ABSTRACT
Grape as one of the important horticultural crops plays significant role in health maintenance. In this study, the effect of nitric oxide (NO) was investigated on some physio-biochemical attributes and antioxidant enzymes activities of grape fruits (cv. Rish Baba) during chilling damage. Grape fruits were treated by 0 (control), 0.25 and 0.5 mM of NO and stored at −0.5°C and 95% relative humidity for 5 weeks. The results showed that NO reduced chilling injury (CI) and decay, ion leakage, lipid peroxidation and accumulation of malon dialdehyde (MDA) and hydrogen peroxide (H2O2) content of grape fruits. The NO also increased vitamin C, organic acids, total soluble solids, and the activity of antioxidant enzymes such as peroxidase (POD), ascorbate peroxidase (APX), superoxide dismutase (SOD) and catalase (CAT) during chilling damage. The results indicated that 0.5 mM showed the best effect. The founding revealed that NO has potential application in postharvest treatment by alleviating CI and maintain of quality. The present study is the first evidence of nitric oxide effects on avoiding chilling damage in grape.
AbbreviationsNitric oxide (NO); Sodium nitroprusside (SNP); Chilling injury (CI); Reactive oxygen species (ROS); Peroxidase (POD); Ascorbate peroxidase (APX); Superoxide dismutase (SOD); Catalase (CAT); Glutathion reductase (GR); Malon dialdehyde (MDA); Hydrogen peroxide (H2O2).
Introduction
Grape (Vitis vinifera L.) is one of the most important horticultural crops in the world. Grape is a valuable crop compared with the various products in terms of production, so it plays an important role in economy of countries. [Citation1] Grape production is about 74 million tons in 2014, that Iran with a production of two million tons has an important place in the world. [Citation2] Grape plays a significant role in protection against inflammation, cardiovascular disease, cancer, age-related disorders and health maintenance. [Citation3]
Chilling injury (CI) and decay are primary postharvest problems of grape and many other horticultural crops during storage. [Citation4–Citation6] Oxidative stress from an excessive reactive oxygen species (ROS) has been related with chilling damage in plants. [Citation7,Citation8] Exceeding of ROS leads to oxidative damages including inactivation of enzymes, lipid peroxidation, protein degradation and DNA damage. [Citation9] Plant antioxidant defense systems including enzymatic and non-enzymatic protection have co-evolved with aerobic metabolism to counteract oxidative damage due to ROS. [Citation10] This involved lipid soluble antioxidant (α-tocopherol and carotenoids), water soluble reductants (glutathion and ascorbate) and enzymes such as catalas (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), peroxidase (POD) and glutathion reductase (GR). [Citation11] Oxidative damage only ensues when the complex systems fail to limit ROS accumulation. Previous studies have shown that a positive relationship exists between the antioxidant enzymes activity and chilling tolerance in harvested fruits. [Citation8,Citation12] These results suggest that enhanced antioxidant enzymes systems and reduced peroxidation of membrane lipid may be involved in chilling tolerance in harvested fruit.
Several methods have been developed to increase shelf life of grape fruit. These include postharvest physical treatments [Citation13,Citation14], modified atmosphere packaging [Citation15–Citation17], temperature conditioning [Citation18] and chemical treatments with plant growth regulators. [Citation19–Citation22] In this case, nitric oxide (NO) due to its properties (small size, no charge, short-lived, free radical and highly diffusible across biological membranes) has multifunctional roles in plant growth and mechanisms [Citation23] and involved many plant physiological processes. [Citation24,Citation25] NO protects plant cells against oxidative stress by reducing ROS accumulation. [Citation26–Citation28] It has been reported that NO can counteract oxidative damage and has protective effect against various stressful conditions. [Citation9,Citation27,Citation29] In some studies, NO has regulated the multiple plant responses towards a variety of biotic and abiotic stresses and alleviating some consequences provoked by oxidative stresses. [Citation30–Citation33] For example when NO applied exogenously, it improved chilling tolerance and reduced incidence of CI in several fruits. [Citation34–Citation36] In this study, the effect of NO was investigated on some physio-biochemical and antioxidant enzymes activities of grape cv. Rish Baba during chilling damage. The aim of study was to determine how NO improve the mechanisms of chilling tolerance and shelf life of grape.
Materials and methods
Plant materials and treatments
Rish Baba grape fruits were harvested at commercial maturity from a commercial orchard in Kerman, Iran, and then transported to the laboratory on the same day. Fruits without wounds or rot were selected based on uniformity of size and absence of physical injury or disease. The fruits were disinfected with 1% sodium hypochlorite (v/v) for 2 min, washed, and dried in air. Subsequently, they were randomly divided into three groups. Two of the groups were immersed in aqueous solution containing, respectively 0.25 and 0.5 mM of sodium nitroprusside (SNP) as NO for 5 min, based on our preliminary experiments. The third group was immersed in distilled water for 5 min and served as a control. It should be noted that SNP was purchased from Sigma-Aldrich (St. Louis, MO, USA). In the preliminary experiment, we tested a series of SNP concentrations and found that a concentration of 1 mM contain about 2.5 µM NO in solution. Therefore, SNP at 0.25 and 0.5 mM or 0.62 and 1.25 µM NO was used in the following experiments. All fruits were enclosed in plastic boxes with polyethylene film bags to maintain the relative humidity at about 95% and stored at −0.5°C. We selected the temperature according to ROBERTS et al.. [Citation18] Finally, fruit physio-biochemical and antioxidant enzymes activities were measured during 5 weeks as follow:
Chilling injury (CI) and decay
The symptoms of CI include surface pitting and browning. The CI index was determined according to the method described by OBENLAND et al.. [Citation37] Grade levels were classified as follow: 0 (the orange fruits are unaffected); 1 (less than 25% of the fruit area shows CI symptoms in the peel); 2 (25–50% of the fruit area shows CI symptoms); and 3 (more than 50% of the fruit area shows CI symptoms). For this purpose, three replicates were performed for each treatment, and each replicate contained ten clusters. The CI index is calculated using the following formula:
CI index % = (CI grade × number of fruit at this level)/(highest level × total fruit number) × 100
Ion leakage
Cell membrane stability index was measured according to PAKKISH et al. [Citation38] The fruits were separated from the twig samples, placed in tubes with 15 ml distilled water and kept in a water bath for 24 h at room temperature (25◦C). Then conductivity (EC1) measured using EC meter (model: Metrhom). The samples were then frozen (−20◦C) for 24 h and kept in 25◦C for 24 h and remeasured for conductivity (EC2). The relative conductivity (EC) of each sample was calculated as: EC % = (EC1/EC2) × 100
Lipid peroxidation and accumulation of malon dialdehyde (MDA)
Lipid peroxidation was determined and expressed as malon dialdehyde (MDA) equivalents, according to RAJINDER et al. [Citation39] with some modification. To do so, the fruit pulp and peel tissue (4 g) were homogenised with 20 mL of 10% trichloroacetic acid and then centrifuged for 10 min at 5000 × g. 1 mL of the supernatant was mixed with 3 mL of 0.5% thiobarbituric acid (TBA) dissolved previously in 10% trichloroacetic acid. The reaction mixture solution was heat-treated for 20 min at 95◦C, quickly cooled, and then centrifuged for 10 min at 10000 × g to clarify precipitation. Then, the absorbance was measured at 532nm and subtracted from the nonspecific absorbance at 600nm. The amount of MDA was calculated using an extinction coefficient of 155 mM−1cm−1 and expressed as mg g−1 of fresh weight.
Hydrogen peroxide (H2O2) content
H2O2 content assay was done by the method described by PRASSAD. [Citation40] Fresh tissues (2 g) were homogenised with 10 ml of acetone at 0°C. After centrifugation at 6000 × g for 15 min at 4°C, the supernatant phase was collected. The supernatant (1 ml) was mixed with 0.1 ml of 5% titanium sulphate and 0.2 ml ammonia, and then centrifuged at 6000 × g for 10 min at 4°C. The pellets were dissolved in 3 ml of 10% (v/v) H2SO4 and centrifuged at 5000 × g for 10 min. Absorbance of the supernatant phase was measured at 410nm. H2O2 content was calculated using H2O2 as a standard and then expressed as µg g−1 on fresh weight.
Ascorbic acid (vitamin C), total acids (TA) and total soluble solids (TSS) analysis
The ascorbic acid (vitamin c) was determined according to BASIOUNY. [Citation41] To do so, iodine (1.269 g) and potassium iodide (KI) (16.6 g) were homogenised with distilled water and the volume increased up to 1 liter. In this solution, iodine normalisation is 0.01%. The mixture was kept for 1 to 2 days and then 20 ml of it was added to 2 ml of starch solution (1%). The mixture was titrated with pure ascorbic acid solution (100 mg of ascorbic acid powder/100 ml of distilled water) to change its colour to gray. The iodine factor was calculated as following:
F = A/B × N × 88.1
F: iodine mixture factor (mg L−1); N: iodine mixture normality; A: amount of the pure ascorbic acid (mg); B: amount of the used iodine (mg). Subsequently, total acids (TA) was determined by neutralisation of fruit juice using 0.1 N NaOH [Citation41,Citation42] and data presented as mg 100ml−1. Total soluble solids (TSS) was also measured using digital refractometer (Atago Co, Ltd, Tokyo, Japan) with a range of 0–32%, by placing 1–2 drops of fruit juice. [Citation43]
Antioxidant enzymes activity
For measuring antioxidant enzymes activity, pulp and peel tissue samples (4 g) was homogenised with 0.1 g PVPP in 10 mL of ice-cold PBS (25 mM) containing 1 mM EDTA. The homogenate was centrifuged at 12000 × g for 20 min at 4°C, and the resulting supernatant was collected for the enzyme assay [Citation44] as follow:
Peroxidase (POD)
Peroxidase (POD) activity was analysed according to XING et al. [Citation45] with some modification. 0.5 mL enzyme extract was incubated in 2 mL buffered substrate (100 mM sodium phosphate, pH 6.4 and 8 mM guaiacol) for 5 min at 30◦C and the increasing absorbance measured at 460nm every 30 s for 120 s after adding 1 mL of H2O2 (24 mM). POD activity was expressed as U per mg of protein, where U = ΔA at 470 nm s−1.
Ascorbate peroxidase (APX)
Ascorbate peroxidase (APX) activity was assayed according to NAKONA and ASADA. [Citation46] Reaction mixture in a total volume of 1 ml contained 50 mM K-phosphate buffer (pH 7.0), 0.2 mM ascorbic acid, 0.2 mM EDTA, 20 mM H2O2 and enzyme. H2O2 was the last component to be added and the decrease in absorbance was recorded at 290nm (extinction coefficient of 2.8 mM−1cm−1) using a UV-vis spectrophotometer. Correction was made for the low, non-enzymicoxidation of ascorbic acid by H2O2. APX specific activity expressed as unit mg protein−1.
Superoxide dismutase (SOD)
Superoxide dismutase (SOD) activity was analysed according to MISRA and FRIDOVICH. [Citation47] About 200 mg of fresh tissues were homogenised in 5 ml of 100 mM K-phosphate buffer (pH 7.8) containing 0.1 mM EDTA, 0.1% (v/v) triton X-100and 2% (w/v) polyvinyl pyrrolidone (PVP). The extract was filtered through muslin cloth and centrifuged at 22000 × g for 10 min 4–8 ◦C. The supernatant was dialysed in cellophane membranetubings against the cold extraction buffer for 4 h with carbon-ate/bicarbonate buffer and then used for the assay. The assay mixture in a total volume of 3 ml contained 50 mM sodium carbonate/bicarbonate buffer (pH 9.8), 0.1 mM EDTA, 0.6 mM epinephrineand enzyme. Epinephrine was the last component to be added. The adrenochrome formation in the next 4 min was recorded at 475nm in a spectrophotometer. One unit of SOD activity is expressed as the amount of enzyme required to cause 50% inhibition of epinephrine oxidation under the experimental conditions. The specific activity of SOD expressed as unit mg protein−1.
Catalase (CAT)
Catalase (CAT) activity was assayed according to XING et al. [Citation45] with some modification. The reaction mixture consisted of 2 mL sodium phosphate buffer (50 mM, pH 7.0), 0.5 mL H2O2 (40 mM) and 0.5 mL enzyme extract. The decomposition of H2O2 was measured by the decline in absorbance (A) at 240nm. CAT specific activity was expressed as U kg−1 of FW, where
U = Δ - A at 240 nm s−1.
Statistical analysis
The experiment was as a factorial randomised complete design with three replications. Data was analysed by one-way ANOVA and mean comparison were performed by Duncan’s new multiple range test using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Analysis of variance revealed significant differences (P ≤ 0.05) in the studied traits in both NO application and the time of use it (). The mean values of CI and decay, ion leakage, lipid peroxidation and accumulation of MDA, and H2O2 content have increased during 5 weeks at cold storage that the application of NO reduced them significantly. Moreover, the NO increased vitamin C, TA, TSS, an antioxidant enzymes activity during cold storage.
Table 1. Variance analysis of physico-biochemical and antioxidant enzymes activity of ‘rish baba’ grape in response to nitric oxide (NO) during cold storage.
Chilling injury (CI) and decay
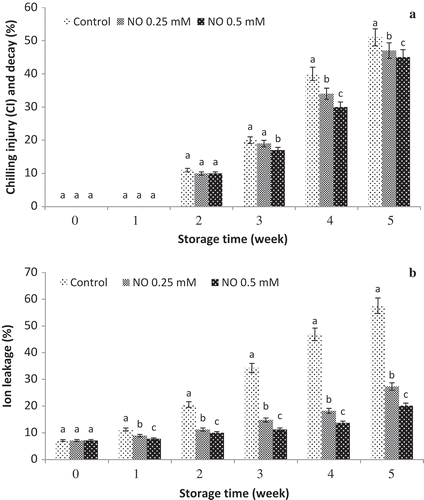
The effect of NO on CI and decay of grape fruits during cold storage is shown in .
CI and decay symptoms (surface pitting and browning) increased during 5 weeks at cold storage and NO-treated fruit had the lowest damage. In the untreated control grape fruit, CI and decay symptoms occurred at 2 weeks after storage and it has been increased at 5 weeks (50.21%). However, CI and decay symptoms in NO-treated fruit occurred at 2 weeks after storage and it was significantly lower than the index in non-treated fruits. Based on these results, the lowest CI and decay values were observed in NO-treated fruits as compared to non NO-treated fruits. So, the NO at 0.5 mM was the most effective treatment among the all treated fruits, in alleviating CI and decay percentage.Ion leakage
As shown in , ion leakage increased during 5 weeks at cold storage of grape fruits and NO-treatment reduced leakage as compared to the control. The highest and lowest ion leakage was related to untreated and NO-treated grape fruits, respectively. In the untreated control grape fruits, ion leakage was occurred at 1 week after storage and it has been increased at 5 weeks (57.55%). However, the ion leakage in NO-treated fruits occurred at 2 weeks after storage and it was significantly lower compared to the control. According to the results, the lowest ion leakage was observed in NO-treated fruits specially NO at 0.5 mM was the most effective treatment.
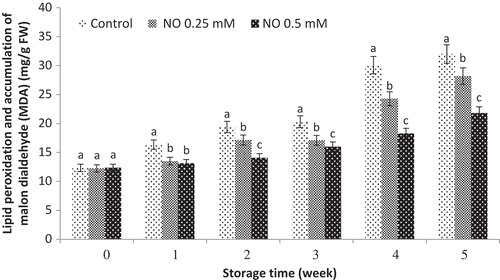
Lipid peroxidation and accumulation of malon dialdehyde (MDA)
Lipid peroxidation, used as direct indicator of membrane injury, is often associated with CI. Regarding data of MDA accumulation that indicates lipid peroxidation, a continuous increase was observed in lipid peroxidation both in control and treated grape fruits. Although NO significantly delayed the increase of lipid peroxidation during storage, at the end of storage period (day 35), the lipid peroxidation of samples treated with NO was significantly lower compared to the control samples. Based on the results, the lowest lipid peroxidation was observed in NO-treated fruits and NO at 0.5 mM was the most effective treatment ().
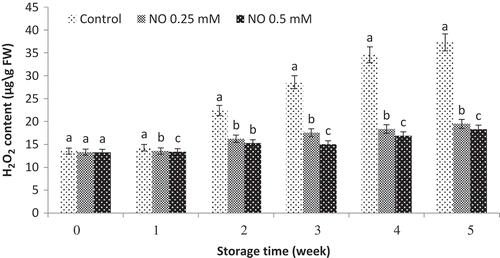
Hydrogen peroxide (H2O2) content
Changes in H2O2 content of grape fruits are presented in . In general, H2O2 content increased during storage in both control and NO-treated grape fruits. The increasing of H2O2 content in the control group was much higher than that in the NO-treated grapes. According to the results, the lowest H2O2 content was observed in NO-treated fruits and NO at 0.5 mM
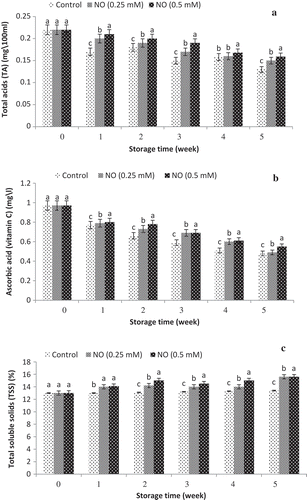
was the most effective treatment.Ascorbic acid (vitamin C), total acids (TA) and total soluble solids (TSS)
As shown in . vitamin C and TA decreased during 5 weeks at cold storage of grape fruits and NO-treatment increased them as compared to the control. The lowest and highest traits were related to the untreated and NO-treated grape fruits, respectively. In addition, the NO increased TSS during cold storage of grape fruits. Based to the results, the highest vitamin C, TA and TSS were observed in NO-treated fruits specially NO at 0.5 mM was the most effective treatment.
Antioxidant enzymes activity
Antioxidant enzymes activity increased in grape fruits stored at −0.5°C. In control and NO treatments, activity of POD, APX, SOD and CAT peaked at the end of storage period (35 day). These enzymes had a similar activity during storage. The results show, NO treatment at 0.25 and 0.5 mM (especially 0.5 mM) induced the activity of these enzymes in grape fruits ().
Table 2. Effect of nitric oxide (NO) treatment on antioxidant enzymes activity of ‘rish baba’ grape fruit during cold storage.
Discussion
In the present study we found that treatment with nitric oxide (NO) reduced significantly CI and decay of grape fruits during storage at −0.5°C. CI and decay are major factors in reducing the quality and limiting the storage time of horticultural crops. To prevent CI development and extend shelf life, a number of strategies including physical and chemical treatments have been evaluated. [Citation19–Citation21,Citation48,Citation49] In this case, NO has been applied to reduce development of CI and decay symptom in some horticultural crops including broccoli, green bean and bok choy [Citation50], tomato [Citation51], strawberry [Citation52], Arabidopsis [Citation53], peach and plums. [Citation35,Citation36,Citation54] Our results are consistent with the findings of these studies.
Moreover, there was a continuous increasing in tissue lipid peroxidation and ion leakage content in all fruits, but application of NO significantly delayed the increase of lipid peroxidation and ion leakage. Also, change in membrane permeability (revealed by H2O2 content) showed trends similar to lipid peroxidation content; that is tissue H2O2 content increased with storage duration and NO markedly delayed the increase. H2O2 content is as an indicator of membrane integrity. It has been reported that CI occurrence is often accompanied by oxidative damage which can be followed through lipid peroxidation content since it is a final product of lipid peroxidation. [Citation28] Our results showed that the membrane integrity was maintained as a result of NO treatment under cold stress. NO has been considered to be involved in a network of interacting signal transduction pathways which regulate defense responses to abiotic stress. [Citation9,Citation27,Citation55] HUANG et al. [Citation53] demonstrated that NO-mediated stress tolerance in Arabidopsis.
In addition, the NO increased ascorbic acid (vitamin C), total acids (TA) and total soluble solids (TSS) under chilling stress. Vitamin C is important, because it supports normal tissue during wound healing. Deficiency in vitamin C causes the development of many degenerative diseases. [Citation56] Vitamin C in non-enzymatic antioxidant that is synthesised under stress conditions in live cells. [Citation57] BELIGNI et al. [Citation30] has reported that vitamin C deficit is a critical factor in shelf life of some crops. Since the role of NO in increasing the resistance of live cells to stress due to its antioxidant activity and the gene expression [Citation33], an increase in vitamin C as a non-enzymatic antioxidant is natural in the treated fruits. [Citation35] Furthermore, TA and TSS are indicators of fruit quality attributes. [Citation42] Most of organic acids are secondary metabolites that their amounts decrease during storage. Treatment with NO delays senescence. Chemical compounds reduce the reduction process of the soluble solids via slowing down the fruits respiration and senescence. [Citation58] So, the use of these compounds increases the fruits qualitative characteristics during storage. Probably, NO treatment protects the soluble solids via slowing down the respiration rate and senescence. In other studies, NO delays senescence and increased the shelf life of citrus fruits [Citation59] and peach [Citation36] after harvest. These findings confirm the results of this study.
Moreover, the NO significantly increased POD, APX, SOD and CAT activity under chilling stress. These results suggest that the effect of NO in reducing the occurrence of CI and decay were correlated to enhance antioxidant enzyme activity. Totally, low temperature disrupts the destruction of scavenging enzymes such as POD, APX, SOD and CAT. [Citation60,Citation61] When horticultural crops are exposed to severe abiotic stresses including cold stress, large amounts of intracellular ROS are generated. [Citation62,Citation63] The accumulation of ROS would induce lipid peroxidation, damage membrane structure, and cause solute leaking. [Citation50,Citation64] The detoxification of ROS is dependent on antioxidant enzymes such as CAT and POD. [Citation48,Citation65] The increasing of these enzymes activity contributes to the adaptation of plants to cold stress and ameliorates oxidative damage such as lipid peroxidation and H2O2 content. [Citation48,Citation66] A number of postharvest treatments that induce chilling and decay tolerance and alleviate CI and decay also enhance antioxidant enzyme activity. [Citation8,Citation12,Citation67,Citation68] In this case, low concentration of NO induces the expression or activation of antioxidant enzyme which was proven in plants. [Citation9,Citation27,Citation55] SALA and LAFUENTE [Citation8] found that the chilling tolerant mandarins have a higher antioxidant enzyme activity that the chilling sensitive plants. YANG et al. [Citation69] demonstrated that exogenous NO was effective in reducing CI and decay in cucumber by elicitation of CAT, POD, APX and polyphenol oxidase. SHI et al. [Citation9] reported that NO enhanced the activity of CAT, APX and SOD in cucumber roots, and apoplastic H2O2 in NO-induced antioxidant defense. ZHU et al. [Citation70] has also reported that NO improved the antioxidant enzymes activity and reduced the ROS in kiwifruit during storage. Based on our results, chilling and decay tolerance of grape fruits was enhanced by postharvest treatment with NO. We suggest that the antioxidant enzyme activity in grape fruits induced by the NO may be a key factor in lowering oxidative damage caused by cold stress, thus improving the cold tolerance and alleviating CI of grape stored at −0.5°C. So, NO application can detoxify ROS such as H2O2 and O2-.
Conclusion
The findings of the study show NO reduced CI and decay of grape fruits stored at −0.5°C and maintained its quality as well. The lipid peroxidation and peroxide hydrogen content were significantly reduced by NO especially at 0.5 mM. Vitamin C, organic acids, total soluble solids, APX and SOD enzymes activity has also showed higher levels in grapes treated with NO. NO treatments at 0.25 and 0.5 mM (especially 0.5 mM) induced the activity of these enzymes in grape fruits. It seems that NO-induced cold resistance may be due to stimulation of antioxidant enzymes and protection against membrane oxidative damage, decreased lipid peroxidation and H2O2 content in grape fruits. These results may have implications for the use of NO in alleviating CI and maintaining of grape quality or other temperate fruits stored at low temperature. This is the first report in which NO has been shown to have beneficial effects against CI and decay of postharvest grape fruits.
References
- Jalili-Marandi, R.;. Small Fruits. Academic Center for Education Culture and Research of West Azarbaijan Urmia. 2003.
- FAO. 2017: Grapes production in 2014 mostly based on FAOSTAT. Available online at http://www.faostat.fao.org. Accessed 4 January 2017.
- Weston, L. A.; Grape and Wine Tannins and Phenolics, Their Roles in Flavor, Quality and Human Health. Proc. 29th Annual New York Wine Industry Workshop, NY. 2005, pp. 6–15.
- Artes-Hernndez, F.; Aguayo, E.; Artés, F. Alternative Atmosphere Treatments for Keeping Quality of ‘Autumn Seedless’ Table Grapes during Long-Term Cold Storage. Postharvest Biol. Technol. 2004, 31, 59–67.
- Artes-Hernndez, F.; Toms-Barbern, F. A.; Artés, F. Modified Atmosphere Packaging Preserves Quality of SO2-free ‘Superior Seedless’ Table Grapes. Postharvest Biol. Technol. 2006, 39, 146–154.
- Wang, C. Y.;. Approaches to Reduce Chilling Injury of Fruits and Vegetables. Hortic Rev (Am Soc Hortic Sci). 1993, 15, 63–132.
- Hodges, D. M.; Lester, G. E.; Munro, K. D.; Toivonen, P. M. Oxidative Stress: Importance for Postharvest 4 Ual: Ty. Hort Science. 2004, 39(5), 924–929.
- Sala, J. M.; Lafuente, M. Catalase Enzymes Activity Is Related to Tolerance of Mandarin Fruits to Chilling. Postharvest Biol. Technol. 2000, 20, 81–89.
- Shi, Q. H.; Ding, F.; Wang, X. F.; Wei, M. Exologenous Nitric Oxide Protect Cucumber Roots against Oxidative Stress Induced by Salt Stress. Plant Physiology Bio Chemistry. 2007, 45(8), 542–550.
- Davar, R.; Darvishzadeh, R.; Majid, A. Changes in Antioxidant Systems in Sunflower Partial Resistant and Susceptible Lines as Affected by Sclerotinia Sclerotiorum (Lib.). De Bary. Biologia. 2013, 68, 821–829.
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism Oxidative Stress, and Signal Transduction. Annu Rev Plant Biol. 2004, 55, 373–399.
- Wang, C. Y.;. Effect of Temperature Preconditioning on Catalase, Peroxidase and Superoxide Dismutase in Chilled Zuechini Squash. Postharvest Biol. Technol. 1995, 5, 67–76.
- Lichter, A.; Zutahy, Y.; Kaplunov, T. Evaluation of Table Grape Storage in Boxes with Sulfur Dioxide-Releasing Pads with either an Internal Plastic Liner or External Wrap. HortTechnology. 2008, 18(2), 206–214.
- Zutkhi, Y.; Kaplunov, T.; Lichter, A.; Ben Arie, R.; Lurie, S.; Kosto, I.; Raban, E. Extended Storage of ‘Redglobe’ Grapes. Acta Hortic. 2007, 553, 617–618.
- Kader, A. A.; Ben-Yehoshua, S. Effect of Super Atmospheric Oxygene Levels on Postharvest Physiology and Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 2000, 20, 1–13.
- Ke, D.; Mateos, M.; Kader, A. A. Regulation of Fermrntative Metabolism in Fruits and Vegetables by Controlled Atmospheres. Proceedings from the sixth International Controlled Atmosphere Researches Conference NRAES-71, Cornell University, Ithaca, NY, 1993, pp. 63–77.
- Lichter, A.; Zutahy, Y.; Kaplunov, T.; Aharoni, N.; Lurie, S. The Effect of Ethanol Dip and Modified Atmosphere on Prevention of Botrytis Rot of Table Grapes. HortTechnology. 2005, 15, 284–291.
- Roberts, K. P.; Sargent, S. A.; Fox, A. J. Effect of Storage Temperature on Ripening and Postharvest Qulity of Grape and Mini-Pear Tomatoes. Proc. Fla. State Hortic. Soc. 2002, 115, 80–84.
- Cantin, C. M.; Fidelibus, M. W.; Crisosto, C. H. Application of Abscisic Acid (ABA) at Veraison Advanced Red Color Development and Maintained Postharvest Quality of ‘Crimson Seedless’ Grapes. Postharvest Biol. Technol. 2007, 46, 237–241.
- Chervin, C.; Westercamp, P.; El-Kereamy, A.; Rache, P.; Tournaire, A.; Roger, B.; Goubran, F.; Salib, S.; Holmes, R. Ethanol Vapours to Complement or Suppress Sulfite Fumigation of Table Grapes. Acta Hortic. 2003, 628, 779–784.
- Karabulut, O. A.; Smilanick, J. L.; Gabler, F. M.; Mansour, M.; Droby, S. Near140 Harvest Applications of Metschnikowia Fructicola, Ethanol, and Sodium Bicarbonate to 141 Control Postharvest Diseases of Grape in Central California. Plant Dis. J. 2003, 87, 1384–1389.
- Skog, L. J.; Chu, C. L. Effect of Ozone on Qualities of Fruits and Vegetables in Cold Storage. Can. J. Plant Sci. 2001, 81, 773–778.
- Siddiqui, M. H.; Al-Whadibi, M. H.; Basalah, M. O. Role of Nitric Oxide in Tolerance of Plants to Abiotic Stress: A Review. Protoplasma. 2010, DOI: 10.1007/s00709-010-0206-9.
- Crawford, N. M.;. Mechanisms for Nitric Oxide Synthesis in Plants. J. Exprimental Bot. 2006, 57, 471–478.
- Leshem, Y. Y.;. Nitric Oxide in Plants. Occurrence, Function and Use; In: Leshem, Y. Y. (Eds.); Kluwer Academic Publishers:Dordrecht, the Netherlands, 2000.
- Leshem, Y. Y.; Wills, R. B. H.; Ku, V. V. V. Evidence for the Function of the Free Radical Gas Nitric Oxide (NO) as an Endogenous Maturation and Senescence Regulating Factor in Higher Plants. Plant Physiology and Biochemistry. 1998, 36, 825–833.
- Zhang, L. G.; Zhou, S.; Xuan, Y.; Sun, M.; Zhao, L. Protective Effect of Nitric Oxide against Oxidative Damage in Arabidopsis Leaves under ultraviolet-B Irradiation. J. Plant Biol. 2009, 52, 135–140.
- Xu, W.; Peng, X.; Luo, Y.; Wang, J.; Guo, X.; Huang, K. Physiological and Biochemical Responses of Grapefruit Seed Extract Dip on ‘Redglobe’ Grape. LWT- Food Sci. Technol. 2009, 42, 471–476.
- Song, L. L.; Ding, W.; Zhao, M. G.; Sun, B. T.; Zhang, L. X. Nitric Oxide Protects against Oxidative Stress under Heat Stress in the Calluses from Two Ecotypes of Reed. Plant Sci. 2006, 171(4), 449–458.
- Beligni, M. V.; Lamattina, L. Nitric Oxide Counteracts Cytotoxic Processes Mediated by Reactive Oxygen Species in Plant Tissues. Planta. 1999a, 208, 337–344.
- Beligni, M. V.; Lamattina, L. Is Nitric Oxide Toxic or Protective? Trends Plant Sci. 1999b, 4, 299–300.
- Crawford, N. M.; Guo, F. Q. New Insights into Nitric Oxide Metabolism and Regulatory Functions. Trends Plant Sci. 2005, 10, 195–200.
- Delledonne, M.;. NO News Is Good News for Plants. Curr. Opin. Plant Biol. 2005, 8, 390–396.
- Pfeiffer, S.; Mayer, B.; Hemmens, B. Nitric Oxide: Chemical Puzzles Posed by a Biological Messenger. Angewandte Chemie International Edition. 1999, 38, 1714–1731.
- Singh, S. P.; Singh, Z.; Swinng, E. E. Postharvest Nitric Oxide Fumigation Delays Fruit Ripening and Alleviates Chilling Injury during Cold Storage of Japanese Plums (Prunus Saliciana L.). Postharvest Biol. Technol. 2009, 53, 101–108.
- Zhu, L. Q.; Zhou, J.; Zhu, S. H. Effect of a Combination of Nitric Oxide Treatment and Intermittent Warming on Prevention of Chilling Injury of ’Feicheng‘ Peach Fruit during Storage. Food Chem. 2010, 121, 165–170.
- Obenland, D.; Collin, S.; Sievert, J.; Fjeld, K.; Doctor, J.; Arpaia, M. L. Commercial Packing and Storage of Navel Oranges Alters Aroma Volatiles and Reduces Flavor Quality. Postharvest Biol. Technol. 2008, 47, 159–167.
- Pakkish, Z.; Tabatabaienia, M. The Use and Mechanism of NO to Prevent Frost Damage to Flower of Apricot. Sci. Hortic. 2016, 198, 318–325.
- Rajinder, S. D.; Dhindsa, P. P.; Thorpe, T. A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101.
- Prassad, T. K.;. Mechanisms of Chilling-Induced Oxidative Stress Injury and Tolerance in Developing Maize Seedlings: Changes in Antioxidant System, Oxidation of Proteins and Lipids, and Protease Activities. Plant J. 1996, 10, 1017–1026.
- Basiouny, F. M.;. Blueberry Fruit Quality and Storability Influenced by Postharvest Application of Polyamines and Heat Treatments. Proccding Fland State Horticulturae Society. 1996, 109, 269–272.
- Najafzadeh, R.; Arzani, K.; Bouzari, N.; Hashemi, K. Identification of New Iranian Sour Cherry Genotypes with Enhanced Fruit Quality Parameters and High Antioxidant Properties. New Zealand J. Crop Hortic. Sci. 2014, 42, 275–287.
- Mirdehghan, S. H.; Valero, D. Bioactive Compounds in Tomato Fruit and Its Antioxidant Activity as Affected by Incorporation of Aloe, Eugenol, and Thymol in Fruit Package during Storage, International Journal of Food Properties. 2016, DOI: 10.1080/10942912.2016.1223128.
- Egley, G. H.; Paul, R. N.; Vaughn, K. C.; Duke, S. O. Role of Peroxidase in the Development of Water Impermeable Seed Coats in Sida Spinosa L. Planta. 1983, 157, 224–232.
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of Chitosan Coating Enriched with Cinnamon Oil on Qualitative Properties of Sweet Pepper (Capsicum Annuum L.). Food Chem. 2011, 124, 1443–1450.
- Nakona, Y.; Asada, K. Hydrogen Peroxide Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplast. Plant . Physiol. 1981, 22(5), 867–880.
- Misra, H. P.; Fridovich, I. The Role of Superoxide Anion in the Auto-Oxidationof Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175.
- Aghdam, M. S.; Asghari, M.; Farmani, B.; Mohayeji, M.; Moradbeygi, H. Impact of Postharvest Brassinosteroids Treatment on PAL Activity in Tomato Fruit in Response to Chilling Stress. Sci. Hortic. 2012, 144, 116–120.
- Zhang, C. F.; Tian, S. P. Peach Fruit Acquired Tolerance to Low Temperature Stress by Accumulation of Linolenic Acid and N-Acylphosphatidylethanolamine in Plasma Membrane. Food Chem. 2010, 120, 864–872.
- Soegiarto, L.; Wills, R. B. H. Short Term Fumigation with Nitric Oxide Gas in Air to Extend the Postharvest Life of Broccoli, Green Bean and Bok Choy. HortTechnology. 2004, 14, 538–540.
- Lai, T.; Wang, Y.; Li, B.; Qin, G.; Tian, S. Defense Responses of Tomato Fruit to Exogenous Nitric Oxide during Postharvest Storage. Postharvest Biol. Technol. 2011, 62, 127–132.
- Wills, R. B. H.; Ku, V. V. V.; Leshem, Y. Y. Fumigation with Nitric Oxide to Extend the Postharvest Life of Strawberries. Postharvest Biol. Technol. 2000, 18, 75–79.
- Huang, X.; Von Rad, U.; Durner, J. Nitric Oxide Induce Transcriptional Activation of the Nitric Oxide-Tolerant Alternative Oxidase in Arabidopsis Suspension Cell. Planta. 2002, 215(6), 914–923.
- Asbahi Sis, S.; Mostofi, Y.; Boojar, M. M. A.; Khalighi, A. Effect of Nitric Oxide on Ethylene Biosynthesis and Antioxidant Enzymes on Iranian Peach (Prunus Persica Cv. Anjiri). J. Food, Agric. Environment. 2012, 10(2), 125–129.
- Laspina, N. V.; Groppa, M. D.; Tomaro, M. L.; Behavides, M. P. Nitric Oxide Protects Sunflower Leaves against Cd- Induced Oxidative Stress. Plants Sci. 2005, 169(2), 323–330.
- Silva, S. F.; Blank, D. E.; Peixoto, C. R.; Silveira Moreira, J. J.; DeMoura, N. F. Bioactive Compounds and Antioxidant Activity of Bunchosia Glandulifera. Int. J. Food Properties. 2016, 19, 467–473.
- Pignocchi, C.; Foyer, C. H. Apoplasticascorbate Metabolism and Its Role in the Regulation of Cell Signaling. Curr. Opin. Plant Biol. 2003, 6, 379–389.
- Kelebek, H.; Selli, S.; Canbasand, A.; Cabarogl, T. HPLC Determination of Organic Acids, Sugars, Phenolic Composition and Antioxidant Capacity of Orange Wine Made from a Turkish Cv, Kozan. Microchemical J. 2009, 91, 187–192.
- Montesinos-Herrero, C.; Palou, L. Combination of Physical and Low-Toxicity Chemical Postharvest Treatments for the Management of Citrus Fruit: A Review. Stewart Postharvest Review. 2010, 1, 1.
- Kang, H. M.; Saltveit, M. E. Antioxidant Defense System and DPPH-radical Scavenging Activity in Chilled and Heat Shocked Rice (Oryza Sativa L.) Seedlings Radicales. J. Agric. Food Chem. 2002a, 50(3), 513–518.
- Kang, H. M.; Saltveit, M. E. Effect of Chilling on Antioxidant Enzymes and DPPH-radical Scavenging Activity of High and Low Vigor Cucumber Seedling Radicals. Plant . Environ. 2002b, 25(10), 1233–1238.
- Gualanduzzi, S.; Baraldi, E.; Braschi, I.; Carnevali, F.; Gessa, C. E.; DeSantis, A. Respiration, Hydrogen Peroxide Levels and Antioxidant Enzyme Activities during Cold Storage of Zucchini Squash Fruit. Postharvest Biol. Technol. 2009, 52, 16–23.
- Wagstaff, C.; Bramke, I.; Breeze, E.; Thornber, S.; Harrison, E.; Thomas, B.; Buchanan- Wollaston, V.; Stead, T.; Rogers, H. A. Specific Group of Genes Respond to Cold Dehydration Stress in Cut Alstroemeria Flowers Whereas Ambient Dehydration Stress Accelerates Developmental Senescence Expression Patterns. J. Exp. Bot. 2010, 61, 2905–2921.
- Xi, Z. X.; Duan, L. S.; Tian, X. L.; Wang, B. M.; Eheji, A. E.; Li, Z. H. Coronatine Alleviates Salinity Stress in Cotton by Improving the Anti-Oxidative Defense System and Radical- Scavenging Activity. J. Plant Physiol. 2008, 165(4), 375–384.
- Ding, Z.; Tian, S.; Zheng, X.; Zhou, Z.; Xu, Y. Responses of Reactive Oxygen Metabolism and Quality in Mango Fruit to Exogenous Oxalic Acid or Salicylic Acid under Chilling Temperature Stress. Physiologia Plantarum. 2007, 130, 112–121.
- Huang, M.; Guo, Z. Responses of Antioxidative System to Chilling Stress in Two Rice Cultivars Differing in Sensitivity. Biologia Plantarum. 2005, 49, 81–84.
- Wendehenne, D.; Pugin, A.; Klessig, D. F.; Durner, J. Nitric Oxide: Comparative Synthesis and Signaling in Animal and Plant Cells. Trends Plant Science. 2001, 6, 177–183.
- Zheng, Y. H.; Raymond, W. F.; Wang, S. Y.; Wang, C. Y. Transcript Levels of Anti-Oxidative Genes and Oxygen Radical Scavenging Enzyme Activities in Chilled Zucchini Squash in Response to Superutmospheric Oxygen. Postharvest Biol. Technol. 2008, 47, 151–158.
- Yang, H.; Wu, F.; Cheng, J. Reduced Chilling Injury in Cucumber by Nitric Oxide and the Antioxidant Response. Food Chem. 2011, 127(3), 1237–1242.
- Zhu, S.; Sun, L.; Liu, M.; Zhou, J. Effect of Nitric Oxide on Reactive Oxygen Species and Antioxidant Enzymes in Kiwifruit during Storage. J. Sci. Food Agric. 2008, 88, 2324–2331.