ABSTRACT
The objective of this study was to produce and characterise biodegradable thermoplastic starch (TPS) derived from the fruit of pejibaye palm (Bactris gasipaes Kunth) plasticised with glycerol and sorbitol. The plasticised starch yielded a strength (σ) of 1.3 ± 0.2 MPa, deformation (ε) of 9.4 ± 1.6%, and Young’s modulus (E) of 191 ± 72.0 MPa. The thermal analysis showed a 61.14% mass loss over the temperature range 290–388°C and an endothermic peak at 130°C. X-ray diffractograms of the TPS revealed peaks corresponding to crystallites of type Vh at 19.4° and type V at 22.6°. Morphological studies, by scanning electron microscopy, showed the plasticised starch had a homogeneous surface, without phase separation and without cracks, with few granules not gelatinised. The analysis of its biodegradation by soil burial tests showed a total mass loss of 84.4 ± 4.4%, after 18 weeks. Biodegradable TPS was successfully obtained from the pejibaye palm fruit, presenting resistant to traction and to the thermal degradation.
Introduction
There is a worldwide demand to reduce the amount of plastic materials discarded into the environment, in addition to a great incentive to recycle. As an alternative, the use of biodegradable thermoplastics as a substitute for conventional plastics has received considerable attention.[Citation1–Citation5] Bioplastics can be used in garbage bags, as films to protect food and to cover soil, in infant diapers, as flexible stems, in plant containers, capsule preparation, an expanded polystyrene (Isopor®) replacement, in the production of cutlery, plates and disposable cups, in the manufacture of pens, pencils, toys, and in other applications, for which the biodegradable character is desirable.[Citation6–Citation9]
The use of starch as the raw material of biodegradable thermoplastics is promising because it is abundant in nature, is renewable, and has relatively low production costs.[Citation10,Citation11] The technology of the use of native starch for production of thermoplastic materials is a trend in the market of disposable plastics, because the polysaccharide confers biodegradability to the composite, without significantly lessening its functionality.[Citation9,Citation12,Citation13] Thermoplastic starch (TPS), also known as cockle starch or plasticised starch, is a bioplastic product obtained by fusion of the starch granule with a plasticiser agent (e.g. water, glycerol, sorbitol), under a combination of pressure, shear, and temperature (90–180ºC). In this condition, the chains of amylose and amylopectin of the starch become interwoven and the original semi-crystalline structure of the granule is destroyed.[Citation4,Citation14,Citation15] The processing of TPS promotes changes, allowing its use as a raw material that improves the degradability of packages.
Among the existing starches, the fruit of pejibaye or peach palm (Bactris gasipaes Kunth) presents as an alternative to produce bioplastics, because of its chemical and functional properties, as well as its thermal and morphological characteristics, allowing the obtention of a thermoplastic. This starch has 12.4% amylose and 66.6% amylopectin, low water solubility and gelatinisation temperature in the range of 65–75ºC.[Citation16] In addition, it presents oval granules of 8.40 µm, and a high degree of association between its components that form the matrix and confer its pseudoplastic rheological behaviour.[Citation17]
Considering that the starch of pejibaye palm fruit has great potential for applications in various industrial sectors, the aim of this work was to produce and characterise a biodegradable thermoplastic of starch obtained from the fruit of peach palm (B. gasipaes Kunth), plasticised with glycerol and sorbitol.
Materials and methods
A Doehlert experimental design comprising two variables, with nine formulations, and triplicates at the central point, was used. The thermoplastic was characterised by the traction test, for tension (σ), deformation (ε) and Young’s modulus (E), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), x-ray diffraction (XRD), scanning electron microscopy (SEM), and biodegradation analysis.
Materials
Pejibaye palm fruit starch, with approximately 66.6% amylopectin, 12.4% amylose, 0.5% protein, and 11% moisture, was produced and assigned by the Federal Institute of Bahia. Bi-distilled glycerol (pure, anhydrous) and sorbitol (70% solution) were both supplied by Synth, Brazil.
Methods
Preparation of the thermoplastics
The pejibaye palm fruit starch was added to a mixture of plasticiser (glycerol + sorbitol) at a proportion of 30% plasticiser relative to the mass of starch. The values encoded from the experimental design were proportionally adjusted to obtain a final mass equal to 30 g plasticiser (). The mixtures were sieved (1 mm mesh) to obtain a homogeneous powder, which was then placed into a stainless steel mould, according to the Brazilian Association of Technical Standard (ABNT) D638. The obtained samples were compressed in a hydraulic press, with a heating/cooling system, under the following conditions: 150 ± 2.5°C, 10 min pressing time and 8 t press, then cooled to 40°C, for demoulding.
Table 1. Coded values, predicted by the model and adjusted proportionately to 30g plasticisers (actual values) and results of the dependent variable Fmax (N), Tdeg (°C), and Tfusion (°C) for Doehlert design.
Experimental delineation
The effect of the plasticiser mixture (glycerol + sorbitol) in the thermoplastics was evaluated by a Doehlert design, comprising nine formulations, with three repetitions at the central point.[Citation18] Based on the literature and preliminary tests, the variable responses investigated were the maximum force of rupture (Fmax) by the traction test, the degradation temperature (Tdeg) by the TGA, and the fusion temperature (Tfusion) by the exploratory calorimetry differential.[Citation8,Citation11,Citation14,Citation15] The compositions studied and the codes used to designate each of the ratios of the plasticisers are presented in . Analysis of variance (ANOVA) was used to test the statistical significance of the following linear model in the parameters:
where Y is the output variable, β0 is the average, Βi is the linear coefficient, βii is the quadratic coefficient, βij is the coefficient of interaction, and Xi and Xj represent the independent variables (glycerol and sorbitol). This common and well-established technique aims to simultaneously determine the input variables that can give the optimum performance levels for one or more responses. First, the individual desirability function is calculated that varies from 0 to 1
, where 1 is the maximum desirability and 0 is for non-desirable situations or minimum. The individual desirability scores are then combined into an overall desirability (D) by computing the geometric mean of different desirability values (di):
where is the importance of each variable on the other. All results were evaluated using Statistica® 8.0 (Statsoft, USA).
Traction test
The traction of the thermoplastics was tested according to the American Society for Testing and Materials (ASTM) D638 standard, in an EMIC DL3000N universal testing machine, with a loading speed of 1.5 mm/min. The tests were conducted at ambient temperature.
TGA and DSC
The thermal stability of the thermoplastics was evaluated using a thermogravimetric analyzer (Perkin Elmer 6000 STA), assisted by Pyris software. The TGA thermograph was obtained over a temperature range of 20–600°C, at a heating rate of 10°C/min, in a nitrogen atmosphere (20 mL/min flow rate). A Shimadzu CSD-60 differential calorimeter was used to define the initial temperature (Tonset), fusion temperature (Tfusion), and the enthalpy (ΔH) of the samples. The analysis was performed over a temperature range of 10–250°C, at a heating rate of 10°C/min.
XRD and SEM
The thermoplastics were characterised by XRD using a Shimadzu Xdr-7000 diffractometer, operating at a power of 30 kV and 10 mA. The test was performed at ambient temperature (25°C), observing the diffraction angle of 2Ɵ between 5 and 40° (0.5° min−1). SEM observations (FEI Company Quanta 400) were performed at a maximum operating voltage of 30 kV, 1.2 nm spatial resolution, in a high vacuum, and an acceleration voltage of 20 kV.
Biodegradation analysis
The biodegradability test in soil simulation was performed according to the ASTM G160-03 standard, controlling soil moisture (range between 20 and 30%), and the temperature conditions. Each condition contained a sample of each composition. The conditions were formed by adding the prepared soil and plates of each thermoplastic coating to 600-mL beakers. The beakers were placed in a greenhouse (with air circulation and cooling) maintained at 30 ± 2°C. The samples were cleaned and weighed after storage for 2, 4, 8, 10, 12, 14, 16, and 18 weeks.[Citation22]
Results and discussion
Optimisation of production of the thermoplastic
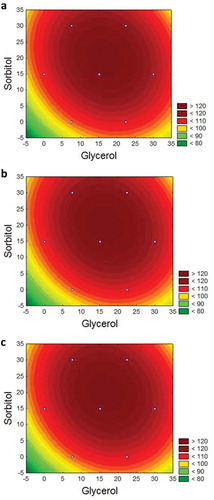
The results of nine experiments performed on TPS derived from the pejibaye palm fruit using the Doehlert design are presented in . The effect of each plasticiser, their respective mixtures, as well as the ANOVA, are presented in , , and . Both the glycerol and sorbitol exerted a positive effect on the Fmax of the TPS samples, as well as an interaction effect. The presence of sorbitol conferred the samples more resistant to traction, whereas those prepared with higher glycerol concentrations presented lower Fmax values. This behaviour can be associated with the hygroscopic character of glycerol.[Citation23] According to[Citation8] thermoplastics plasticised with glycerol are generally more fragile and flexible than those with sorbitol.
Table 2. Estimated regression coefficients and ANOVA (p = 0.05) for Fmax (N) of pejibaye starch thermoplastic.
Table 3. Estimated regression coefficients and ANOVA (p ≤ 0.05) for Tdeg (°C) of peach palm fruit starch thermoplastic.
Table 4. Estimated regression coefficients and ANOVA (p ≤ 0.05) for Tfusion (°C) of peach palm fruit starch thermoplastic.
Regarding the Tdeg, it is observed that there was neither a linear nor interaction effect of sorbitol, however, glycerol exerted a significant and negative effect. Samples plasticised with glycerol had a lower thermal stability compared to those containing sorbitol, which has been associated with the loss of glycerol during processing.[Citation1] With respect to the Tfusion, both glycerol and sorbitol exerted positive linear effects, however, the interaction effect was negative. This negative effect may be associated with the phenomenon known as anti-plasticisation that occurs when the plasticisers glycerol, sorbitol, and water are used in small quantities.[Citation24] The causes of this effect on materials based on starch have not been clearly elucidated but molecular mobility has a preponderant role in the mechanisms involved.
The Doehlert delineation showed significant (p ≤ 0.05) in all models. Both the traction (Fmax), and the thermal properties (Tdeg and Tfusion) were affected by sorbitol and glycerol proportions used in the plasticiser mixture, corroborating previous literature results for other thermoplastics based on starch.[Citation8,Citation23,Citation24] From the ANOVA, it was verified that the regression models generated were significant, while the lack of adjustment was not significant for any of the variables. On the basis of significant effects and considering the 95% confidence level, the mathematical models proposed are given by the following equations:
The graphics for Fmax, Tdeg, and Tfusion are illustrated in . It can be observed that the excellent values determined for Fmax were X1 = 15.2 (13.5 g glycerol) and X2 = 18.5 (16.5 g sorbitol). For Tdeg, these were X1 = 12.4 (13.1 g glycerol) and X2 = 15.9 (16.9 g sorbitol) and, for Tfusion, X1 was 16.7 (12.1 g glycerol) and X2 was 20.0 (17.9 g sorbitol). Considering that all models were valid, the desirability for the variables studied was 13.2 g glycerol and 16.8 g sorbitol. The masses obtained tended to a maximum desirability because the total value was 0.98.
Characterisation of the thermoplastics
Traction test
reveals a strength (σ) of 1.3 ± 0.2 MPa, deformation (ε) of 9.4 ± 1.6%, and Young’s modulus (E) of 191 ± 72.0 MPa. For σ, a similar value was found by [Citation15], for TPS from maize (0.8 MPa). In contrast, for TPS from wheat[Citation25] and cassava[Citation26] higher values (4.5 and 4.0 MPa, respectively) were reported. Also,[Citation26] studying TPS from maize and yam, documented σ values of 17 and 30 MPa, respectively. Thermoplastics plasticised with glycerol have a lower σ compared to sorbitol, due to the hygroscopic character of glycerol, which tends to provide additional water to the system.[Citation23] Based on[Citation25], thermoplastics plasticised with sorbitol in concentrations below 27%, are usually rigid and brittle, however, the σ are superior. Increasing the sorbitol concentration above this limit makes the material plastic, reducing the σmax. This behaviour can be observed in the TPS derived from pejibaye palm fruit. The deformation (ε) values found in this study were similar to those found by[Citation27], of 8.9 and 10.6%, for thermoplastics of Amaranthus cruentus dried at 40 and 50°C, respectively. However,[Citation28] reported a comparatively higher deformation (27%) for TPS from modified potatoes.
Table 5. Mechanical properties of the thermoplastic peach palm fruit starch.
In the current study, E was lower than 800 MPa, suggesting that the material was not fully in a vitreous state.[Citation29,Citation30] A similar behaviour was also observed by [Citation26], for the TPS from cassava, where E ranged between 9 and 737 MPa. However,[Citation30] also studied the TPS derived from cassava and encountered an E of 2600 MPa. For these authors, the effect of plasticisers, particularly glycerol, on E was difficult to understand, however, it was believed that the samples suffered a direct influence of the moisture/water activity present in the material. A similar phenomenon has been described for thermoplastics of wheat starch, with a small amount of sorbitol incorporated.[Citation25]
Thermal analysis
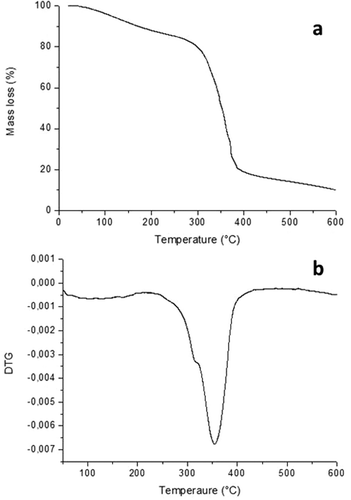
Three thermal events were observed in the TGA curve of the TPS (). The first event occurred between 23 and 290°C and was accompanied by a mass loss of 18.50%. This initial event presented a gradual mass loss that was associated with the evaporation of water and the plasticisers (glycerol and sorbitol).[Citation15] The second event occurred between 290 and −388°C, with a mass loss of 61.14%. This event is considered the main stage of decomposition of the material (TPS) and presents an intense weight loss.[Citation15] The third event was observed between 388 and 600°C, with a mass loss of 20.35%. This event corresponded to the degradation of waste (residual material). The same thermogravimetric profile in thermoplastics based on starch. The observed decomposition temperature is thought to be due to the observed crystalline structure in this polymer.[Citation31–Citation34]
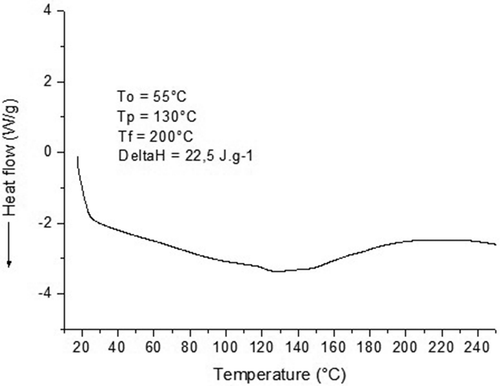
In the DSC curve (), the presence of a very large peak of endothermic fusion was observed between 55 to 245°C, with a Tonset of 55°C and Tfusion of 130°C, with an enthalpy change (ΔH) of 22.5 J g–1, it was not possible to detect the vitreous transition temperature (Tg) over the range of analysis established. In accordance with[Citation35,Citation36] the Tfusion found was directly associated with the evaporation of water and the glycerol, demonstrating the semi-crystalline character of the TPS processed from the pejibaye palm fruit. The TGA evaluation corroborates with this observation because the material presented a loss of mass over the same temperature range. The presence of an interval of extended temperature can be associated with the presence of plasticised sorbitol, which promotes the formation of more resistant links during the heating process. A similar thermal behaviour was also observed by[Citation24] in TPS from cassava.
XRD
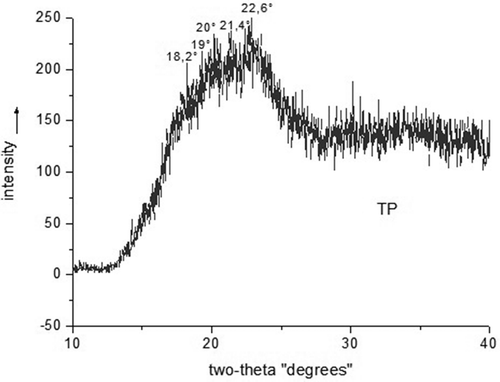
The XRD pattern of the TPS of the pejibaye palm fruit () presented a standard native crystalline structure, with the corresponding Bragg (2Ɵ) diffraction angles of 15°, 17°, and 23°, which are characteristic of type A crystals.[Citation17] The result shows that the conditions applied in the preparation of the thermoplastic were sufficient to rupture the starch granules. Starch has two typical components, amylose and amylopectin.[Citation37] In granular starch, the crystallinity is attributed to the amylopectin because the amylose is found in an amorphous state. However, in the bioplastics, the amylose rapidly crystallises into type B and V structures. The type B crystal structure is associated with the crystallisation of amylose due to its linear structure and to the amylopectin during long storage periods.[Citation14,Citation38] Type V crystalline structures are formed only by amylose, directly after processing.[Citation39] The XRD of the bioplastics showed a peak near 19° (2Ɵ). This peak is attributed to the crystallinity of the amylose uni-helical and is denoted Vh. A similar peak was observed by[Citation37,Citation40] in bioplastics based on cassava and potatoes, respectively. There were no type B crystalline structures observed. However, the literature reports that starch-based bioplastics are sensitive to storage conditions and tend to suffer recrystallisation, consequently, type B crystalline structures are formed due to the amylose and amylopectin.[Citation11,Citation14] Comparing the results of the XRD and thermal analysis, only one crystalline peak and one melting peak, respectively, were noted. This demonstrates the reliability of the results obtained for the evaluation of the polymer structure.
SEM
The micrographs of the fractured thermoplastic are observed in . At 400x magnification, a homogeneous surface was revealed, without separation of phases and cracks, as reported by[Citation41], in analyses of bioplastics on the basis of cassava starch and emulsifier. Under extension of 1.000x, it was possible to view the presence of non-gelatinised and partially gelatinised (not completely broken) granules, in addition to the presence of tiny pores in the material. These pores may have originated from the expulsion of air during processing, as reported by[Citation40,Citation42] in bioplastics based on maize and cassava, respectively. Same characteristics in relation to total non-gelatinisation of maize starch granules in bioplastics and concluded that this may be associated with the parameters of this process, such as the gelatinisation temperature and the water concentration, which may have reduced the effect of shear.[Citation43] According to [Citation44,Citation45], the swelling of the granules and the leaching of amylose into the intergranular space may also occur. Consequently, due to their close proximity, hydrogen bonds can form between the amylose chains, creating “ghosts” of granules that were not disintegrated during the process of gelatinisation. Thus, through the analysis of the micrographs, it can be concluded that the conditions applied were not efficient to cause the rupture of the majority of the starch granules, however, there were no compromises on the other properties studied.
Biodegradation properties
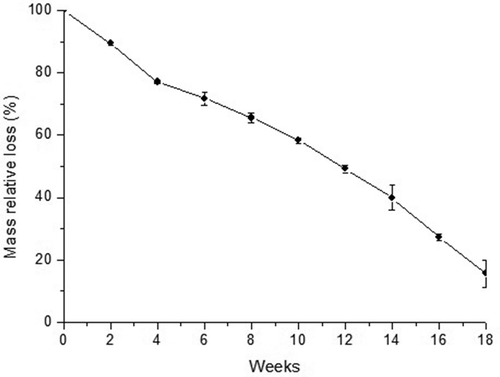
The weight loss percentages found during the biodegradability test in soil are shown in . As expected, it was observed that the weight loss during the biodegradation test increased with increasing storage duration. It was verified that the thermoplastic suffered degradation over the 18 weeks, with a linear mass loss over the biodegradation test period, in agreement with the literature.[Citation46] The thermoplastic presented a total mass loss of 84.4 ± 4.4%, with a residual mass of 15.6 ± 1.2% after 126 days. According to [Citation47], the main change that a degradable polymer suffers is the decrease in molecular weight as smaller products are being formed. The starch can be degraded by fungi and/or bacteria due to the action of enzymes, resulting in the formation of carbon dioxide, water, and sugar.[Citation48] The starch degrades rapidly, facilitating access by microorganisms, to other components of this mixture, which can be fully or partially biodegradable.[Citation22] In this study, all components added to the polymeric matrix of starch are biodegradable, this is, the plasticisers glycerol and sorbitol.
Conclusions
Biodegradable TPS was successfully obtained from the pejibaye palm fruit. The Doehlert experimental design proved effective to optimise the concentrations of glycerol and sorbitol, as well as the desirability function, which allowed to obtain the optimal point for the three responses simultaneously. The thermoplastic obtained, presented resistance to traction and thermal degradation, similar to traditional plasticised starches, such as cassava and maize. Bioplastic produced is part of a new generation of materials with the potential to reduce the environmental impact caused by the indiscriminate use of conventional plastic in the packaging, especially of food, since they are easily integrated into the composting cycle. Its thermoplastic properties suggest a great potential of application in minimally processed foods packages, edible ice creams, and baked products, however, its application depends on the suitability of the industrial scale.
Additional information
Funding
References
- Róz, A. L.; Carvalho, A. J. F.; Gandini, A.; Curvelo, A. A. S. The Effect of Plasticizers on Thermoplastic Starch Compositions Obtained by Melt Processing. Carbohydrate Polymers. 2006, 63, 417–424.
- Cyras, V. P.; Manfredi, L. B.; Ton-That, M. T.; Vázquez, A. Physical and Mechanical Properties of Thermoplastic Starch/Montmorillonite Nanocomposite Films. Carbohydrate Polymers. 2008, 73, 55–63.
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal Processing of Starch-Based Polymers. Progress in Polymer Science. 2009, 34, 1348–1368.
- Miranda, V. R.; Carvalho, A. J. F. Blendas Compatíveis De Amido Termoplástico E Polietileno De Baixa Densidade Compatibilizadas Com Ácido Cítrico. Polímeros. 2011, 21, 353–360.
- Mościcki, L.; Mitrus, M.; Wójtowicz, A.; Oniszczuk, T.; Rejak, A.; Janssen, L. Application of Extrusion-Cooking for Processing of Thermoplastic Starch (TPS). Food Research International. 2012, 47, 291–299.
- Bastioli, C.;. Properties and Application of Mater-Bi Starch-Based Materials. Polymer Degradation and Stability. 1998, 59, 263–272.
- Coutinho, B. C.; Miranda, G. B.; Sampaio, G. R.; De Souza, L. B. S.; Santana, W. J.; Coutinho, H. D. M. A Importância E as Vantagens Do Polihidroxibutirato (Plástico Biodegradável). Holos. 2007, 3, 76–81.
- Shimazu, A. A.; Mali, S.; Grossmann, M. V. E. Efeitos Plastificante E Antiplastificante Do Glicerol E Do Sorbitol Em Filmes Biodegradáveis De Amido De Mandioca. Semina: Ciências Agrárias. 2007, 28, 79–88.
- Henrique, C. M.; Cereda, M. P.; Sarmento, S. B. S. Características Físicas De Filmes Biodegradáveis Produzidos a Partir De Amidos Modificados De Mandioca. Ciência E Tecnologia De Alimentos. 2008, 28, 231–240.
- Rocha, T. S.; Demiate, I. M.; Franco, C. M. L. Características Estruturais E Físico-Químicas De Amidos De Mandioquinha-Salsa (Arracacia Xanthorrhiza). Ciência Tecnologia De Alimentos. 2008, 28, 620–628.
- Mali, S.; Grossmann, M. V. E.; Yamashita, F. Filmes De Amido: Produção, Propriedades E Potencial De Utilização. Semina: Ciências Agrárias. 2010, 31, 137–156.
- Avérous, L.;. Formulation and Development of Biodegradable and Bio‐Based Multiphase Materials: Plasticized Starch‐Based Materials. Environmental Impact of Polymers. 2014, 9, 155–199.
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Critical Reviews in Food Science and Nutrition. 2014, 54, 1353–1370.
- Corradini, E.; Lotti, C.; Medeiros, E. S.; Carvalho, A. J. F.; Curvelo, A. A. S.; Mattoso, L. H. C. Estudo Comparativo De Amidos Termoplásticos Derivados Do Milho Com Diferentes Teores De Amilose. Polímeros: Ciência E Tecnologia. 2005, 15, 268–273.
- Campos, A. D.; Teodoro, K. B.; Marconcini, J. M.; Mattoso, L. H.; Martins-Franchetti, S. M. Efeito Do Tratamento Das Fibras Nas Propriedades Do Biocompósito De Amido Termoplástico/Policaprolactona/Sisal. Polímeros. 2011, 21, 217–222.
- Neto, B. A. M.; Barbosa, A. A.; Leite, C. X. S.; Almeida, P. F.; Bonomo, R. C. F.; Pontes, K. V. Chemical Composition and Functional Properties of Starch Extracted from the Pejibaye Fruit (Bactris Gasepaes Kunth.). Acta Scientiarum. Technology. 2015, 37, 105–110.
- Valencia, G. A.; Moraes, I. C. F.; Lourenço, R. V.; Bittante, A. M. Q. B.; Sobral, P. J. D. A. Physicochemical. Morphological, and Functional Properties of Flour and Starch from Peach Palm (Bactris Gasipaes Kunth.) Fruit. Starch‐Stärke. 2015, 67, 163–173.
- Ferreira, S. L.; Santos, W. N.; Quintella, C. M.; Neto, B. B.; Bosque, J. M. S. Doehlert Matrix: A Chemometric Tool for Analytical Chemistry—Review. Talanta. 2004, 63, 1061–1067.
- Massart, D. L.; Vandeginste, B. G. M.; Buydens, L. M. C.; Jong, P. J. S.; Lewi, S. J. V. Handbook of Chemometrics and Qualimetrics, Part A; Elsevier: Amsterdam, 2003.
- Barros, J. M.; Bezerra, M. A.; Valasques, G. S.; Junior, N.; Souza, A. S.; Aragão, N. M. Multivariate Optimization of an Ultrasound-Assisted Extraction Procedure for Cu, Mn, Ni and Zn Determination in Ration to Chickens. Anais Da Academia Brasileira De Ciências. 2013, 85, 891–902.
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. Journal of Quality Technology. 1980, 12, 214–219.
- Leite, M. C.; Furtado, C. R.; Couto, L. O.; Oliveira, F. L.; Correia, T. R. Avaliação Da Biodegradação De Compósitos De Poli (ε-Caprolactona)/Fibra De Coco Verde. Polímeros. 2010, 20, 339–344.
- Mali, S.; Sakanaka, L. S.; Yamashita, F.; Grossmann, M. V. E. Water Sorption and Mechanical Properties of Cassava Starch Films and Their Relation to Plasticizing Effect. Carbohydrate Polymers. 2005, 60, 283–289.
- Schlemmer, D.; Sales, M. J. A.; Resck, I. S. Preparação, Caracterização E Degradação De Blendas PS/TPS Usando Glicerol E Óleo De Buriti Como Plastificantes. Polímeros. 2010, 20, 6–13.
- Gaudin, S.; Lourdin, D.; Le Botlan, D.; Ilari, J. L.; Colonna, P. Plasticisation and Mobility in Starch-Sorbitol Films. Journal of Cereal Science. 1999, 29, 273–284.
- Mali, S.; Grossmann, M. V. E.; García, M. A.; Martino, M. N.; Zaritzky, N. E. Effects of Controlled Storage on Thermal, Mechanical and Barrier Properties of Plasticized Films from Different Starch Sources. Journal of Food Engineering. 2006, 75, 453–460.
- Tapia-Blácido, D. R.;. Do Amaral. P.J.; Menegalli, F.C. Effect of Drying Conditions and Plasticizer Type on Some Physical and Mechanical Properties of Amaranth Flour Films. LWT-Food Science and Technology. 2013, 50, 392–400.
- Thunwall, M.; Kuthanova, V.; Boldizar, A.; Rigdahl, M. Film Blowing of Thermoplastic Starch. Carbohydrate Polymers. 2008, 71, 583–590.
- Ollett, A. L.; Parker, R.; Smith, A. C. Deformation and Fracture Behaviour of Wheat Starch Plasticized with Glucose and Water. Journal of Materials Science. 1991, 26, 1351–1356.
- Chang, Y. P.; Karim, A. A.; Seow, C. C. Interactive Plasticizing-Antiplasticizing Effects of Water and Glycerol on the Tensile Properties on Tapioca Starch Films. Food Hydrocolloids. 2006, 20, 1–8.
- Curvelo, A. A. S.; De Carvalho, A. J. F.; Agnelli, J. A. M. Thermoplastic Starch–Cellulosic Fibers Composites: Preliminary Results. Carbohydrate Polymers. 2001, 45, 183–188.
- Thiré, R. M.; Ribeiro, T. A.; Andrade, C. T. Effect of Starch Addition on Compression‐Molded Poly (3‐Hydroxybutyrate)/Starch Blends. Journal of Applied Polymer Science. 2006, 100, 4338–4347.
- Lai, S. M.; Don, T. M.; Huang, Y. C. Preparation and Properties of Biodegradable Thermoplastic Starch/Poly (Hydroxy Butyrate) Blends. Journal of Applied Polymer Science. 2012, 100, 2371–2379, 2006.
- Yulian, A. M.; Huynh, L. H.; Ho, Q. P.; Truong, C. T.; Ju, Y. H. Defatted Cashew Nut Shell Starch as Renewable Polymeric Material: Isolation and Characterization. Carbohydrate Polymers. 2012, 87, 2576–2581.
- Mothé, C. G.; Azevedo, A. D. Análise Térmica De Materiais. São Paulo: Editora. 2002, 113–115.
- Innocentini‐Mei, L. H.; Bartoli, J. R.; Baltieri, R. C. Mechanical and Thermal Properties of Poly (3‐Hydroxybutyrate) Blends with Starch and Starch Derivatives. Macromolecular Symposia. 2003, 197, 77–88.
- Brito, L. M.; Tavares, M. I. B. Desenvolvimento De Nanocompósitos À Base De Amido De Batata. Polímeros. 2013, 23, 771–775.
- Hulleman, S. H. D.; Janssen, F. H. P.; Feil, H. The Role of Water during Plasticization of Native Starches. Polymer. 1998, 39, 2043–2048.
- Van Soest, J. J. G.; Essers, P. Influence of Amylose-Amylopectin Ratio on Properties of Extruded Starch Plastic Sheets. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry. 1997, 34, 1665–1689.
- Marengo, V. A.; Vercelheze, A. E. S.; Mali, S. Compósitos Biodegradáveis De Amido De Mandioca E Resíduos Da Agroindústria. Química Nova. 2013, 36, 680–685.
- Jensen, S.; Grossmann, M. V. E.; Mali, S. Microestrutura E Estabilidade De Filmes De Amido De Mandioca Adicionados De Emulsificantes Com Diferentes Equilíbrios Hidrofílico/Lipofílico. Brazilian Journal of Food Technology. 2009, 12, 97–105.
- Rosa, D. S.; Franco, B. L.; Calil, M. R. Biodegradabilidade E Propriedades Mecânicas De Novas Misturas Poliméricas. Polímeros: Ciência E Tecnologia . 2001, 11, 82–88.
- Meuser, F.; Wittig, J.; Huster, H. Effects of High Pressure Disintegration of Steeped Maize Grits on the Release of Starch Granules from the Protein Matrix. Starch‐Stärke. 1989, 41, 225–232.
- Biliaderis, C. G.;. The Structure and Interactions of Starch with Food. Canadian Journal of Physiology and Pharmacology. 1991, 69, 60–78.
- Conde‐Petit, B.; Nuessli, J.; Handschin, S.; Escher, F. Comparative Characterization of Aqueous Starch Dispersion by Light Microscopy, Reometry and Iodine Binding Behaviour. Starch/Stärke. 1998, 50, 184–192.
- Machado, B. A. S.; Reis, J. H. O.; Silva, J. B.; Cruz, L. S.; Nunes, I. L.; Pereira, F.; Druzian, J. I. Obtenção De Nanocelulose Da Fibra De Coco Verde E Incorporação Em Filmes Biodegradáveis De Amido Plastificados Com Glicerol. Química Nova. 2014, 37, 1275–1282.
- Jayasekara, R.; Harding, I.; Bowater, I.; Lonergan, G. Biodegradability of a Selected Range of Polymers and Polymer Blends and Standard Methods for Assessment of Biodegradation. Journal of Polymers and the Environment. 2005, 13, 231–251.
- Franchetti, S. M. M.; Marconato, J. C. Polímeros Biodegradáveis-Uma Solução Parcial Para Diminuir a Quantidade Dos Resíduos Plásticos. Química Nova. 2006, 29, 811–816.