ABSTRACT
In this study, effect of sugars (sucrose and trehalose) and their amount on the volatile profile of sour cherry puree was evaluated. Results were compared to sour cherry puree without addition of sugars. Volatiles amount depended on type and amount of sugar. This indicated that the sugars showed an effect on flavour profile. Volatiles with the ethereal, alcoholic flavour note were present in the highest amount in the puree while with the addition of sugars, amount of those volatiles was lower. Sweet, floral volatiles were determined in higher amount when sugars were added in comparison to puree. Addition of sucrose did not have significant impact on the volatiles with sweet flavour note, while with addition of trehalose lower amount of those compounds were determined than in puree. Sweet, fruity and fresh, green volatiles were determined in higher amount in samples with the addition of sugars, especially when trehalose was added. Addition of sugar did not have impact on pungent volatiles.
Introduction
Sour cherries, rich sources of phenolics and anthocyanins[Citation1–Citation3] are well known for their specific flavour. The flavour of sour cherry (Prunus cerasus) is composed of a great number of organic components, including carbonyls, alcohols, esters, acids and terpenes. Benzaldehyde is a character-impact compound, but other compounds, like benzyl alcohol, 2-phenyl ethyl alcohol, eugenol, 2-hexenal, α-ionone and β-ionone, have very important role in formation of sour cherry flavour.[Citation4–Citation6] Chemical composition, macro- and micro-structure of foods and particle size are known to influence volatiles and non-volatiles.[Citation7] Fragility and high post-harvest respiration rate of fruits results in nutritional and microbiological deterioration and limited shelf-life, as well as loss of quality and health benefits. To minimise those negative effects, fruits are converted into different products (like frozen, dried, and canned products) or processed into jams, jellies, and juices for longer storage to satisfy various markets and consumer demands. It is important that fruit properties are transferred from raw material to product. Flavour of fruits and fruit products is the result of the mixture of different volatile compounds. The equilibrium between the different volatile compounds and their concentrations are responsible for overall flavour profile of fruits and their products.[Citation8] The stability of the volatile compounds in the products during preparation as well as during storage are of increasing interest to the food industry. The retention and the stability of the products flavour are in the strong relationship with the consumer’s acceptability of the products, and it is necessary to control it. In order to retain as much as possible of volatile compounds especially the most typical ones for the selected fruit, the huge efforts are made during the fruit product formulation. Sugars are very often used in the foods formulation for the preservation but also in order to achieve desired properties.
In this study, sucrose and alternatively trehalose, two sugars that are chemical isomers, were used. Sucrose is commonly used sugar in the fruit product formulation. Application of trehalose in fruit product formulation is getting more and more attention due to its positive effect on the products quality.[Citation9–Citation16] In addition, some health benefits were related to the trehalose. Studies showed a substantially reduced cariogenic potential[Citation17] compared with sucrose and therefore can be used in the formulation of ‘kind to teeth’ and ‘tooth-friendly’ products but without the laxative effects of other low-cariogenic bulk sweeteners and those products are especially beneficial for children. Recent study showed that trehalose is digested more slowly, and thus has a lower glycemic index, with a lower insulin release than sucrose.[Citation18] Also, trehalose inhibits lipid and protein misfolding and has become subject in studies on neurodegenerative diseases characterised by protein misfolding and aggregate pathology like Alzheimer’s, Parkinson’s and Huntington’s disease, and occulopharyngeal muscular dystrophy.[Citation19] The aim of this study was to evaluate influence of sucrose and trehalose in different amount on volatile profile of sour cherry puree.
Materials and methods
Materials
Sour cherries were obtained from a local market. Benzaldehyde-d6 were purchased from Sigma-Aldrich (Germany). Sucrose was obtained from Kemika (Croatia) and trehalose were obtained from Hayashibara, Nagase group (Japan).
Preparation of sour cherry puree
Prior to disintegration (Braun Multiquick Professional 600 Watt Turbo), sour cherries were washed and the pits were removed. After disintegration of fruits, sugars (sucrose or trehalose) in different amounts (5 or 10 g/100 g) were added and the mixtures were well homogenised. Prepared samples were left for 5 days for stabilizstion prior to evaluation of volatile compounds by gas spectrometry.
SPME extraction
In glass vial, 8 g of sample was weighted, followed by 2.5 mL of saturated solution of CaCl2 and 40 µL of benzaldehyde-d6 solution (22.92 µg/100 mL) as internal standard. The extraction of volatiles was carried out using a solid-phase microextraction (SPME) fiber coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) sorbent (1 cm long, 50/30 µm thickness, StableFlex™, Supelco, USA).[Citation20] The sample vials were conditioned in a temperature-controlled heating module at 40°C for 45 min and agitated at 350 rpm. After extraction, the fiber was removed from the sample and the volatiles were thermally desorbed in the injector port of the GC.
Gas chromatography/mass spectrometry (GC/MS) analysis
Analysis was carried out on a GC 7890A gas chromatograph (Agilent Technologies, CA, USA) equipped with a MPS2 Multipurpose autosampler (Gerstel GmbH, Mülheim van der Ruhr, Germany) and 5975C mass spectrometer (Agilent Technologies). Volatile compounds were desorbed into a GC injector port at 250°C in splitless mode for 2 min. The gas chromatograph was fitted with a ZB-WAX capillary column, 60 m × 0.32 mm i.d. with 1 μm film thickness. Helium was the carrier gas at a flow rate of 1.2 mL/min at 40°C. Oven temperature was programmed as follows: initial temperature 40°C held for 5 min, then 4°C/min to 230°C. The volatile compounds were identified with a mass selective detector (5975C, Agilent Technologies, CA, USA). The detector operated in the m/z range between 30 and 250, ion source and quadrupole temperature were maintained at 250 and 150°C, respectively. Identification of compounds was performed by comparison of their mass spectra with those of available commercial standards. All compounds were also confirmed by matching of their mass spectra with NIST 2.0 mass spectral database (National Institute of Standards and Technology, USA). Results were expressed as μg of volatile per 100 g of sour cherry puree (μg/100 g).
Statistical analysis
Results were expressed as the mean values of five repetitions ± standard deviation. Data of the volatile compounds amount were analysed by analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) with the significance defined at P < 0.05. All statistical analyses were carried out using software program STATISTICA 7 (StatSoft, Inc, USA).
Results and discussion
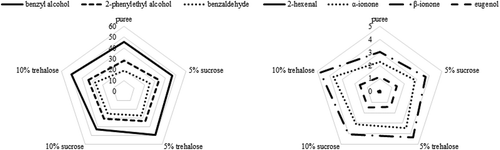
Results of evaluation of volatile compounds amount in sour cherry purees without and with addition of sugars are presented in . Comparison of influence of sugars on key volatile compounds (benzyldehyde, benzyl alcohol, phenyl ethyl alcohol, 2-hexenal, α-ionone, β-ionone and eugenol) is given in . Twelve alcohols were determined in the samples. Control sample had 52. 6 μg/100 g of 1-propanol. Addition of 5% of sucrose had no effect on this volatile, while with addition of 5% of trehalose and 10% of both sugars lower amount was determined. The lowest amount of this volatile was determined when 10% of sucrose was added. With increase of sugar amount decrease of 1-propanol amount occurred. Similar tendency was also observed with 2-methyl-1-propanol, only in this case the lowest amount was determined with addition of trehalose (241.6 μg/100 g and 239.5 μg/100 g for 5% and 10% of trehalose addition, respectively). 1-butanol was determined in control sample in amount of 29.2 μg/100 g. Addition of sucrose caused increase, while addition of trehalose decrease of amount of 1-butanol in comparison to control sample. The highest amount of 3-methyl-1-butanol was determined in control sample, 242.6 μg/100 g. With increase of sugar amount decrease of this volatile amount occurred. In the case of 2-methyl-3-buten-1-ol only sample with the addition of 10% of trehalose had the same amount of this volatile while all other samples had much lower amount. On the amount of 1-pentanol and 1-penten-3-ol there was no effect of added sugars. Addition of sugars had positive effect on 2-heptanol, with trehalose having higher impact. Control sample had 5.33 μg/100 g of 2-heptanol, samples with sucrose addition 6.5 μg/100 g and samples with trehalose addition 7.5 μg/100 g. the same tendency was observed for 1-hexanol. Control sample had 8.518 μg/100 g of 1-hexanol, samples with sucrose addition 9.6 μg/100 g and samples with trehalose addition 10.5 μg/100 g. Sugars addition had positive effect on 1-octanol. With increase of sugar amount, amount of this volatile increased but there was no difference between sucrose and trehalose effect. 2-phenyl ethyl alcohol was determined in amount of 28.61 μg/100 g in the puree while in samples with addition of sugars higher amount was determined. Addition of 5% of sugars caused increase of 2-phenyl ethyl alcohol, around 34 μg/100 g, while with addition of 10% of sucrose lower amount was evaluated (30.77 μg/100 g) and there was no difference in sample with trehalose addition. In puree, benzyl alcohol was determined in amount of 45.43 μg/100 g. Trehalose addition had positive effect on benzyl alcohol, with 5 and 10% of trehalose higher amount was evaluated, 48.84 μg/100 g and 50.42 μg/100 g, respectively in comparison to control sample. 5% of sucrose had no effect on benzyl alcohol while in sample with 10% lower amount (42.38 μg/100 g) was determined in comparison to control sample.
Table 1. Volatile compounds amount (μg/100 g) in sour cherry puree with addition of sucrose and trehalose.
Figure 1. Graphical display of influence of sugars and their amount on key volatile compounds of sour cherry puree.
From ester group, five volatiles were determined. Hexyl acetate was determined in control sample in amount of 0.172 μg/100 g, while with addition of sugars higher amount was determine (0.26 μg/100 g) with no difference between sugars and their amount. Another acetate ester that was determined was 1-butanol-3-methyl-acetate. In control sample, its amount was 1.056 μg/100 g, while with sugar addition higher amount was determined. In this case, higher amount was determined with lower sugar amount, but there was no difference between sugars. Three derivates of butanoate esters were determined. 2-methyl butanoate was the same in control sample and samples with trehalose addition, around 8 μg/100 g, while in samples with sucrose addition higher amount was determined, 8.7 μg/100 g. 2-methyl-buthyl butanoate, was determined in lowest amount in control sample, 0.049 μg/100 g. In samples with sugar addition, higher amount was determined but amount of sugar had no effect on the amount of volatile. Samples with addition of trehalose had the highest amount of 2-methyl-buthyl butanoate, 0.120 μg/100 g. On contrary, 3-methyl-buthyl butanoate, was determined in the highest amount in control sample, 0.565 μg/100 g. in the samples with sugar addition much lower amount was determined, 0.13 μg/100 g and 0.06 μg/100 g for sucrose and trehalose addition, respectively.
Amount of benzaldehyde in the puree was 19.42 μg/100 g. In the samples with sugars addition, higher amount of this volatile was determined. Sugar amount had no effect on amount of benzaldehyde. In samples with sucrose addition amount of benzaldehyde was 24.8 μg/100 g while in samples with trehalose addition higher amount was determined, 27.4 μg/100 g. In the control sample, 2-hexenal was determined in amount of 0.053 μg/100 g. Both sugars had positive effect on 2-hexenal, but there was no difference between used amounts of sugars. In samples with trehalose addition lower amount of 2-hexenal was determined (around 0.08 μg/100 g) than in sample with sucrose addition (0.115 μg/100 g). β-cyclocitral, in control sample was determined in amount of 0.709 μg/100 g. in all samples with sugar addition higher amount of this volatile was determined, around 1 μg/100 g. Amount of o-cymene in puree was 1.288 μg/100 g while samples with sugars addition had higher amount than control sample. With addition of 5% of sugars, higher amount was determined when trehalose was added, 2.835 μg/100 g, while addition of 10% of sugars did not cause difference in amount of this volatile. In the case of sucrose addition with increase of its amount higher retention was observed, while in the case of trehalose reverse effect was obtained. α-ionone was determined in amount of 2.27 μg/100 g in control sample. With addition of sugars positive effect was observed with trehalose having higher positive influence (3.44 μg/100 g and 3.70 μg/100 g for 5 and 10% of trehalose addition). In both cases with increase of sugars amount, higher amount of α-ionone was determined. Similar results were obtained for β-ionone. In control sample β-ionone was determined in amount of 3.01 μg/100 g while with addition of sugars positive effect was observed. Trehalose had higher positive influence (4.31 μg/100 g and 4.76 μg/100 g for 5 and 10% of trehalose addition) than sucrose (3.69 μg/100 g and 4.00 μg/100 g for 5 and 10% of sucrose addition). Eugenol in control sample was determined in amount of 1.119 μg/100 g. Addition of sugars caused increase of this volatile compound. With addition of 5% of sugars, higher amount was determined when trehalose was added (1.448 μg/100 g) while with addition of 10% of sugars difference between sucrose and trehalose was not observed (1.5 μg/100 g).
Control sample had 0.093 μg/100 g of 2-decanone, while addition of sugars caused increase of amount of this volatile. Samples with addition of sugars had lower amount (from 12.12 μg/100 g to 14.56 μg/100 g) of acetoin than control sample (17.10 μg/100 g). Comparing sucrose and trehalose, lower amount was determined when trehalose was added.
In control sample diacetyl was determined in 6.52 μg/100 g. Addition of 5% of sucrose had no effect on diacetyl, while with addition of 5% of trehalose (6.0 μg/100 g) and 10% of sucrose and trehalose lower amount (5.91 μg/100 g and 5.70 μg/100 g, respectively) was determined. In samples with addition of sugars, geranic oxide was determined in higher amount than in the control sample (1.459 μg/100 g). Especially high amount was determined in samples with trehalose addition (around 5.6 μg/100 g). In the case of sucrose, with increase of amount of added sugar increase in amount of this volatile occurred (2.740 μg/100 g and 4.275 μg/100 g for 5 and 10% of sucrose addition).
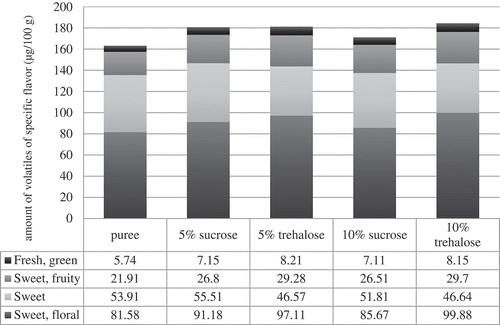
Type of sugar as well as their amount had influence on volatile compounds () and flavour profile, as well as on specific flavour note (). Volatiles determined in the sour cherry puree samples can be divided according the flavour note into six groups: etherial, alcoholic; sweet, floral; sweet; sweet, fruity; fresh, green and pungent. Volatiles with the ethereal, alcoholic flavour note are present in the highest amount in the puree. With the addition of sugars, amount of those volatiles decreased, especially with addition of 5 g/100 g of trehalose and 10 g/100 g of both sugars (20%). Sweet, floral volatiles were determined in the samples with sucrose addition in higher amount (from 5 to 10%), and in trehalose samples around 17% in comparison to the puree. Addition of sucrose didn’t have significant impact on the volatiles with sweet flavour note, while with addition of trehalose lower amount of those compounds were determined (around 13%) than in control sample. Sweet, fruity volatiles were determined in higher amount in samples with the addition of sugars. With addition of sucrose, their amount was higher for 18% and with addition of trehalose for 25%. Fresh, green volatiles were also determined in higher amount when sugars were added. With addition of sucrose, their amount was higher for 19% and with addition of trehalose for 30%. Addition of sugar didn’t have impact on pungent volatiles.
Figure 2. Amount of volatiles of specific flavour note in sour cherry puree without and with addition of sugars.
Effect of sugars addition on volatile compounds in different fruit products was investigated through several studies. Generally, positive trehalose effect on volatiles was observed for different dehydrates puree samples like strawberry, apricot and pear.[Citation10–Citation12] Zlatić et al.[Citation16] reported that type of sugar and their amount had influence on key volatiles in freeze-dried sour cherry puree. They investigated influence of sucrose, maltose and trehalose addition (5, 10 or 20 g/100 g) on benzaldehyde, benzyl alcohol and 2-hexenal. The highest amount of benzaldehyde and 2-hexenal was determined in samples with trehalose addition. However, sugars addition didn’t have positive effect on the benzyl alcohol. Authors concluded that among investigated disaccharides, samples with addition of sucrose had the lowest amount of examined volatile compounds. Positive influence of sugars, especially trehalose addition on benzaldehyde and 2-hexenal was also observed in freeze-dried strawberry puree and apricot puree.[Citation10,Citation11] In the study of Galmarini et al.[Citation9], who investigated volatiles of freeze-dried strawberry puree, majority of volatiles were determined in higher amount when trehalose was added (30%). They also, reported that there were some exceptions and some volatiles, such as nerodilol, octyl butyrate, γ-dodecalactone, were determined in higher amount in the samples with the sucrose addition.[Citation9] Strawberry cream fillings with the trehalose addition had higher amount of fruity esters, however its positive influence was not reported for γ-decalactone and furaneol.[Citation13,Citation14] Also, the same group reported that with increase of the trehalose addition proportional increase of the amount of fruity esters wasn’t observed, as it was the case with results reported in this study and study of Zlatić et al..[Citation16]
Water has a very specific and important role as a solvent and a reactant in many chemical reactions in foods. When water activity is high, reactions limited by diffusion are enhanced. Those reactions together with enzymatic activity, oxidation, and molecular mobility, which are all consequence of more available water, are cause of degradation of sensitive compounds.[Citation21] Investigated sugars, sucrose and trehalose, are chemical isomers but as it is evident from results of volatiles, their behavior in complex matrix can be different. Even if these sugars are chemical isomers they are showing different behavior in much simpler media like water. Sugar molecules change water dynamics. Trehalose has superior effect on “destructing” the network of water and in this way on slowing down its dynamics.[Citation22] This change of water dynamics could have great effect on volatile compounds. Determination of hydration number revealed that trehalose binds to a larger number of water molecules in comparison to sucrose and consequence of this behavior is that trehalose affects water structure in higher extent. Cluster analyses showed that investigated disaccharides have different tendency to form clusters and trehalose is able to form larger clusters than sucrose. Based on hydration number, the radius of gyration, the glycosidic dihedral angles and cluster formation, Lerbret at al.[Citation23] suggested that trehalose-water mixtures would be more homogeneous than the sucrose water solutions. Studies showed that fruit volatiles retention due to addition of sugars depends on physical and chemical properties of sugars and volatile flavour compound, and steric and other characteristics, which are connected to different diffusion and sorption ability inside the microstructure of fruit product matrix. Sugars have different diffusion coefficients in the water, but they also change diffusion coefficient of water[Citation24] thus they are probably responsible for change of diffusion coefficient of volatile compounds and in this way on their retention in fruit product matrix. In addition, trehalose can form a stable intramolecular complex with unsaturated compounds that possess the cis-type olefinic double bond.[Citation25,Citation26] Other compounds with similar structure like benzene and p-cresol can bind to trehalose in aqueous solutions but not with other disaccharides.[Citation27] These phenomena could be responsible for stabilising effect of trehalose on volatiles that are having similar structure since it is possible that aromatic ring of volatiles approaches to the dehydrated, hydrophobic pocket of trehalose forming the complex.[Citation27] Change of water dynamics and diffusion of volatiles as well as formation of weak complexes between sugars and volatiles could be the reason for retention of volatiles due to addition of sugars, especially trehalose.
Conclusion
Sour cherry purees were prepared with addition of different amounts of sucrose and trehalose in order to evaluate their effect on volatile compounds. Type of sugar and its amount had an effect on sour cherry puree volatiles. Compering trehalose and sucrose, trehalose had higher positive effect on the key volatile compounds of sour cherries and all together on volatiles with desired flavour note. Both investigated sugars are chemical isomers but probably their structure and behavior influenced water dynamics in different way causing higher impact of trehalose on volatiles.
Acknowledgements
This work has been supported by the Croatian Science Foundation under the project Trehalose: fruit product quality improvement project (6949). We are grateful to Hayashibara, Nagase Group, Japan for generous donation of trehalose.
References
- Siddiq, M.; Iezzoni, A.; Khan, A.; Breen, P.; Sebolt, A. M.; Dolan, K. D.; Ravi, R. Characterization of New Tart Cherry (Prunus Cerasus L.): Selections Based on Fruit Quality, Total Anthocyanins, and Antioxidant Capacity. Int. J. Food Properties. 2011, 14, 471–480.
- Taghadomi-Saberi, S.; Omid, M.; Emam-Djomeh, Z.; Ahmadi, H. Development of an Intelligent System to Determine Sour Cherry’s Antioxidant Activity and Anthocyanin Content during Ripening. Int. J. Food Properties. 2014, 14, 1169–1181.
- Kopjar, M.; Oršolić, M.; Piližota, V. Anthocyanins, Phenols and Antioxidant Activity of Sour Cherry Puree Extracts and Their Stability during Storage. Int. J. Food Properties. 2014, 17, 1393–1405.
- Schmid, W.; Grosch, W. Quantitative Analyse Flüchtiger Aromastoffe Mit Hohen Aromawerten in Sauerkirschen (Prunus Cerasus L.), Süßkirschen (Prunus Avium L.) Und Kirschkonfitüren. Zeitschrift Für Lebensmittel-Untershuncung Und Forschung. 1986, 183, 39–44.
- Poll, L.; Barixtofte, M. Influence of Harvest Year and Harvest Time on Soluble Solids, Titrateable Acid, Anthocyanin Content and Aroma Components in Sour Cherry (Prunus Cerasus L. Cv. “Stevnsbær”). Eur. Food Res. Technol. 2003, 216, 212–216.
- Xiao, Z.-B.; Zhang, N.; Niu, Y.-W.; Fena, T.; Tian, H.-X.; Zhu, J.-C.; Yu, H.-Y. Multivariate Classification of Cherry Wines Based on Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry of Volatile Compounds. Int. J. Food Properties. 2015, 18, 1272–1287.
- Frank, D. C.; Eyres, G. T.; Piyasiri, U.; Delahunty, C. M. Effect of Food Matrix Structure and Composition on Aroma Release during Oral Processing Using in Vivo Monitoring. Flavour Fragr. J. 2012, 27, 433–444.
- Seuvre, A.-M.; Philippe, E.; Rochard, S.; Voilley, A. Retention of Aroma Compounds in Food Matrices of Similar Rheological Behaviour and Different Compositions. Food Chem. 2006, 96, 104–114.
- Galmarini, M. V.; Van Baren, C.; Zamora, M. C.; Chirife, J.; Di Leo Lira, P. Bandoni A. Impact of Trehalose, Sucrose And/Or Maltodextrin Addition on Aroma Retention in Freeze Dried Strawberry Puree. Int. J. Food Sci. Technol. 2011, 46, 1337–1345.
- Komes, D.; Lovrić, T.; Ganić, K. K.; Gracin, L. Study of Trehalose Addition on Aroma Retention in Dehydrated Strawberry Puree. Food Technol. Biotechnol. 2003, 41, 111–119.
- Komes, D.; Lovrić, T.; Ganić, K. K.; Kljusurić, J. G.; Banović, M. Trehalose Improves Flavour Retention in Dehydrated Apricot Puree. Int. J. Food Sci. Technol. 2005, 40, 425–435.
- Komes, D.; Lovrić, T.; Kovačević Ganić, K. Aroma of Dehydrated Pear Products. LWT Food Sci. Technol. 2007, 40, 1578–1586.
- Kopjar, M.; Piližota, V.; Hribar, J.; Simčič, M.; Zlatič, E.; Tiban, N. N. Influence of Trehalose Addition and Storage Conditions on the Quality of Strawberry Cream Filling. J. Food Eng. 2008, 87, 341–350.
- Kopjar, M.; Hribar, J.; Simčič, M.; Zlatić, E.; Tomaž, P.; Piližota, V. Effect of Trehalose Addition on Volatiles Responsible for Strawberry Aroma. Nat. Prod. Communicastions. 2013, 8, 1767–1770.
- Lončarić, A.; Pichler, A.; Trtinjak, I.; Piližota, V.; Kopjar, M. Phenolics and Antioxidant Activity of Freeze-Dried Sour Cherry Puree with Addition of Disaccharides. Lwt. 2016, 73, 391–396.
- Zlatić, E.; Pichler, A.; Lončarić, A.; Vidrih, R.; Požrl, T.; Hribar, J.; Piližota, V.; Kopjar, M. Volatile Compounds of Freeze-Dried Sour Cherry Puree Affected by Addition of Sugars. Int. J. Food Properties. 2017, 20(S1), S449–S456.
- Neta, T.; Takada, K.; Hirasawa, M. Low-Cariogenicity of Trehalose as a Substrate. J. Dent. 2000, 28, 571–576.
- Van Can, J. G. P.; Van Loon, L. J. C.; Brouns, F.; Blaak, E. E. Reduced Glycaemic and Insulinaemic Responses following Trehalose and Isomaltulose Ingestion: Implications for Postprandial Substrate Use in Impaired Glucose-Tolerant Subjects. Br. J. Nutr. 2012, 108, 1210–1217.
- Patra, F.; Tomar, S. K.; Arora, S. Technological and Functional Applications of Low-Calorie Sweeteners from Lactic Acid Bacteria. J. Food Sci. 2009, 74, R16–23.
- Howerd, K. L.; Mike, J. H.; Riesen, R. Validation of a Solid-Phase Microextraction Method for Headspace Analysis of Wine Aroma Components. Am. J. Enol. Vitic. 2005, 56, 37–45.
- Syamaladevi, R. M.; Sablani, S. S.; Tang, J.; Powers, J.; Swanson, B. G. Stability of Anthocyanins in Frozen and Freeze-Dried Raspberries during Long-Term Storage: In Relation to Glass Transition. J. Food Sci. 2011, 76, 414–421.
- Bordat, P.; Lerbret, A.; Demaret, J.-P.; Affouard, F.; Descamps, M. Comparative Study of Trehalose, Sucrose and Maltose in Water Solutions by Molecular Modelling. Europhys. Lett. 2004, 65, 41–47.
- Lerbret, A.; Bordat, P.; Affouard, F.; Descamps, M.; Migliardo, F. How Homogeneous are the Trehalose, Maltose, and Sucrose Water Solutions? an Insight from Molecular Dynamics Simulations. J. Phys. Chem. B. 2005, 109, 11046–11057.
- Engelsena, S. B.; Monteiro, C.; De Penhoat, C. H.; Pérez, S. The Diluted Aqueous Solvation of Carbohydrates as Inferred from Molecular Dynamics Simulations and NMR Spectroscopy. Biophys. Chem. 2001, 93, 103–127.
- Oku, K.; Watanabe, H.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tujisaka, Y.; Komori, M.; Inoue, Y.; Sakurai, M. NMR and Quantum Chemical Study on the OH…pi And CH…O Interactions between Trehalose and Unsaturated Fatty Acids: Implication for the Mechanism of Antioxidant Function of Trehalose. J. Am. Chem. Soc. 2003, 125, 12739–12748.
- Oku, K.; Kurose, M.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tujisaka, Y.; Okabe, A.; Sakurai, M. Combined NMR and Quantum Chemical Studies on the Interaction between Trehalose and Dienes Relevant to the Antioxidant Function of Trehalose. J. Phys. Chem. B. 2005, 109, 3032–3040.
- Sakakura, K.; Okabe, A.; Oku, K.; Sakurai, M. Experimental and Theoretical Study on the Intermolecular Complex Formation between Trehalose and Benzene Compounds in Aqueous Solution. J. Phys. Chem. B. 2011, 115, 9823–9830.