ABSTRACT
In the last years, the attention to the potential carcinogenic effects of the 5-hydroxymethylfurfural in the human health is increased. This compound is used as a marker of honey adulteration and as an indication of thermal treatment of food containing sugars. In this study, we evaluated the content of 5-hydroxymethylfurfural, furfural, 2-furoic acid, in sapa syrup, Marsala wines and bakery products containing sapa. Average levels of 5-hydroxymethylfurfural in sapa syrup were 2.3 ± 0.8 g/kg while in bakery products were 167 ± 133 mg/kg. Moreover, levels of 5-hydroxymethylfurfural in Marsala wine were 175 ± 150 mg/L. In addition, bakery products showed levels of furfural and furoic acid equal to 7.0 ± 4.5 and 48 ± 46 mg/kg, respectively, while furfural and furoic acid levels of 3.7 ± 1.7 and 27 ± 18 mg/L in Marsala wines. The present study suggested that 5-hydroxymethylfurfural can be used as a marker to fingerprint the use of sapa in food preparation.
Abbreviations: DAD: diode array detector, F: furfural, FA: 2-furoic acid, HPLC: high performance liquid chromatography, HMF: 5-hydroxymethylfurfural; LOD: limit of detection, LOQ: limit of quantification, SMF: 5-sulfoxymethylfurfural, TCA: trichloroacetic acid.
Introduction
5-hydroxymethylfurfural (HMF) is a water soluble heterocyclic aldehyde which is commonly present at low level in fresh food containing sugars. HMF is formed during thermal treatment of food by acid-catalysed dehydration of carbohydrates, Maillard reaction, or heat induced caramelisation.[Citation1] HMF formation is favoured by levels of fructose, sucrose and a lesser extent glucose from which furfural is predominantly formed.[Citation2,Citation3] The main factors influencing the formation of HMF in foods during storage are temperature, water activity and pH.[Citation4]
Foods known to contain HMF are honey, dried fruits, jams, bread and coffee with levels spanning from 3 to 4100 mg/kg while for fruit juices, beer and wine between 2 and 22 mg/L, respectively.[Citation2,Citation4] On the other hand, the content of HMF can be higher during food storage thus this parameter may be used as a marker of food heat treatment and spoilage. Nursten reported that during storage of orange juices, ascorbic acid was the main precursor of furfural and 2-furoic acid while HMF is formed principally from fructose.[Citation5] High levels of HMF in honey indicate a probable adulteration with inverted syrup.[Citation4] Codex Alimentarius fixes at 40 mg/kg the maximum level of HMF in honey with an exception of 80 mg/kg for honey originated from tropical regions.[Citation6]
Nguyen et al. studied HMF levels in bakery products produced with different sugars demonstrating that HMF concentration was highest in biscuits with glucose and fructose and suggested that HMF is formed via caramelisation.[Citation7] Anciently, Romans were used to reduce unfermented grape juice at different extent producing a syrup called sapa. Sapa, defrutum, or caroenum were prepared after heating or simmering the must in a leaden vessel by one third, half volume, and two third respectively. Pliny the Elder and Columella gave detailed instructions for preparing sapa and preserving wines, while Caelius Apicius illustrated the uses of sapa in Roman cooking.[Citation8] Nowadays, sapa syrup is produced in different Italian regions and it is used as a sweetener in different food such as cheese, ice-creams and ricotta cheese or to prepare local bakery products.
Moreover, sapa syrup is used in winemaking to produce Marsala fine ambra and Marsala superiore ambra wines with addition level of reduced must not lower than 1%.[Citation9] Marsala is a wine fortified with alcohol produced in the homonymous area of western Sicily in Italy, made following a process called in perpetuum, which is similar to the solera system used to produce Sherry in Jerez, Spain. Since 1969 Marsala wine is sold as PDO status that is a recognition of quality. Marsala wine can be produced as oro, rubino and ambra; only the latter is prepared with the addition of sapa. The three levels of sweetness are secco, semisecco and sweet with a maximum level of reducing sugars of 40, 41–100 and over 100 g/L, respectively. The classification of aging wine is fine, superiore and superiore riserva with aging not lower of a year, two years and four years, respectively. Amber and oro Marsala wine is produced using different white grape cultivar, specially Grillo is one of the most used.[Citation10] To our knowledge no data reported the presence of HMF, F and FA in the commodities analysed in this work.
From a toxicological point of view, HMF is in vitro and vivo converted into the reactive metabolite 5-sulfoxymethylfurfural (SMF), that showed to be mutagenic in Ames test[Citation11] and initiated papillomas in mouse skin test after topical application.[Citation12] Moreover, HMF was found genotoxic in vitro experiments when metabolic preconditions for the formation of the reactive metabolite 5-sulphoxymethylfurfural were met[Citation2] and in vivo studies induced and promoted aberrant crypt foci in rat’s colon.[Citation13,Citation14] On the other hand, Sachse et al. reported that humans may be less sensitive regarding HMF sulfo-conjugation compared with the rodent models.[Citation15]
Several analytical methods for the determination of HMF levels in food have been reported, i.e. spectrophotometry[Citation16], high performance liquid chromatography (HPLC),[Citation17–Citation20], ion-exclusion chromatography (IEC)[Citation21] and capillary electrophoresis coupled with UV detection.[Citation22] Xu et al.[Citation23] used a high-performance anion-exchange chromatography method with pulsed amperometric detection to quantify HMF and dextrose in aqueous dextrose solutions, while other authors analysed HMF by gas chromatography with flame ionisation detection and by gas chromatography-mass spectrometry.[Citation24,Citation25]
Taking into consideration the carcinogenic potential of HMF aims of this work were: a) to evaluate the simultaneous content of HMF and furfural as markers of thermal treatment of sugars, as well as 2-furoic acid as oxidation product of furfural, in selected food commodities such as sapa syrup, bakery products containing sapa and Marsala wines; b) to find a correlation between sugars and HMF, furfural, 2-furoic acid by measuring levels of sugars such as glucose, fructose and sucrose in the same commodities.
Materials and methods
Chemicals
5-Hydroxymethylfurfural (HMF), furfural (F), 2-furoic acid (FA), glucose, sucrose, fructose, sodium hydroxide solution 50% w/w, acetonitrile, ammonium formate and trichloroacetic acid (TCA) were obtained from Sigma Aldrich Italy. Water used was purified on a Milli-Q apparatus (Millipore, Milan, Italy). Acetonitrile was of HPLC grade and sodium hydroxide of IC grade. Standard stock solutions were prepared daily in a mixture consisting of acetonitrile and aqueous ammonium formate 10 mM (50:50, v/v) and stored at −20°C until use.
HPLC-DAD analysis of furanic compounds
The analysis was performed on HPLC using an Agilent Technologies 1100 series (Waldbronn, Germany) equipped with a quaternary pump, autosampler, a degasser system and a diode array detector (DAD). A reverse phase column Varian Pursuit Xrs C18 (250 mm x 4.6 mm x 5µm) was employed and a guard column of the same material was used. The separation was carried out in gradient at room temperature using aqueous ammonium formate 10 mM and acetonitrile as mobile phase. The gradient program was as follows: starting from aqueous ammonium formate for 5 min then linear gradient to 65% acetonitrile in 25 min. The flow rate was 1.0 mL/min and the injection volume was 10 µL. HMF and F were detected at 280 nm while FA at 254 nm.
Five sapa syrup samples, five Marsala wines and five typical bakery products containing sapa were purchased at a local food market (Cagliari, Italy). Samples for analysis were prepared diluting 1 mL of sapa into 150 mL of the mobile phase. Furthermore, bakery products were prepared weighting 2 g in polyethylene test tubes. Then, 3 mL of TCA 4% (w/v) were added and mixed with a rotary shaker for 20 min. Afterwards the samples were centrifuged at 4000 rpm for 25 min. 1 mL of the supernatant was recovered and diluted with 10 mL of mixture consisting of acetonitrile and aqueous ammonium formate 10 mM (50:50, v/v). Additionally, 1 mL of Marsala wines were diluted with 10 mL of mobile phase. All samples were filtered through a 0.45 µm nylon membrane filter and injected for HPLC-DAD analysis.
IC-PAD analysis of sugars
The chromatographic separation of sugars was performed on HPLC using a Dionex ICS 3000 DC series (Sunnyvale, California) equipped with a dual pistol pump with a vacuum degasser, and a pulsed amperometric detector (PAD). A column Carbopac PA20 C18 (150 mm x 3 mm x 6.0 µm) packed with a hydrophobic, polymeric anion exchange resin was employed and a guard column of the same material was used. The separation was carried out isocratically with constant helium purge at 30°C using sodium hydroxide solution 10 mM as mobile phase. The flow rate was 0.5 mL/min and the injection volume was 10 µL.
Sapa samples for analysis were prepared dissolving 100 µL of sapa syrup into 1 L of Milli-Q water. Moreover, bakery products were prepared weighting 500 mg of matrix into 100 mL of Milli-Q water. Samples were homogenised and filtered. 1 mL of the solution was diluted with 20 mL of Milli-Q water. Furthermore, 100 µL of Marsala wine were diluted with 100 mL of Milli-Q water and homogenised. Before analysis samples were filtered through a 0.45 µm nylon membrane filter.
Linearity of calibration curves
Adequate amounts of HMF, F and FA were dissolved in 10 mL mixture consisting of acetonitrile and aqueous ammonium formate 10 mM (50:50, v/v). Stock solutions were diluted with the same mixture and injected in triplicate for the preparation of calibration curves.
HMF, F and FA in Marsala wines, sapa syrup and bakery products were quantified by interpolation in calibration curves of matrix matched standards in the range of 0.6–100 mg/L for HMF, FA and 0.6–120 mg/L for F, respectively. Samples were fortified at the desired level of HMF, F and FA and directly injected for HPLC analysis after dilution with blank sample. Calibration curves were created by plotting the concentration of these compounds against the standard peak area following the standard addition method. To overcome matrix interference, quantification of HMF, F and FA was performed by the standard additions analysis. The linearity of the method was demonstrated using blank sample spiked with the standards at concentration level of 0.6–100 mg/L for HMF, FA and 0.6–120 mg/L for F, respectively.
Statistical analysis
Each analysis was conducted five times. Data were expressed as means ± standard error. Data were statistically analysed using Minitab 16 software (Minitab Inc., State College, PA).
Results and discussion
HPLC analysis of furanic compounds and IC analysis of sugars
Levels of furanic compounds in bakery products, Marsala wines and sapa syrup were measure by an HPLC-DAD method, without the need of liquid-liquid extraction of chemical derivatisation steps. We applied a reversed-phase HPLC method with photodiode detection proposed by Hu et al.[Citation19] and Petisca et al.[Citation26] with slight modifications. Since HMF and F showed a strong UV absorption at 280 nm while FA showed a maximum at 254 nm () we used these wavelengths for quantitation. Considering that HMF and F were unstable under light conditions all samples were analysed fresh. The optimal injection volume was 10 µL while using 50 µL we observed a signal saturation. and report respectively the HPLC-DAD chromatograms of sapa syrup and Marsala wine, fortified at 10 mg/L for all tested compounds. HPLC retention times of HMF, F and FA in Marsala wines were 14.9, 16.0 and 4.1 min, respectively (). Moreover, no interfering peaks were detected at the retention time of all compounds analysed. The limit of quantitation of this method (LOQ) calculated as signal noise ratio (S/N) of ten were 0.12 mg/L for HMF and F and 0.3 mg/L for FA, respectively. The limit of determination (LOD) calculated as signal noise ratio (S/N) of three were 0.04 mg/L for HMF and 0.02 mg/L for FA and F (). The linearity of the calibration curves for FA, HMF and F in spiked Marsala wine, sapa syrup and bakery products are reported in . Calibration curves consisting of concentration values in the range of 0.6–100 mg/L for HMF, FA and 0.6–120 mg/L for F. The mean correlation coefficients (r2) of calibration curves for HMF, F and FA were 0.993, 0.999 and 0.999, respectively. Moreover, recovery percent of HMF, F, FA on sapa bakery products were in the range of 83 – 124.
Table 1. Regression equation for HMF, F and FA in Marsala wines, sapa syrup and bakery products using the standard addition method.
Table 2. Regression equation, limit of detection (LOD) and quantification (LOQ) for furanic compounds and sugars.
Figure 2. HPLC-DAD chromatogram for the analysis of HMF and F at 280 nm (A) and FA at 254 nm (B) in sapa syrup.
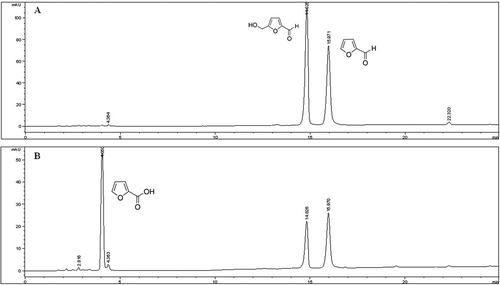
Figure 3. HPLC-DAD chromatogram for the analysis of HMF and F at 280 nm (A) and FA at 254 nm (B) in Marsala wine.
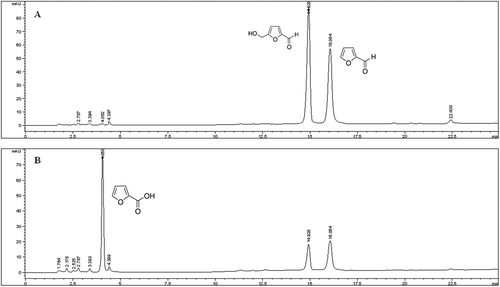
For the analysis of sugars by IC-PAD, no interfering peaks were detected at the retention time of all compounds analysed. In this case LOQ calculated were 0.20 mg/L for glucose and sucrose and 0.15 mg/L for fructose, respectively. LOD were 0.10 mg/L for glucose and sucrose and 0.07 mg/L for fructose (). Calibration curves consisting of concentration values for all sugars analysed were in the range of 0.05–40 mg/L. The mean correlation coefficients (r2) of calibration curves for glucose, sucrose and fructose was 0.997.
Furanic and sugars levels in sapa syrup and bakery products
With this HPLC method average levels of HMF in sapa syrup were 2.3 ± 0.8 g/kg while F and FA showed 0.05 ± 0.04 g/kg and 0.37 ± 0.26 g/kg, respectively (). Moreover, average levels of HMF in bakery products were 167 ± 133 mg/kg on dry matter basis while F and FA showed respectively 7.0 ± 4.5 and 48 ± 46 mg/kg on a dry matter basis. For comparison, Petisca et al.[Citation27] reported in a survey in bread and bakery products pointed that biscuits showed the highest content (7.8 mg/kg fw) whereas cake/pastry samples showed the lowest HMF content (3.0 mg/kg fw). Regarding furfural, bread samples presented the highest furfural content (5.3 mg/kg fw) (p < 0.05), cake/pastry and biscuits showed the lowest content (1.9 and 3.0 mg/kg fw, respectively).
Table 3. Quantification of furanic compounds and sugars in Marsala wines, sapa syrup and bakery products.
Moreover, levels of HMF found in sapa syrup and sapa bakery products were elevated with a maximum of 3.3 g/kg and 395 mg/kg, respectively. De Andrade et al.[Citation28] reported HMF levels for corn syrup between 0.41 and 2.12 g/kg while for cane syrup between 0.11 and 0.89 g/kg, respectively. Cocchi et al. measured the levels of different sugars, F and HMF during the preparation of the “Italian aceto balsamico tradizionale”. Authors reported that sugars levels increased during cooking following almost a linear trend; on the contrary, furfural compounds, produced by monosaccharides degradation, significantly increased with an exponential trend only in the final step of the process corresponding to a reduction of water content and a prolonged thermal treatment.[Citation29]
As regard IC-PAD data, average levels of glucose and fructose in sapa syrup were 230 ± 38 g/kg and 283 ± 31 g/kg while sucrose showed 33 ± 13 g/kg (). In addition, bakery products showed average levels of glucose and fructose equal to 53 ± 11 g/kg and 53 ± 14 g/kg of dry matter, respectively; while sucrose was 8.5 ± 2.9 g/kg.
Furanic and sugars levels in Marsala wine samples
Average levels of HMF in Marsala wine were 175 ± 150 mg/L while F and FA showed 3.7 ± 1.7 and 27 ± 18 mg/L, respectively (). The maximum level of HMF in Marsala wine samples corresponds to the sweet Marsala type (511 ± 157 mg/L) followed by semisecco ambra and secco ambra Marsala type. The maximum level of HMF in Marsala wine samples corresponds to the sweet Marsala type that can be produced with reducing sugars over 100 g/L followed by semisecco ambra and secco ambra Marsala type with a maximum amount of reducing sugars of 41–100 and 40 g/L, respectively. Serra-Cayuela et al.[Citation30] reported an average amount of HMF for brut champagne and cava wine between 0.25 and 12.81 mg/L while Morales[Citation4] reported HMF levels between 1 and 1.3 mg/L for red wine, 1.5 and 4.8 mg/L for brandy, 20 and 340 mg/L for sherry and 130 and 1245 mg/L for sweet sherry.
Average levels of glucose and fructose in Marsala wines were 27 ± 8 and 30 ± 9 g/L, respectively. while sucrose levels were 2.1 ± 1.0 g/L (). The maximum level of glucose and fructose in Marsala wine samples corresponds to sweet Marsala type following by semisecco ambra and secco ambra Marsala type. On the other hand, grillo wine showed low levels of glucose and fructose (1.3 and 1.8 g/L, respectively) while sucrose was not detected. A positive correlation between fructose and HMF (r = 0.93; **P = 0.003) was observed in Marsala wine. This correlation observed in Marsala wine confirms that HMF formation is favoured by high levels of fructose. When comparing Marsala wine with sapa syrup we found a positive correlation between F and FA levels (r = 0.87 **P = 0.01; r = 0.94 *P = 0.02). This correlation confirms a common origin for both compounds; while no correlation was found for bakery products containing sapa, this is probably due to other source of F and FA. Moreover, no matrix effect was detected for all commodities studied. To produce Marsala different vineyards are commonly used such as the grillo cultivar. After HPLC analysis of the latter wine we detected 0.80 ± 0.04 mg/L of HMF and 1.2 ± 0.4 mg/L of F, well below levels found in the Marsala wine and thus confirming that the origin of HMF is certainly derived from the addition of sapa syrup and sugars. High levels of HMF and furfural compounds in food are an index of sugars thermal degradation and consequently of remarkable product degradation.
Conclusion
Considering the assumption on the potential carcinogenic effects of the 5-hydroxymethylfurfural and the fact that this compound is used as an adulteration marker and an index of food thermal treatment we evaluated, for the first time, levels of this compound in Marsala wines, sapa syrup and bakery products obtained from sapa syrup. In the same commodities, we evaluated levels of furfural and 2-furoic acid, a product of sugars degradation and oxidation derivative of furfural respectively. Further translational studies are needed to estimate human toxicity. In addition, HMF, F and FA can be potentially used as a marker to fingerprint the use of sapa in food preparation and the positive correlation between sugars and furanic compounds suggest a prolonged thermal treatment.
References
- Danehy, J. P.;. Maillard Reactions: Nonenzymatic Browning in Food Systems with Special Reference to the Development of Flavor. In Advances in Food Research; Chichester, C. O., Mrak, E. M., Stewart, G. F., Eds.; Academic Press: Boston, 1986; pp. 77–124.
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, C. E. Toxicology and Risk Assessment of 5-Hydroxymethylfurfural in Food. Mol. Nutr. Food Res. 2011, 55, 667–668.
- Lopes De Souza, R.; Yu, H.; Rataboul, F.; Essayem, N. 5-Hydroxymethylfurfural (5-HMF) Production from Hexoses: Limits of Heterogeneous Catalysis in Hydrothermal Conditions and Potential of Concentrated Aqueous Organic Acids as Reactive Solvent System. Challenges. 2012, 3, 212–232.
- Morales, F. J.; Hydroxymethylfurfural (HMF) and Related Compounds. In Process-Induced Food Toxicants: Occurrence, Formation, Mitigation and Health Risks; Stadler, R. H., Lineback, D. R., Eds.; John Wiley & Sons: New York, 2009; pp. 134–175.
- Nursten, H.;. The Maillard Reaction: Chemistry, Biochemistry and Implications; University of Reading: UK, 2005.
- Codex Alimentarius, Alinorm 01/25 2000. Draft Revised Standard for Honey at Step 8 of the Codex Procedure, EU Directive/1/110/2001 of 02/ 12/2001(L 10/47).
- Nguyen, H. T.; Van Der Fels-Klerx, H. J.; Peters, R. J.; Van Boekel, M. A. Acrylamide and 5-Hydroxymethylfurfural Formation during Baking of Biscuits: Part I: Effects of Sugar Type. Food Chem. 2016, 192, 575–585.
- Eisinger, J.;. Lead and Wine Eberhard Gockel and the Colica Pictonum. Med. Hist. 1982, 26, 279–302.
- Disciplinare Di Produzione Della Denominazione Di Origine Controllata Del Vino “Marsala” Modificato Con D.M. 07.03.2014.
- Settanni, L.; Sannin, C.; Francesca, N.; Guarcello, R.; Moschetti, G. Yeast Ecology of Vineyards within Marsala Wine Area (Western Sicily) in Two Consecutive Vintages and Selection of Autochthonous Saccharomyces Cerevisiae Strains. J. Biosci. Bioeng. 2012, 114, 606–614.
- Lee, Y. C.; Shlyankevich, M.; Jeong, H. K.; Douglas, J. S.; Surh, Y. J. Bioactivation of 5-Hydroxymethyl-2-Furaldehyde to an Electrophilic and Mutagenic Allylic Sulfuric Acid Ester. Biochem. Biophys. Res. Commun. 1995, 209, 996–1002.
- Surh, Y. J.; Liem, A.; Miller, J. A.; Tannenbaum, S. R. 5-Sulfooxymethylfurfural as a Possible Ultimate Mutagenic and Carcinogenic Metabolite of the Maillard Reaction Product, 5-Hydroxymethylfurfural. Carcinogenesis. 1994, 15, 2375–2377.
- Archer, M. C.; Bruce, W. R.; Chan, C. C.; Corpet, D. E.; Medline, A.; Roncucci, L.; Stamp, D.; Zhang, X. M. Aberrant Crypt Foci and Microadenoma as Markers for Colon Cancer. Environ. Health Perspect. 1992, 98, 195–197.
- Zhang, X. M.; Chan, C. C.; Stamp, D.; Minkin, S.; Archer, M. C.; Bruce, W. R. Initiation and Promotion of Colonic Aberrant Crypt Foci in Rats by 5-Hydroxymethyl-2-Furaldehyde in Thermolyzed Sucrose. Carcinogenesis. 1993, 14, 773–775.
- Sachse, B.; Meinl, W.; Glatt, H.; Monien, B. H. Conversion of Suspected Food Carcinogen 5-Hydroxymethylfurfural by Sulfotransferases and Aldehyde Dehydrogenases in Postmitochondrial Tissue Preparations of Humans, Mice, and Rats. Toxicolog. Sci. 2016, 149, 192–201.
- White, J. W., Jr;. Spectrophotometric Method for Hydroxymethylfurfural in Honey. J. Assoc. Off. Anal. Chemists. 1979, 3, 509–514.
- Smith, R. M.;. Determination of 5-Hydroxymethylfurfural and Caffeine in Coffee and Chicory Extracts by High Performance Liquid Chromatography. Food Chem. 1981, 7, 41–45.
- Lo Coco, F.; Valentini, C.; Novelli, V.; Ceccon, L. High Performance Liquid Chromatographic Determination of 2-Furaldehyde and 5-Hydroxymethyl-2-Furaldehyde in Processes Citrus Juices. J. Liq. Chromatogr. 1994, 17, 603–617.
- Hu, G.; Hernandez, M.; Zhu, H.; Shao, S. An Efficient Method for the Determination of Furan Derivatives in Apple Cider and Wine by Solid Phase Extraction and High Performance Liquid Chromatography - Diode Array Detector. J. Chromatogr. 2013, 1284, 100–106.
- Zappalà, M.; Fallico, B.; Arena, E.; Verzera, A. Methods for the Determination of HMF in Honey: A Comparison. Food Contr. 2005, 16, 273–277.
- Kim, H. J.; Richardson, M. Determination of 5-Hydroxymethylfurfural by Ion-Exclusion Chromatography by Uv Detection. J. Chromatogr. 1992, 593, 153–156.
- Chen, Z.; Yan, X. Simultaneous Determination of Melamine and 5-Hydroxymethylfurfural in Milk by Capillary Electrophoresis with Diode Array Detection. J. Agric. Food Chem. 2009, 57, 8742–8747.
- Xu, H.; Templeton, A. C.; Reed, R. A. Quantification of 5-HMF and Dextrose in Commercial Aqueous Dextrose Solutions. J. Pharm. Biomed. Anal. 2003, 32, 451–459.
- Teixido, E.; Santos, F. J.; Puignou, L.; Galceran, M. T. Analysis of 5-Hydroxymethylfurfural in Foods by Gas Chromatography–Mass Spectrometry. J. Chromatogr. 2006, 1, 85–90.
- Gaspar, E. M.; Lopes, J. F. Simple Gas Chromatographic Method for Furfural Analysis. J. Chromatogr. 2009, 1216, 2762–2767.
- Petisca, C.; Henriques, A. R.; Pérez-Palacios, T.; Pinho, O.; Ferreira, I. M. P. L. V. O. Study of Hydroxymethylfurfural and Furfural Formation in Cakes during Baking in Different Ovens Using a Validated Multiple Stage Extraction Based Analytical Method. Food Chem. 2013, 141, 3349–3356.
- Petisca, C.; Henriques, A. R.; Pérez-Palacios, T.; Pinho, O.; Ferreira, I. M. P. L. V. O. Assessment of Hydroxymethylfurfural and Furfural in Comercial Bakery Products. J. Composition Anal. 2014, 33, 20–25.
- De Andrade, J. K.; Komatsu, E.; Perreault, H.; Torres, Y. R.; Da Rosa, M. R.; Felsner, M. L. In House Validation from Direct Determination of 5-Hydroxymethyl-2-Furfural (HMF) in Brazilian Corn and Cane Syrups Samples by HPLC-UV. Food Chem. 2016, 190, 481–486.
- Cocchi, M.; Ferrari, G.; Manzini, D.; Marchetti, A.; Sighinolfi, S. Study of the Monosaccharides and Furfurals Evolution during the Preparation of Cooked Grape Musts for Aceto Balsamico Tradizionale Production. J. Food Eng. 2007, 79, 1438–1444.
- Serra-Cayuela, A.; Castellari, M.; Bosch-Fusté, J.; Riu-Aumatell, M.; Buxaderas, S.; López-Tamames, E. Identification of 5-Hydroxymethyl-2-Furfural (5-HMF) in Cava Sparkling Wines by LC-DAD-MS/MS and NMR Spectrometry. Food Chem. 2013, 141, 3373–3380.