ABSTRACT
Microwave blanching (MB) and hot water blanching (HB) samples of L.edodes were first analysed by electronic tongue and then analysed by high performance liquid chromatography (HPLC) for the content of non-volatile flavour components (soluble sugars (mannitol), organic acids, 5ʹ-nucleotides, and free amino acids). The results showed that contents of bitterness and astringency changed markedly (p < 0.05) when the blanching conditions were 60 s (HB) and 300 w 90 s (MB), respectively. The contents of non-volatile flavour components in HB samples (60 s) were relatively low, whereas contents in MB samples (300w 90s) were significantly higher (p < 0.05) as compared to HB, especially the taste-active amino acids and 5ʹ-nucleotides, which were attributed to the equivalent umami concentration (EUC) values. In addition, the EUC values of MB samples did not differ significantly from the fresh ones, suggesting that MB could effectively preserve the MSG (monosodium glutamate)-like components of L.edodes compared to HB.
Introduction
L.edodes is one of most important traditional medicinal and edible delicacies in Asia on account of their nutritional characteristics, desirable complex flavour, and their medicinal properties in preventing tumor and cancer, helping lower blood pressure, and enhancing the human immunity.[Citation1–Citation5] With the rapid development of industrialized cultivation techniques, L.edodes has been the second widely cultivated mushroom around the world, which represented about 25% of mushroom production and increased more than any other mushroom species.[Citation6,Citation7] L.edodes has a high nutritional value and the contents of amino acids in stipes and caps are different.[Citation8,Citation9] About 18 types of amino acids are found in L.edodes, which provides nearly the ideal ratios of all essential amino acids for human nutrition.[Citation10] In addition, L.edodes also is one of the foods with strong umami taste, contributed by aspartic acid, glutamic acid, and 5ʹ-nucleotides, especially 5ʹ-GMP, as well as 5ʹ-XMP, 5ʹ-IMP, and 5ʹ-AMP, which have a remarkable influences on the umami taste.[Citation2] Umami substances are naturally found in various food including sea foods, vegetables, meat and cheese, and they are the typical flavour components responded for the taste of stored mushrooms.[Citation11] However, the content of bitterness and astringency in L.edodes cannot be ignored. Therefore, it requires appropriate pretreatment methods to reduce the content of bitterness and astringency.
Blanching, a kind of pretreatment method of fruits and vegetables processing or cooking could briefly be described as the process of heating vegetables and fruits to a high temperature. It not only destroys the enzymes, reduces the quantity of total microorganism on the surface of fruit and vegetable, excludes air within the organization, but also softens tissue, reduces the nitrate content, and prevents oxidation in vegetables and fruits.[Citation12,Citation13] Some common blanching methods have already been used in vegetables and fruits, such as HB, MB, high temperature steam blanching and others. HB is one of the most traditional pretreatment methods, which is usually used by people to reduce or eliminate the bitterness and astringency of the vegetables. However, one of its disadvantages is the consumption of large water volumes and loss of nutrients. Compared with HB, MB is a new technology used widely in the field of food in recent years. Uniform treatment, better penetrating power, and lower nutrient losses are its significant advantages.[Citation14,Citation15] Some studies have reported that HB or MB could significantly increase the content of phenol, reduce the quantity of microorganism and enhance the antioxidant activity in vegetables.[Citation12,Citation16] Jaworska and Bernaś[Citation17] also reported that different pretreatment methods in mushroom could markedly change the contents of vitamin C, riboflavin, thiamine, and total acidity during the frozen storage period. However, the changes of non-volatile flavour components in mushroom after blanching have not been reported.
In recent years, electronic tongue and high-performance liquid chromatography (HPLC) have widely been used to analyse the non-volatile flavour components of food. Electronic tongue, based on the sensors array, is a special type of instrument, which can mimic human taste and offer a satisfactory taste results close to the human sensory evaluation.[Citation18,Citation19] Electronic tongue has widely been used for the classification of food products especially in fruit juice, cocoa beans, and wine.[Citation20–Citation22] In addition, chemical analysis by HPLC provides a quantitative data that cannot be explained according to the overall taste on account of the detection of the method which each taste substance being detected separately and this cannot reveal taste-substance interactions such as suppression and synergistic effects.[Citation19] Therefore, the combination of electronic tongue and HPLC could provide a comprehensive flavour characterisation of L.edodes.
In the present study, we aimed to find the changes of non-volatile flavour components in L.edodes blanched by hot water and microwave. The contents of non-volatile flavour components including soluble sugar and polyols, organic acids, 5ʹ-nucleotides, and free amino acids, were examined and compared after HB and MB. Similarly, the EUC values of mushroom after the treatment of hot water and microwave were also evaluated.
Materials and methods
Materials
Raw L.edodes purchased from a local market (Nanjing, China) with an internal moisture content of 91.95 ± 0.37% (w.b) was of uniform size and then washed and cut into pieces with a mushroom slicer (MSC International Co., Montreal, Canada).
Blanching methods
Hot water blanching: Same quality of raw L.edodes was blanched in boiling water (10%, w/v, 100 °C) for 30 s, 60 s, 90 s, 120 s, 150 s, respectively.
Microwave blanching: In order to investigate the effect of MB on the non-volatile flavour components of L.edodes, different blanching time and microwave powers were performed by microwave oven (HC238-AV, Midea, Guangdong, China) in this experiment. The same size pieces of L.edodes were blanched at 60 s for 150 w, 300 w, 450 w, 600 w, and 800 w, respectively. Another experiment was done with the same microwave power (300 w) but different microwave time (30 s, 60 s, 90 s, 120 s, and 150 s, respectively). In addition, small amount of distilled water (5 mL) was added to prevent mushroom coking prior to the microwave blanching.
Electronic tongue measurement
The Insent taste system (SA402B, Beijing, China), which includes multichannel lipid/polymer membrane electrodes, reference electrodes, electronic unit for date acquisition, auto-sampler and personal computer with a progressive chemometric software package was. In addition, the response intensity of each sensor was measured with an Ag/AgCl reference electrode, which is most commonly used in this field. The potentiometric differences between each coated sensor and reference electrode contributed to the intensity value of the measure samples.[Citation23] In this experiment, the same quantity (5 g) of L.edodes blanched by hot water or microwave was smashed with a refiner (MJ-25BM05B, Midea, Guangdong, China) and then centrifuged using a refrigerated centrifuge (Allegra-64R, America). Lastly, the liquid supernatant was taken for electronic tongue analysis. Furthermore, the same volume of water was added before smashing. Prior to analysis, electronic tongue sensors were cleaned by cleaning solution (1, 2, 3) for 90 s, 90 s, 120 s, respectively. Afterwards, conditioning solution and samples were measured by electronic tongue sensor for 30 s, respectively.
Soluble sugar assay
The soluble sugars were extracted and analysed as described by Tsai, Tsai.[Citation24] Different blanching samples were ground sufficiently and extracted with 50 mL of 80% aqueous ethanol. The suspension was stirred by glass rod for 40 min in the water bath, and centrifuged at 5000 rpm for 15 min. The residual was re-extracted three times. Afterwards, the pooled supernatants were concentrated by rotary evaporators (EYELA OIL BATH OSB-2000, Shanghai, China) and re-dissolved with 75% acetonitrile solution to a final volume of 10 mL. The extract was filtered through a 0.45 μm microfiltration membrane before analysis. Each soluble sugar or polyol was confirmed by a reference compound (Yuanye Bio-Technology Co., Ltd, Shanghai, China) and quantified by a calibration curve.
The soluble sugar and polyols were analysed by Agilent 1200 HPLC system (Agilent technologies, Palo Alto, CA, USA) equipped with the Alltech 3300 evaporative light scattering detectors (ELSD, Alltech Associate, Deerfield, IL, USA). The samples were run on a Sugar-D column (4.6 mm × 250 mm, 5 μm; Nacalai Tesque Inc. Kyoto, Japan) and the mobile phase was composed of deionised water and acetonitrile (LC grade) (25:75, v/v) at a flow rate of 0.8 mL/min. The injected volume was 20 μL and ELSD was operated at 55°C with the nitrogen as the nebulizing gas.
Organic acid assay
Organic acids were extracted as described by Li, Gu[Citation25] with modifications. Different blanching samples were weighed, ground sufficiently and extracted by Ultrasonic extraction apparatus (KQ-250DB, Jiangsu, China) with 10 mL of 0.01 M KH2PO4 (pH = 2.86) solution at 45°C for 30 min. Then, the solution was centrifuged at 5000 rpm for 15 min and the supernatant filtered through a 0.45 μm micro-pore filter membrane prior to analysis.
The assay was performed on a Zorbax Eclipse XDB C18 column (250 × 4.6 mm, 5 μm, Agilent) with the Agilent 1200 HPLC system (Agilent technologies, Palo Alto, CA, USA). The injection volume was 20 μL and mobile phase was KH2PO4 (0.01 M, pH = 2.86)/methanol (95/5, v/v) at a flow rate of 0.5 mL/min. In addition, the detection wavelength of organic acid was 210 nm by UV and each organic acid was identified by an authentic standard (Yuanye Bio-Technology Co, Ltd, Shanghai, China). Lastly, all the organic acids were quantified use of a calibration curve prepared from the external standards.
5ʹ-nucleotides assay
5ʹ-Nucleotides were determined as described by Pei, Shi[Citation26] with some modifications. Raw L.edodes, HB and MB samples were weighed, ground sufficiently, and extracted with distilled water (20 mL). The extract was boiled for 1 min, cooled and then centrifuged for 20 min at 4500 rpm. The residue was re-extracted thrice and all the pooled supernatants were concentrated, re-dissolved to a final volume of 10 mL with distilled water. Finally, the extract was analysed by HPLC after filtering with a 0.45 μm micro-pore filter membrane.
The Agilent 1200 HPLC analysis system for 5ʹ-Nucleotide determination was the same as that for organic acids, and soluble sugars. The mobile phase was distilled water/methanol/acetic acid/tetrabutylammonium hydroxide (89.35/10/0.6/0.05, %) at a flow rate of 0.6 mL/min performed on a Zorbax Eclipse XDB C18 column (250 × 4.6 mm, 5 μm, Agilent). The injection volume was 20 μL detected at 254 nm by UV. Each 5ʹ-Nucleotide also was identified by the authentic 5ʹ-Nucleotide (Yuanye Bio-Technology Co., Ltd, Shanghai, China) and finally quantified using a calibration curve.
Free amino acid assay
The content of free amino acids in the samples was detected by automatic amino acid analyser (Amino acid analyser, L-8900, Tokyo, Japan). Raw L.edodes, HB and MB samples were weighed, ground, and then sulfosalicylic acid (1 mL, 10 g/L) and EDTA (0.5mL, 10 g/L) gradually added. The free amino acids was extracted by ultrasonic extraction apparatus (KQ-250DB, Jiangsu, China), dissolved, and made up to a final volume of 25 mL with distilled water. Lastly, same volume of the extract was taken for drying with the pressure blowing concentrator (CM-12, Beijing, China) and re-dissolved by hydrochloric acid. The extract was filtered with a 0.45 μm micro-pore filter membrane before analysis.
Equivalent umami concentration (EUC)
The equivalent umami concentration is the concentration of MSG (g/100g), which is equivalent to the umami intensity of that given by a mixture of MSG and 5ʹ-Nucleotide and can be calculated using the following equation.[Citation27]
where Y is the EUC of the sample (g MSG/100g), ai is the concentration (g/100g) of each umami amino acid (glutamic acid (Glu) or aspartic acid (Asp)); aj is the concentration (g/100g) of each umami 5ʹ-Nucleotide (5ʹ-GMP, 5ʹ-AMP or 5ʹ-IMP); bi is the relative umami concentration (RUC) of each umami amino acid to MSG (Asp, 0.077 and Glu, 1); bj is the RUC for each umami 5ʹ-Nucleotide relative to that of MSG (5ʹ-AMP, 0.18; 5ʹ-GMP, 2.3 and 5ʹ-IMP, 1), and 1218 is a synergistic constant based on the concentration (g/100g) used.
Statistical analysis
All the assays were performed in triplicate and the experimental data were subjected to analysis of variance (ANOVA) for a completely randomised design by statistical analysis system software (IMP SPSS statistics 20). The least significant difference (LSD, defined when p < 0.05) were used to analyse the significant differences between raw L.edodes, HB and MB samples. The results were expressed as the mean value ± standard deviation (SD).
Results and discussions
Electronic tongue measurements
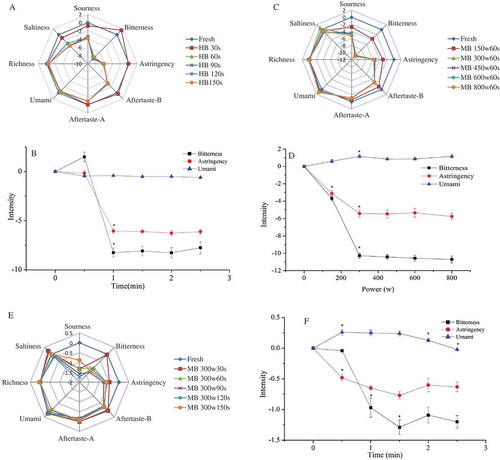
The correlation between electronic tongue and human sensory evaluation was identified by He et al who reported that electronic tongue sensors were correlated best with human sensory evaluation.[Citation23,Citation28] The sensory evaluation of raw L.edodes, HB and MB samples were carried out by the electronic tongue test and the radar fingerprint chart of electronic tongue with different hot water blanching time were presented in . The values of bitterness, astringency, and umami had slight changes compared with raw L.edodes when the HB time was 30 s. However, great changes were observed in the bitterness and astringency values as the HB time increased. When the HB time was 60 s, the values of bitterness and astringency decreased to the minimum (). Afterward, it almost remained unchanged. In addition, the values of umami in the HB samples almost remained the same. The radar fingerprint chart of electronic tongue with different powers of MB was presented in . The values of bitterness, astringency, aftertaste-B and sourness in MB samples were lower than raw L.edodes and aftertaste-A, umami, richness, and saltiness almost overlapped with fresh sample (). The value of astringency in MB samples first decreased, and then remained almost the same with the increase of microwave power. When the microwave power was 300 w, the value of astringency nearly reached the minimum. In addition, the same result was also found in bitterness. However, values of umami in MB samples were almost the same and slight augment was observed in . The radar fingerprint chart of electronic tongue with different times of MB was presented in . Some changes were observed in the values of sourness, bitterness, astringency, and aftertaste-B with different microwave times. The value of astringency and bitterness first decreased to the minimum, and then increased with the extension of MB time (). When the MB time was 90 s, the astringency and bitterness values reached to the lowest level. In contrast, the values of umami in MB samples first increased, remained constant, and finally decreased with the increase of MB time. Similarly, the values of umami in MB samples reached higher level when the MB time was 90 s. It can therefore be concluded that contents of bitterness and astringency significantly changed when the blanching conditions were 60 s (HB) and 300 w 90 s (MB), respectively. In order to further explore the difference of non-volatile flavour components in raw L.edodes, HB and MB samples, three samples were selected from all the blanching samples, raw L.edodes, HB (60 s), and MB (300 w 90 s), respectively.
Figure 1. Radar fingerprint chart and line chart of non-volatile flavor components in L.edodes after hot water blanching (HB) and microwave blanching (MB). A radar fingerprint chart of L.edodes with different hot water blanching time, B changes of bitterness, astringency, and umami in L.edodes with different hot water blanching time, C radar fingerprint chart of L.edodes with the same time and different microwave blanching powers, D changes of bitterness, astringency, and umami in L.edodes with the same time and different microwave blanching powers, E radar fingerprint chart of L.edodes with the same power and different microwave blanching time, F changes of bitterness, astringency, and umami in L.edodes with the same power and different microwave blanching time. *p < 0.05.
Soluble sugar and polyols
The changes of soluble sugar and polyols in L.edodes after HB and MB were presented in . Mannitol was the major soluble mushroom sugar/polyol and the content was 91.99 mg/g dry weight in MB sample, which was significantly higher (p < 0.05) than HB samples (32.82 mg/g dry weight). There was a possibility that the short time hot water blanching dissolved it in the boiling water, which resulted in its decrease. In addition, Mannitol and other soluble sugars also were considered as a taste-active components in mushroom contributed to the sweet perception.[Citation29] Apart from mannitol, the content of trehalose, fructose and glucose in MB sample (10.32 mg/g, 33.86 mg/g and 23.11 mg/g dry weight) were also significantly higher than in HB samples (p < 0.05, 6.80 mg/g, 10.78 mg/g and 11.36 mg/g dry weight). However, the contents of mannitol, fructose, and trehalose in HB and MB samples decreased compared with raw L.edodes, which could be due to the solubility of sugar (polyol) and the Maillard reaction occurred during the heat treatment.[Citation30] The total content of soluble sugars (polyols) in raw L.edodes, MB, HB samples ranged from 61.76 to 203.09 mg/g dry weight and were in the order: raw L.edodes > MB sample > HB sample.
Table 1. The changes of soluble sugar and polyols in L.edodes after hot water blanching (HB) and microwave blanching (MB).
Organic acids assay
Organic acids are strongly related to the synthesis and metabolism of amino acids, aromatic compounds, esters, and phenols. In addition, succinic acid is the common freshener, which plays an important role in the flavour of L.edodes. Similarly, citric acid also is the major organic acid produced by fungal fermentation.[Citation25] The changes of organic acids in L.edodes after HB and MB were shown in . It could be seen that tartaric acid was the major organic acid (68.3 mg/g dry weight) in raw L.edodes, followed by citric acid (31.04 mg/g dry weight), acetic acid (8.33 mg/g dry weight), malic acid (6.08 mg/g dry weight), succinic acid (4.21 mg/g dry weight), and fumaric acid (3.44 mg/g dry weight). The total content of organic acid in L.edodes were significantly (p < 0.05) decreased from 117.4 (raw L.edodes) to 44.76 (HB sample) and 105.21 (MB sample) mg/g dry weight after blanching, respectively. The content of succinic acid in MB sample was 3.35 mg/g dry weight, and it significantly decreased (p < 0.05) compared with raw L.edodes (4.21 mg/g dry weight). However, it was not detected in HB samples, which may attribute to the occurrence of decarboxylation during the blanching of hot water.[Citation31] In addition, the contents of tartaric acid, malic acid, acetic acid, citric acid and fumaric acid also declined in different degrees, which may result in the decrease of astringency value in electronic tongue.[Citation32]
Table 2. The changes of organic acid in L.edodes after hot water blanching (HB) and microwave blanching (MB).
5ʹ-nucleotide
shows the changes of 5ʹ-Nucleotide in L.edodes after HB and MB. The total content of 5ʹ-Nucleotide in MB samples was 4.11 mg/g dry weight, which was significant higher (p < 0.05) than HB sample (0.75 mg/g dry weight) and markedly lower (p < 0.05) than raw L.edodes (6.61 mg/g dry weight). Yang Joan-Hwa[Citation33] reported that the total content of 5ʹ-Nucleotide in L.edodes was 9.51–24.2 mg/g, which was different from the results of this study. The main constituent of 5ʹ-Nucleotide in raw L.edodes, HB and MB samples was 5ʹ-UMP (1.84 mg/g dry weight), 5ʹ-CMP (0.36 mg/g dry weight), and 5ʹ-GMP (1.23 mg/g dry weight), respectively.
Table 3. The changes of 5ʹ-Nucleotide in L.edodes after hot water blanching (HB) and microwave blanching (MB).
5ʹ-GMP and 5ʹ-IMP are regarded as flavour 5ʹ- Nucleotide and 5ʹ-GMP gave a meaty flavour, which is a flavour enhancer much stronger than MSG.[Citation29,Citation33] The content of 5ʹ-GMP in MB samples was 1.23 mg/g dry weight and it was significantly (p < 0.05) higher than in HB samples (0.09 mg/g dry weight). However, 5ʹ-IMP, another typical taste-active component in mushroom and enhances the flavour with other 5ʹ- Nucleotides, was not found.[Citation30] The results may attribute to the effect of thermal decomposition.[Citation34] The content of 5ʹ-AMP in MB sample was 0.74 mg/g dry weight, which was also markedly (p < 0.05) higher than HB samples (0.15 mg/g dry weight). Besides, 5ʹ-AMP could also provide the sweet taste and effectively inhibit the formation of bitter taste in mushroom.[Citation35]
Free amino acids
The content of total free amino acids in MB samples was 251.28 mg/g dry weight, as shown in , which was mildly higher than in raw L.edodes (230.29 mg/g dry weight) and significantly higher than in HB samples (134.68 mg/g dry weight, p < 0.05). There was a possibility that some free amino acids dissolved in water during the progress of HB, and released from the proteolysis occurring during the microwave treatment.[Citation36] Similar results have also been reported by Tian et al who claimed that microwave treatment could increase the content of total free amino acids.[Citation6] Among all the free amino acids, aspartic and glutamic acids were MSG-like components, which contributed to the characteristic umami taste and gave the most mushroom this taste.[Citation37] MSG-like components could also affect the level of EUC in mushroom and those with high concentration of MSG-like components tended to have a high EUC values as well. The content of the MSG-like components in MB samples (29.14 mg/g dry weight) was slightly lower than in HB samples (30.36 mg/g dry weight), but markedly higher than in raw L.edodes (18.73 mg/g dry weight, p < 0.05). Yang Joan-Hwa[Citation33] reported that the content of MSG-like components in mushroom could be divided into three ranges: high (>20 mg/g), middle (5–20 mg/g), and low (<5 mg/g). Obviously, contents of MSG-like components in MB and HB samples were in the high range and raw L.edodes was in the middle range. Moreover, total content of sweet components in MB samples was 80.52 mg/g dry weight and it was significantly higher (p < 0.05) than HB samples (24.23 mg/g dry weight). The total content of bitterness components in HB samples decreased observably (p < 0.05) and a slight increase in MB samples also was observed. Beluhan and Ranogajec[Citation38] reported that MSG-like components, such as glutamic acid, aspartic acid, and sweet components, including serine, threonine, alanine, and glycine were taste-active amino acids, which could be responsible for the pleasant taste of mushrooms. However, none of the bitterness components in mushroom were found to be taste-active. The results indicated that the bitterness could probably be masked by the sweetness from sweet components, which lead to the decrease of bitterness values detected by electronic tongue.[Citation39] In addition, the total content of essential amino acids in MB samples (92.95 mg/g dry weight) was also dramatically higher (p < 0.05) than in HB samples (48.15 mg/g dry weight), which suggested that MB would help to retain more taste-active amino acids in L.edodes.
Table 4. The changes of free amino acids in L.edodes after hot water blanching (HB) and microwave blanching (MB).
Equivalent umami concentration
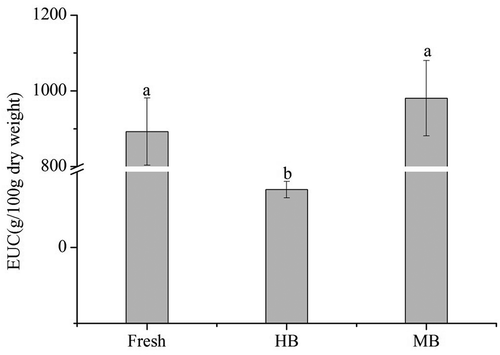
Yamaguchi, Yoshikawa[Citation27] reported that the synergistic effect between MSG-like components and 5ʹ-nucleotides may enhance the umami taste and the EUC values of flavour components in mushroom could be grouped into four levels as: (1) >1000 g MSG/100g dry weight, (2) 100–1000 g MSG/100g dry weight, (3) 10–100 g MSG/100g, and (4) <10 g MSG/100g.[Citation40] The values of EUC in L.edodes were presented in . It was obvious that the value of EUC in raw L.edodes (892.53 g MSG/100g dry weight) was at the second level. However, with the treatment of HB, the EUC value in HB samples (76.58 g MSG/100g dry weight) was significantly (p < 0.05) decreased to the third level. There was a possibility that short time hot water blanching makes the non-volatile flavour components dissolved in the boiling water, which resulted in the decrease of EUC values. In contrast, the value of EUC in MB samples increased to 980.62 g MSG/100g dry weight, which was significantly higher (p < 0.05) than HB samples, and no significant difference was observed in EUC values between MB samples and raw L.edodes. This indicated that the microwave treatment not only maintained the umami taste components but also enhanced the umami value.
Conclusion
Based on results of our study, the contents of non-volatile flavour components in L.edodes significantly changed (p < 0.05) when the blanching conditions were 60 s (HB) and 300 w 90 s (MB), respectively. The contents of non-volatile flavour components in MB samples, including soluble sugar (mannitol), organic acids, and 5ʹ-nucleotides were significantly higher (p < 0.05) than in HB samples. More importantly, the contents of some free amino acids in MB samples (especially the taste-active amino acids, which contributed to the EUC values), were also markedly (p < 0.05) higher than in HB samples. However, these non-volatile flavour components decreased after the microwave and hot water treatment compared with raw L.edodes. In addition, the EUC values of MB samples did not remarkably differ from fresh ones, which suggested that microwave treatment could effectively preserve the MSG-like components of L.edodes. Accordingly, MB is an effective blanching method, which not only maintained the taste-active compounds in L.edodes but also decreased the bitterness and astringency levels compared to HB.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (NO.2016YFD0400405).
References
- Chen, W.; Li, W.; Yang, Y.; Yu, H.; Zhou, S.; Feng, J.; Li, X.; Liu, Y. Analysis and Evaluation of Tasty Components in the Pileus and Stipe of Lentinula Edodes at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 795–801.
- Dermiki, M.; Phanphensophon, N.; Mottram, D. S.; Methven, L. Contributions of Non-Volatile and Volatile Compounds to the Umami Taste and Overall Flavour of Shiitake Mushroom Extracts and Their Application as Flavour Enhancers in Cooked Minced Meat. Food Chem. 2013, 141, 77–83.
- Matsui, S.; Nakazawa, T.; Umegae, Y.; Mori, M. Hypersensitivity Pneumonitis Induced by Shiitake Mushroom Spores. Intern. Med. 2012, 31, 1204–1206.
- Solanki, A. K.; Rathore, Y. S.; Badmalia, M. D.; Dhoke, R. R.; Nath, S. K.; Nihalani, D. Ashish. Global Shape and Ligand Binding Efficiency of the HIV-1-neutralizing Antibodies Differ from Those of Antibodies that Cannot Neutralize HIV-1. J. Biol. Chem. 2014, 289, 34780–34800.
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P. C. K. Advances in Lentinan: Isolation, Structure, Chain Conformation and Bioactivities. Food Hydrocoll. 2011, 25, 196–206.
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of Different Drying Methods on the Product Quality and Volatile Compounds of Whole Shiitake Mushrooms. Food Chem. 2016, 197, 714–722.
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with Essential Oils Improves Sensory Quality and Enhanced Antioxidant Ability of Shiitake Mushroom (Lentinus Edodes). Food Chem. 2015, 172, 692–698.
- Zhang, N.; Chen, H.; Zhang, Y.; Ma, L.; Xu, X. Comparative Studies on Chemical Parameters and Antioxidant Properties of Stipes and Caps of Shiitake Mushroom as Affected by Different Drying Methods. J. Sci. Food Agric. 2013, 93, 3107–3113.
- Cağlarirmak, N.;. Chemical Composition and Nutrition Value of Dried Cultivated Culinary-Medicinal Mushrooms from Turkey. Int. J. Med. Mushrooms. 2011, 13, 351–356.
- Turło, J.; Gutkowska, B.; Herold, F.; Krzyczkowski, W.; Blazewicz, A. Optimizing Vitamin B12 Biosynthesis by Mycelial Cultures of Lentinula Edodes (Berk.) Pegl. Enzyme Microb. Technol. 2008, 43, 369–374.
- Chen, G.; Wu, F.; Pei, F.; Cheng, S.; Muinde, B.; Hu, Q.; Zhao, L. Volatile Components of White Hypsizygus Marmoreus Detected by Electronic Nose and HS-SPME-GC-MS: Influence of Four Drying Methods. Int. J. Food Prop. 2016. DOI: 10.1080/10942912.2016.1258575.
- Oboh, G.;. Effect of Blanching on the Antioxidant Properties of Some Tropical Green Leafy Vegetables. LWT Food Sci. Technol. 2005, 38, 513–517.
- Rossi, M.; Giussani, E.; Morelli, R.; Lo Scalzo, R.; Nani, R. C.; Torreggiani, D. Effect of Fruit Blanching on Phenolics and Radical Scavenging Activity of Highbush Blueberry Juice. Food Res. Int. 2003, 36, 999–1005.
- Giese, J.;. Advances in Microwave Food Processing. Food Technol. 1992, 46, 118–123.
- Ramesh, M. N.; Wolf, W.; Tevini, D.; Bognár, A. Microwave Blanching of Vegetables. J. Food Sci. 2002, 67, 390–398.
- Dorantes-Alvarez, L.; Jaramillo-Flores, E.; González, K.; Martinez, R.; Parada, L. Blanching Peppers Using Microwaves. Procedia Food Sci. 2011, 1, 178–183.
- Jaworska, G. Y.; Bernaś, E. Qualitative Changes in Pleurotus Ostreatus (Jacq.: Fr.) Kumm. Mushrooms Resulting from Different Methods of Preliminary Processing and Periods of Frozen Storage. J. Sci. Food Agric. 2009, 89, 1066–1075.
- Lu, L.; Deng, S.; Zhu, Z.; Tian, S. Classification of Rice by Combining Electronic Tongue and Nose. Food Anal. Methods. 2014, 8, 1893–1902.
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced Taste Sensors Based on Artificial Lipids with Global Selectivity to Basic Taste Qualities and High Correlation to Sensory Scores. Sensors. 2010, 10, 3411–3443.
- Ciosek, P.; Brzózka, Z.; Wróblewski, W. Electronic Tongue for Flow-Through Analysis of Beverages. Sens. Actuators B. 2006, 118, 454–460.
- Kundu, P. K.; Chatterjee, A.; Panchariya, P. C. Electronic Tongue System for Water Sample Authentication: A Slantlet-Transform-Based Approach. IEEE Trans. Instrum. Meas. 2011, 60, 1959–1966.
- Teye, E.; Huang, X.; Han, F.; Botchway, F. Discrimination of Cocoa Beans according to Geographical Origin by Electronic Tongue and Multivariate Algorithms. Food Anal. Methods. 2013, 7, 360–365.
- Phat, C.; Moon, B.; Lee, C. Evaluation of Umami Taste in Mushroom Extracts by Chemical Analysis, Sensory Evaluation, and an Electronic Tongue System. Food Chem. 2016, 192, 1068–1077.
- Tsai, S.; Tsai, H.; Mau, J. Non-Volatile Taste Components of Agaricus Blazei, Agrocybe Cylindracea and Boletus Edulis. Food Chem. 2008, 107, 977–983.
- Li, W.; Gu, Z.; Yang, Y.; Zhou, S.; Liu, Y.; Zhang, J. Non-Volatile Taste Components of Several Cultivated Mushrooms. Food Chem. 2014, 143, 427–431.
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A. M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F.; Hu, Q. Changes in Non-Volatile Taste Components of Button Mushroom (Agaricus Bisporus) during Different Stages of Freeze Drying and Freeze Drying Combined with Microwave Vacuum Drying. Food Chem. 2014, 165, 547–554.
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the Relative Taste Intensity of Some L‐α‐amino Acids and 5′‐Nucleotides. J. Food Sci. 1971, 36, 846–849.
- He, W.; Hu, X.; Zhao, L.; Liao, X.; Zhang, Y.; Zhang, M.; Wu, J. Evaluation of Chinese Tea by the Electronic Tongue: Correlation with Sensory Properties and Classification according to Geographical Origin and Grade Level. Food Res. Int. 2009, 42, 1462–1467.
- Litchfield, J.;. Morel Mushroom Mycelium as a Food-Flavoring Material. Biotechnol. Bioeng. 1967, 9, 289–304.
- Li, Q.; Zhang, H. H.; Claver, I. P.; Zhu, K. X.; Peng, W.; Zhou, H. M. Effect of Different Cooking Methods on the Flavour Constituents of Mushroom (Agaricus Bisporus (Lange) Sing) Soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108.
- Handschumacher, R. E.;. Orotidylic Acid Decarboxylase: Inhibition Studies with Azauridine 5-Phosphate. J. Biol. Chem. 1960, 235, 2917–2919.
- Sowalsky, R. A.; Noble, A. C. Comparison of the Effects of Concentration, pH and Anion Species on Astringency and Sourness of Organic Acids. Chem. Senses. 1998, 23, 343–349.
- Yang, J.; Lin, H.; Mau, J. Non-Volatile Taste Components of Several Commercial Mushroom. Food Chem. 2001, 72, 465–471.
- Boekel, M.;. Formation of Flavour Compounds in the Maillard Reaction. Biotechnol. Adv. 2006, 24, 230–233.
- Leksrisompong, P.; Gerard, P.; Lopetcharat, K.; Drake, M. Bitter Taste Inhibiting Agents for Whey Protein Hydrolysate and Whey Protein Hydrolysate Beverages. J. Food Sci. 2012, 77, S282–S287.
- Yoneda, C.; Okubo, K.; Kasai, M.; Hatae, K. Extractive Components of Boiled-Dried Scallop Adductor Muscle and Effect on the Taste of Soup after Mixing with Chicken Leg Meat. J. Sci. Food Agric. 2005, 85, 809–816.
- Tsai, S. Y.; Wu, T. P.; Huang, S. J.; Mau, J. L. Nonvolatile Taste Components of Agaricus Bisporus Harvested at Different Stages of Maturity. Food Chem. 2007, 103, 1457–1464.
- Beluhan, S.; Ranogajec, A. Chemical Composition and Non-Volatile Components of Croatian Wild Edible Mushrooms. Food Chem. 2011, 124, 1076–1082.
- Chang, H. L.; Chao, G. R.; Chen, C. C.; Mau, J. L. Non-Volatile Taste Components of Agaricus Blazei, Antrodia Camphorata and Cordyceps Militaris Mycelia. Food Chem. 2001, 74, 203–207.
- Mau, J. L.;. The Umami Taste of Edible and Medicinal Mushrooms. Int. J. Med. Mushrooms. 2005, 7, 119–126.