), 2297–2316.
- Ahn, J.-H.; et al. Optimization of Microencapsulation of Seed Oil by Response Surface Methodology. Food Chem. 2008, 107 (1), 98–105.
- Jafari, S. M.; et al. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Drying Technol. 2008, 26 (7), 816–835.
- Takeungwongtrakul, S.; Benjakul, S. Wall Materials and the Presence of Antioxidants Influence Encapsulation Efficiency and Oxidative Stability of Micro‐Encapsulated Shrimp Oil. Eur. J. Lipid Sci. Technol. 2015, 117 (4), 450–459.
- Tonon, R. V.; Brabet, C.; Hubinger, M. D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe Oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88 (3), 411–418.
- Gupta, P. K.; Jadhav, S. B.; Singhal, R. S. Development of Shrikhand Premix Using Microencapsulated Rice Bran Oil as Fat Alternative and Hydrocolloids as Texture Modifier. Food Hydrocolloids 2015, 48, 220–227.
- Sawale, P. D.; et al. Release Characteristics of Polyphenols from Microencapsulated Terminalia Arjuna Extract: Effects of Simulated Gastric Fluid. Int. J. Food Properties 2017, 20 (12), 1–9.
- Alves, A. I.; et al. Morphological Characterization of Pequi Extract Microencapsulated through Spray Drying; International Journal of Food Properties, 2017. 1–8.
- Morales-Medina, R.; et al. Functional and Antioxidant Properties of Hydrolysates of Sardine (S. Pilchardus) and Horse Mackerel (T. Mediterraneus) for the Microencapsulation of Fish Oil by Spray-Drying. Food Chem. 2016, 194, 1208–1216.
- Carneiro, H. C.; et al. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115 (4), 443–451.
- Mohideen, F. W.; et al. Effects of Blueberry (Vaccinium Corymbosum) Juice on Lipid Oxidation during Spray Drying of Microencapsulated Menhaden Oil. Int. J. Food Properties 2015, 18 (5), 1139–1153.
- Wang, Y.; et al. Quality Analysis and Microencapsulation of Chili Seed Oil by Spray Drying with Starch Sodium Octenylsuccinate and Maltodextrin. Powder Technol. 2017, 312, 294–298.
- Da Silva, F. C.; et al. Assessment of Production Efficiency, Physicochemical Properties and Storage Stability of Spray-Dried Propolis, a Natural Food Additive, Using Gum Arabic and OSA Starch-Based Carrier Systems. Food Bioprod. Process. 2013, 91 (1), 28–36.
- Edris, A. E.; et al. Microencapsulation of Nigella Sativa Oleoresin by Spray Drying for Food and Nutraceutical Applications. Food Chem. 2016, 204, 326–333.
- Abedi, A. S.; et al. Microencapsulation of Nigella Sativa Seeds Oil Containing Thymoquinone by Spray‐Drying for Functional Yogurt Production. Int. J. Food Sci. Technol. 2016, 51 (10), 2280–2289.
- Mohammed, N. K.; et al. Process Conditions of Spray Drying Microencapsulation of Nigella Sativa Oil. Powder Technol. 2017, 315, 1–14.
- Mohammed, N. K.; et al. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella Sativa L.) Oil. In Evidence-Based Complementary and Alternative Medicine; 2016, p. 10, 6273817.
- Wanasundara, U. N.; Shahidi, F. Antioxidant and Pro-Oxidant Activity of Green Tea Extracts in Marine Oils. Food Chem. 1998, 63 (3), 335–342.
- Razmkhah, S.; et al. Quality Changes and Antioxidant Properties of Microencapsulated Kenaf (Hibiscus Cannabinus L.) Seed Oil during Accelerated Storage. J. Am. Oil Chemists’ Soc. 2013, 90 (12), 1859–1867.
- Ng, S. K.; et al. Influence of the Inlet Air Temperature on the Microencapsulation of Kenaf (Hibiscus Cannabinus L.) Seed Oil. Eur. J. Lipid Sci. Technol. 2013, 115 (11), 1309–1318.
- Horwitz, W.; Latimer, G. W. AOAC International. Official methods of analysis of AOAC International; 18th ed. Gaithersburg, MD: AOAC International, 2005.
- Blois, M. S.; Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178.
- Ghosheh, O. A.; Houdi, A. A.; Crooks, P. A. High Performance Liquid Chromatographic Analysis of the Pharmacologically Active Quinones and Related Compounds in the Oil of the Black Seed (Nigella Sativa L.). J. Pharm. Biomed. Anal. 1999, 19 (5), 757–762.
- Cocks, L. V.; Van Rede, C. Laboratory Handbook for Oil and Fat Analysts. Laboratory Handbook for Oil and Fat Analysts; London, UK: Academic Press, 1966.
- Ng, S.-K.; et al. Effect of Accelerated Storage on Microencapsulated Kenaf Seed Oil. J. Am. Oil Chemists’ Soc. 2013, 90 (7), 1023–1029.
- Bastıoğlu, A. Z.; et al. Storage Characteristics of Microencapsulated Extra Virgin Olive Oil Powder: Physical and Chemical Properties. J. Food Meas. Characterization 2017, 11 (3), 1–17.
- Gharsallaoui, A.; et al. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40 (9), 1107–1121.
- Ferreira, C. D.; et al. Physicochemical Characterization and Oxidative Stability of Microencapsulated Crude Palm Oil by Spray Drying. Food Bioprocess Technol. 2016, 9 (1), 124–136.
- Ghasemzadeh, A.; Jaafar, H. Z.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber Officinale Roscoe). Molecules 2010, 15 (6), 4324–4333.
- Viuda-Martos, M.; et al. In Vitro Antioxidant and Antibacterial Activities of Essentials Oils Obtained from Egyptian Aromatic Plants. Food Control 2011, 22 (11), 1715–1722.
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella Sativa Essential Oil. Phytotherapy Res. 2000, 14 (5), 323–328.
- Suhag, Y.; Nanda, V. Degradation Kinetics of Ascorbic Acid in Encapsulated Spray-Dried Honey Powder Packaged in Aluminium Laminated Polyethylene and High-Density Polyethylene. Int. J. Food Properties 2017, 20 (3), 645–653.
- Atta, M. B.; Some Characteristics of Nigella (Nigella Sativa L.) Seed Cultivated in Egypt and Its Lipid Profile. Food Chem. 2003, 83 (1), 63–68.
- Khoddami, A.; et al. Physicochemical Characteristics of Nigella Seed (Nigella Sativa L.) Oil as Affected by Different Extraction Methods. J. Am. Oil Chemists’ Soc. 2011, 88 (4), 533–540.
- Lee, E.; Choe, E. Changes in Oxidation-Derived Off-Flavor Compounds of Roasted Sesame Oil during Accelerated Storage in the Dark. Biocatalysis Agric. Biotechnol. 2012, 1 (1), 89–93.
- Rivellese, A. A.; et al. Effects of Dietary Saturated, Monounsaturated and N-3 Fatty Acids on Fasting Lipoproteins, LDL Size and Post-Prandial Lipid Metabolism in Healthy Subjects. Atherosclerosis 2003, 167 (1), 149–158.
- Wang, R.; Tian, Z.; Chen, L. A Novel Process for Microencapsulation of Fish Oil with Barley Protein. Food Res. Int. 2011, 44 (9), 2735–2741.
- Ramadan, M. F.; Mörsel, J. T. Oxidative Stability of Black Cumin (Nigella Sativa L.), Coriander (Coriandrum Sativum L.) And Niger (Guizotia Abyssinica Cass.) Crude Seed Oils upon Stripping. Eur. J. Lipid Sci. Technol. 2004, 106 (1), 35–43.
- Kiralan, M.; et al. Blends of Cold Pressed Black Cumin Oil and Sunflower Oil with Improved Stability: A Study Based on Changes in the Levels of Volatiles, Tocopherols and Thymoquinone during Accelerated Oxidation Conditions. J. Food Biochem., 2016 14 (1), e12272
- Calvo, P.; et al. Effects of Microcapsule Constitution on the Quality of Microencapsulated Walnut Oil. Eur. J. Lipid Sci. Technol. 2011, 113 (10), 1273–1280.
- Hamilton, R.; et al. Effects of Tocopherols, Ascorbyl Palmitate, and Lecithin on Autoxidation of Fish Oil. J. Am. Oil Chemists’ Soc. 1998, 75 (7), 813–822.
- Wang, G.; Wang, T. Oxidative Stability of Egg and Soy Lecithin as Affected by Transition Metal Ions and pH in Emulsion. J. Agric. Food Chem. 2008, 56 (23), 11424–11431.
- Palacios, L. E.; Wang, T. Egg-Yolk Lipid Fractionation and Lecithin Characterization. J. Am. Oil Chemists’ Soc. 2005, 82 (8), 571–578.
- Commission, CA. Recommended Internal Standard for Edible Fats and Oils. Edn 1982, 1, 1–179.
- Lutterodt, H.; et al. Fatty Acid Profile, Thymoquinone Content, Oxidative Stability, and Antioxidant Properties of Cold-Pressed Black Cumin Seed Oils. LWT-Food Sci. Technol. 2010, 43 (9), 1409–1413.
- Ismail, M.; Al-Naqeep, G.; Chan, K. W. Nigella Sativa Thymoquinone-Rich Fraction Greatly Improves Plasma Antioxidant Capacity and Expression of Antioxidant Genes in Hypercholesterolemic Rats. Free Radic. Biol. Med. 2010, 48 (5), 664–672.
- Tuyen, C. K.; et al. A Storage Study of Encapsulated Gac (Momordica Cochinchinensis) Oil Powder and Its Fortification into Foods. Food Bioprod. Process. 2015, 96, 113–125.
- Shamaei, S.; et al. Microencapsulation of Walnut Oil by Spray Drying: Effects of Wall Material and Drying Conditions on Physicochemical Properties of Microcapsules. Innovat. Food Sci. Emerging Technol. 2017, 39, 101–112.
- Sanchez, V.; et al. Freeze-Drying Encapsulation of Red Wine Polyphenols in an Amorphous Matrix of Maltodextrin. Food Bioprocess Technol. 2013, 6 (5), 1350–1354.
- Binsi, P.; et al. Structural and Oxidative Stabilization of Spray Dried Fish Oil Microencapsulates with Gum Arabic and Sage Polyphenols: Characterization and Release Kinetics. Food Chem. 2017, 219, 158–168.