ABSTRACT
This article reports novel and valuable data about three red (Cabernet Sauvignon, Frankovka and Merlot varieties), one rosé (Muscat Hamburg) and three white wines (Italian Riesling, Chardonnay and Sila), together with their related products (grape juices and young wines) produced in Fruška Gora vineyards, Serbia, concerning their in-depth polyphenolic profile and biological activities (antioxidant and neuroprotective). LC-MS/MS analysis of 47 phenolics showed that the phenolic acids, particularly ellagic (up to 8.99 mg/L, Cabernet Sauvignon) and caffeic acids (up to 2.83 mg/L, Frankovka) were generally more abundant in red wines. Also, the content of catechin and epicatechin (0.32–7.70 and 0.4–15.5 mg/L) as the leading flavonoids, malvidin 3-O-glucoside as the dominant anthocyanin (2.7–160 mg/L) or stilbene resveratrol (0.06–2.42 mg/L), were also higher in the red grape products. Overall antioxidant dominance of the red grape products, Merlot variety being the main one, was confirmed through several assays (DPPH• and •NO scavenging ability, reducing potential and inhibition of lipid peroxidation). In addition, grape juices and young wines, which are a good source of dietary natural antioxidants, were also evidenced. Furthermore, the optimised in vitro acetylcholinesterase inhibition assay was applied, indicating the grape juices and red wines as the most promising neuroprotective agents.
Abbreviations: AAE: ascorbic acid equivalents; AChE: acetylcholinesterase; BHT: butylated hydroxytoluene; DPPH: 2,2-diphenyl-1-picrylhydrazyl; dw: dry weight; FA: fatty acids; FRAP: Ferric Reducing Antioxidant Power; LP: lipid peroxidation; MDA: malondialdehyde; SRM: selected reactions monitoring; PCA: principal component analysis; PG: propyl gallate; TBA: 2-thiobarbituric acid; TPTZ: 2,4,6-tripyridil-s-triazine.
Introduction
There has always been an undeniable link between the diet and wine consumption, strengthened both by pleasure and medical benefits of wine—since it is one of the oldest antiseptics, painkillers or remedies for treatment of gastrointestinal disorders. Nowadays, health benefits of wine, the red one in particular, are recognised worldwide by the French Paradox. It states that moderate daily consumption of red wines contributes to the lower incidence of coronary heart diseases in France - despite the high levels of saturated fats in the traditional French diet.[Citation1] Even though some criticism on epidemiological studies related to this phenomenon exist, numerous reports confirm the positive effect of grapes, grape juices and wines in the treatment of various disorders like cardiovascular and neurodegenerative diseases, ischemic stroke, cancer and, even, aging.[Citation2–Citation5] It is considered that the antioxidant activity of the grapes and grape products is principally responsible for the listed health-protective benefits, while the polyphenolic compounds are marked as major carriers of this activity.[Citation6–Citation8] Polyphenolics are an abundant class of natural products which express a broad range of biological activities including a high antioxidant capacity. They influence the oxidation processes engaged in the development of severe diseases, including the aforementioned ones, primarily by preventing the damaging of biological macromolecules such as proteins, lipids and nucleic acids.[Citation9]
Polyphenolic profile of grapes, and consequently of wines, is influenced by several factors: grape variety and maturity, environmental factors (soil, climate), agricultural practice, wine-making technology, wine aging conditions, etc.[Citation10] Also, distribution of different polyphenolics varies in different grape berry parts. For example, anthocyanins and proanthocyanidins are found in skin, seeds are rich in proanthocyanidins, while the pulp contains mostly the phenolic acids. Additionally, there is a crucial difference between the wine making process of red and white wines. During the red wine production, the must (grape juice) is fermented together with the grape skin and other grape parts, while the white wine is made by the fermentation of only the grape juice, without the berry skin. Consequently, tannins and anthocyanins are the major polyphenolics in red wines, whilst hydroxycinnamic acids are the most abundant in addition to lower presence of 3-hydroxyflavones in white wines.[Citation11] From the point of view of enology, these compounds are extremely important since they are responsible for the organoleptic characteristics of wines, such as colour, mouth-feel, astringency and bitterness. Thus, knowledge of the polyphenolic profile of certain wines could help in predicting their sensory properties and oxidative stability.[Citation7] Furthermore, it was found that a polyphenolic profile of wine could be used for its authentication i.e. the classification of wines of a certain variety, geographical region, manufacturing process and vintage.[Citation12–Citation14]
The region of Fruška Gora, the mountain in the northern part of the Republic of Serbia, is famous for its old winemaking tradition. This practice was established in the third century, when the Roman emperor Probus planted the first vines and initiated viticulture in this region. Interestingly, some experts believe that the famous variety of Italian Riesling (syn. Riesling Italico, Olaszrizling, Laški Rizling, Graševina) primarily originated from Fruška Gora mountain. Today, more than a half of local vineyards cultivate this grapevine along with other native varieties such as Vranac or Portugieser, and autochthonous hybrid varieties (Sila, Neoplanta, Petra, Probus, Slankamenka, etc.). Wines from Fruška Gora vineyards are traditionally well-recognised by the local consumers. In addition, their popularity is rising throughout Europe, as well as in the United States.[Citation15] However, to the best of our knowledge, there have been no reports on detailed phytochemical profile or biological activities of the wines from Fruška Gora region, except in a recent publication[Citation16] which deals with phenolic composition and alterations in the main compounds during maceration of some red wines (Probus, Rumenika and Frankovka). In order to support enological qualities, commercial value and health benefits of the Fruška Gora wines, the quality of three red wines (from Cabernet Sauvignon, Frankovka and Merlot varieties), one rosé wine (from Muscat Hamburg variety), and three white wines (from Italian Riesling, Chardonnay and Sila varieties), all grown in Fruška Gora vineyards (Serbia), were evaluated herein by in-depth polyphenolic profile characterisation and determination of biological activities, such as the antioxidant and neuroprotective ones. Also, the related grape products like grape juices (juice obtained by immediate pressing of crushed grapes) and young wines (first wine obtained after fermentation), which are very popular local beverages especially during the harvest season, were also included in this study with aim to valorise their characteristics. An LC-MS/MS technique was applied to evaluate the quantitative content of 47 phenolics, including 16 phenolic acids, 26 flavonoids, three coumarins and two lignans, followed by HPLC-UV/VIS technique for detection of five anthocyanins. In order to assess a potential impact on oxidative stress or neurological disorders, several in vitro assays were performed. The antioxidant potential of the samples was determined using tests related to free radical (DPPH•) and reactive nitrogen species (•NO) scavenging ability, in addition to the potential of lipid peroxidation (LP) inhibition and reducing power measured by Ferric Reducing Antioxidant Power (FRAP) assay. On the other hand, the neuroprotective effect was estimated through a potential of acetylcholinesterase (AChE) inhibition, the enzyme deeply involved in pathogenesis of neurological diseases, such as Alzheimer’s.
Material and methods
Chemicals and reagents
All standards of phenolic compounds were purchased from Sigma-Aldrich Chem (Steinheim, Germany), Fluka Chemie GmbH (Buchs, Switzerland) or from ChromaDex (Santa Ana, USA). Anthocyanins, malvidin 3-O-glucoside (oenin chloride), cyanin 3-O-glucoside chloride (kuromanine chloride) and delphinidin 3-O-glucoside chloride (delphinin, myrtillin) were obtained from AppliChem, petunidin 3-O-glucoside chloride and peonidin 3-O-glucoside chloride from Phytolab. Methanol and acetonitrile HPLC grade were from Promochem LGC (Wesel, Germany), formic acid from Lach-Ner (Neratovice, Czech Republic), and 35% hydrochloric acid from Roth (Karlsruhe, Germany).
The following reagents were purchased from Sigma-Aldrich Chem, Steinheim, Germany: acetylcholinesterase, acetylthiocholine iodide, bovine serum albumin, butylated hydroxytoluene (BHT), 5,5-dithiobis-2-nitrobenzoic acid, propyl gallate (PG). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2-thiobarbituric acid (TBA) and sulfanilamide were obtained from Fluka Chemie GmbH (Buchs, Switzerland). Trichloroacetic acid was purchased from Lach-Ner s.r.o. (Neratovice, Czech Republic), and sodium nitroprusside from Reanal (Budapest, Hungary). Ascorbic acid, N-(1-naphthyl)ethylenediamine dihydrochloride, 2,4,6-tripyridil-s-triazine (TPTZ) were acquired from Merck, Darmstadt, Germany. Tween-80 was obtained from J. T. Baker (Deventer, Holland).
Wine samples
The study included eight related samples of grape juices, young wines and wines from vintage 2014, provided by distinguished viticulturists and wine producers in the region of Fruška Gora mountain (Serbia). Grape juices were collected immediately after pressing the crushed grapes (must), while the samples labeled as ”young wine” were obtained after pressing the fermented must (for red wines), or filtration of the fermented juice (for white and rosé wines). These five local wineries participated: Podrum Bajilo with products of Cabernet Sauvignon, Muscat Hamburg, Sila and Italian Riesling varieties; Vinum with Frankovka; Vinarija Šukac with Merlot, Vinarija Došen with Chardonnnay, and Vinarija Agner with Italian Riesling. In order to define the concentration of each sample, a solvent was evaporated in vacuo at 45°C and crude residue was measured. In all of the analyses and assays applied, the samples were filtered only through the membrane filter (0.45 μm; Sartorius, USA) and used directly.
Polyphenolic profile
LC–MS/MS analysis of the selected polyphenolics
Determination of the selected phenolics in the examined wine samples was carried out according to the previously published procedure,[Citation17] expanded for three phenolics of interest to our analysis (ellagic acid, morin and resveratrol). Briefly, samples and 47 standards (prepared in serial 1:1 dilutions, ranging from 1.53 ng/mL to 25.0 × 103 ng/mL, dissolved in 50% aqueous methanol) were analysed using Agilent Technologies 1200 Series HPLC coupled with Agilent Technologies 6410A QqQ mass spectrometer with electrospray ionisation source and controlled by Agilent Technologies MassHunter Workstation software (ver. B.03.01). Injection volume was 5 μL. Separation was performed using Zorbax Eclipse XDB–C18 (Agilent Technologies) column, 50 mm × 4.6 mm, 1.8 µm, held at 50°C. Mobile phase, consisting of 0.05% aqueous formic acid (phase A) and methanol (B) was delivered at flow rate of 1 mL/min in gradient mode (0 min 30% B, 6 min 70% B, 9 min 100% B, 12 min 100% B, post time 3 min). Ion source parameters were: nebulisation gas pressure 40 psi, drying gas flow 9 L/min and temperature 350°C, capillary voltage 4000 V. All the compounds were detected in negative mode, using dynamic SRM with optimised compound-specific parameters (retention time, precursor ion, product ion, fragmentor voltage, collision voltage) as previously published, in addition to ellagic acid (2.25 min, m/z 301, m/z 301, 152 V, 0 V, respectively), morin (2.94 min, m/z 301, m/z 149, 120 V, 29 V) and resveratrol (2.28 min, m/z 227, m/z 185, 130 V, 15 V). Concentrations of standard compounds in extracts were determined from the peak areas by using the regression equation obtained from the calibration curves.
HPLC analysis of the selected anthocyanins
Stock solutions of individual anthocyanins (malvidin 3-O-glucoside, cyanin 3-O-glucoside delphinidin 3-O-glucoside, petunidin 3-O-glucoside and peonidin 3-O-glucoside) were prepared in methanol acidified with HCl to 1%. Calibration standards were prepared as mixtures of all the five components diluted with initial mobile phase, in concentration range 1–100 mg/L (R2 above 0.998 for all anthocyanins). Wine samples were prepared by filtration through membrane filter (0.45 μm; Sartorius, USA). HPLC analysis was carried out using Agilent 1100 series liquid chromatograph (USA), consisting of quaternary gradient pump, autosampler with injection system (10–200 μL), column heater, UV-VIS detector and software package.
Chromatographic separation of five major anthocyanins in 100 μL of sample was achieved on a reverse phase Poroshell 120 EC-C18 column (4.6 × 100 mm, 2.7 μm; Agilent, USA) heated at 40°C with gradient elution by water/formic acid/acetonitrile mixtures (A – 87:10:3, B - 40:10:50; flow rate 0.8 mL/min; run time 14 min) and followed by detection at 518 nm (method based on Compendium of International Methods of Analysis – OIV[Citation18]). Quantification of the compounds of interest was performed using the calibration curves obtained from injections of calibration solutions.
Antioxidant activity
Neutralisation of DPPH
Samples of grape juice, young wine and wine were tested for the scavenging effect on DPPH• according to the previously published procedure.[Citation19] As a positive control synthetic antioxidants BHT and PG were used. Ten microliters of the sample examined, previously filtered, in series of different concentrations (0.09–232 mg/mL, dilution of original samples with distilled water), was added to 100 µL of 90 µmol/L DPPH solution in methanol and the mixture was diluted with 190 µL of methanol. In control, the exact amount of extract was substituted with the solvent (ethanol) and in blank probe, only methanol (290 µL) and sample (10 µL) were mixed. After 1 hour, measurements of absorbance were done at 515 nm.
NO scavenger capacity
Test of •NO scavenging capacity was performed by the method previously described.[Citation19] The reaction mixture containing sodium nitroprusside (10 mmol/L, 75 µL), phosphate buffer, pH 7.4 (80 µL) and the sample (50 µL, concentration ranging from 0.12 to 232 mg/mL, substituted with solvent in control) was incubated at 25°C for 60 min. Fifty microliters of sample and 150 µL of buffer were added in the blank probe, while the sample was substituted with the solvent in blank probe for control. After incubation, 150 µL of the solution prepared by mixing equal amounts of sulfanilamide (2% in 4% phosphoric acid) and N-(1-naphthyl) ethylenediamine dihydrochloride (0.2%) was added to the reaction mixture and allowed to stand for 3 min. The absorbance of these solutions was measured at 546 nm against the appropriate blanks. As a positive control, synthetic antioxidant PG was used.
FRAP assay
To evaluate the reducing power of samples, FRAP assay was undertaken.[Citation19] Samples were prepared in three different concentrations, ranging from 0.31 to 270 mg/mL, while the ascorbic acid ranging from 1.25 to 160 µg/mL was used to create a standard curve. FRAP reagent was prepared by mixing 10 mmol/L TPTZ in 40 mmol/L HCl, 0.02 mol/L FeCl3 and acetate buffer pH 3.6 in ratio 1:1:10, respectively. Following the addition of the sample or ascorbic acid (10 µL) to 290 µL of FRAP reagent (substituted with distilled water in blank probe), absorbance at 593 nm was determined after 6 min. All samples were made in triplicate and mean values of reducing power were expressed as mg of ascorbic acid equivalents (AAE) per g of dry weight (dw), calculated according to the standard calibration curve. As a positive control synthetic antioxidants BHT and PG were used.
Lipid peroxidation
The extent of Fe2+/ascorbate induced LP was determined by TBA assay according to the previous research,[Citation20] using polyunsaturated fatty acids (FA) as a substrate, obtained from linseed by Soxhlet extraction (69.7% linolenic acid, 13.5% linoleic acid, as determined by GC–MS). FA were added to phosphate buffer pH 7.4 in presence of 0.25% Tween-80 to obtain 0.035% emulsion, and sonicated for 1 hour. This suspension (600 μL) was mixed with 10 μL of FeSO4 (1.83 mmol/L), 10 μL of ascorbic acid (34.9 μmol/L) and 250 μL of the sample (concentration ranging from 0.13 to 232 mg/mL, diluted in distilled water). In control, samples were substituted with the solvent. Phosphate buffer (620 μL) and 250 μL of sample were used as blank probe. After incubation at 37°C for 1 hour, 37.2 mg/mL EDTA solution (40 μL) was added to all samples followed by 400 μL of aqueous mixture containing TBA (3.75 mg/mL), HClO4 (1.3%) and trichloroacetic acid (0.15 g/mL). Following the heating at 100°C for 15 min, the cooled mixtures were centrifuged at 1600g for 15 min and absorbance of the colour product malondialdehyde (MDA) was measured at 532 nm. As a positive control synthetic antioxidants BHT and PG were used.
Anti-acetylcholinesterase activity
Inhibitory potential of the samples towards AChE was evaluated using the modified Ellman’s method[Citation21] adapted for 96-well plates. During the enzyme hydrolysis of the substrate acetylthiocholine, thiocholine is formed and can react with 5,5-dithiobis-2-nitrobenzoic acid (DTNB, Ellman’s reagent), producing the yellow 5-thio-2-nitrobenzoate that is spectrophotometrically detected at 412 nm. Briefly, the mixture containing 110 μL of 20 mmol/L Tris-HCl buffer pH 8, 20 μL of AChE (0.5 U/mL, dissolved in 20 mmol/L Tris-HCl buffer pH 7.5 containing 0.01% BSA) and 10 μL of test samples in series of different concentrations (dissolved in 20 mmol/L Tris-HCl buffer pH 8) was incubated for 15 min at 37 ºC. After incubation, 40 μL of 3 mmol/L DTNB (dissolved in 50 mmol/L Tris-HCl buffer pH 8, with added 0.1 mol/L NaCl, 0.02 mol/L MgCl2 × 6H2O) and 20 μL of 15 mmol/L acetylthiocholine (dissolved in distilled water) were added in the reaction mixture. In the control, the exact amount of the sample was substituted with the appropriate solvent (juice was substituted with 20 mmol/L Tris-HCl buffer pH 8, young wine with 10% ethanol in 20 mmol/L Tris-HCl buffer pH 8 and wine with 15% ethanol in 20 mmol/L Tris-HCl buffer pH 8). In the blank probe, 20 mmol/L of Tris-HCl buffer pH 7.5 was added instead of the enzyme. The absorbance of solutions was measured at 412 nm after 4 min. Galantamine was used as a positive control.
Statistical analysis
The percent of inhibition achieved by different concentration of extracts was calculated by the following equation in the antioxidant assays performed: I(%) = (A0–A)/A0 × 100, where A0 was the absorbance of the control reaction and A was the absorbance of the examined samples, corrected for the value of blank probe. The corresponding inhibition–concentration curves were drawn using the Origin software version 8.0, and IC50 values (concentration of extract that neutralised DPPH• and •NO, reduced MDA or thiocholine formation by 50%) were determined. For each of the assay and extract composition determinations, all of the results were expressed as mean ± SD of three different trials (all samples and the control were done in triplicate in all of the assays applied). Throughout the study, the significant statistical difference between the results was determined by using the Student’s two-tailed unpaired t-test. The statistical significance was set at p ≤ 0.05. The Principal Component Analysis (PCA) was performed by Past, version 3.[Citation22]
Results and discussion
Polyphenolic profile
In order to thoroughly evaluate polyphenolic profile of the investigated samples, the quantitative analysis of 47 polyphenolic compounds was performed using the LC-MS/MS technique, while the quantification of five anthocyanin glucosides was done by HPLC-UV/VIS technique. Concentrations of the detected phenolic acids (), flavonoids, coumarins and stilbenoids () and anthocyanin glucosides () are presented in the corresponding tables.
Table 1. Concentrations* of detected§ phenolic acids in grape juice, young wine and wine.
Table 2. Concentrations* of detected§ flavonoids, coumarin and stilben in grape juice, young wine and wine.
Table 3. Concentrations* of anthocyanins in grape juice, young wine and wine (red and rose wine samples).
The investigated derivatives of hydroxybenzoic and hydroxycinnamic acids were generally more abundant in the samples of wines, particularly in the red ones (). Among the 16 of the investigated phenolic acids, the ellagic and caffeic acids were present in significant amounts in the samples examined, where the Cabernet Sauvignon wine singled out as the richest source of the listed acids, but some others as well: 2,5-dihydroxybenzoic (gentisic), vanillic and syringic acids. Noticeable amounts of the ellagic acid were particularly determined in the Cabernet Sauvignon wine (8.99 mg/L), implicating that this pharmacologically active compound could be, at least partially, responsible for the wine’s health benefits.[Citation23] Interestingly, among the white wines, a noticeable amount of 2,5-dihydroxybenzoic acid, besides the leading caffeic acid, was found in the autochthon hybrid called Sila, suggesting that this compound could be one of the potentially defining characteristics of this variety. Other general conclusions regarding the content of phenolic acids could also be drawn: absence of the vanillic acid in all of the white grape products, higher content of the caffeic acid in wine than in the juices (possibly the consequence of caftaric acid degradation) and the higher content of syringic acid in the red than in the white grape products (possibly the consequence of malvidin degradation).
The quantities of phenolic acids determined in this work slightly differ from those of other samples, such as some samples of grape juices or wines from Spain.[Citation24] Namely, the concentration of p-hydroxybenzoic acid in the grape juices presented ranged from 0.11 to 0.30 mg/L, while it was reported to be over 3.61 mg/L in grape juices from Spain. Furthermore, the content of caffeic acid in white wines was found to be between 1.24 and 4.19 mg/L, while the average content in the Spanish samples was 0.61 mg/L.[Citation24] If we compare the results of a particular variety, such as Merlot or Cabernet Sauvignon, with the data previously published,[Citation8,Citation25] the determined amounts of phenolic acids remain different, pointing out that the grape variety is not solely responsible for the polyphenolic profile of a wine.
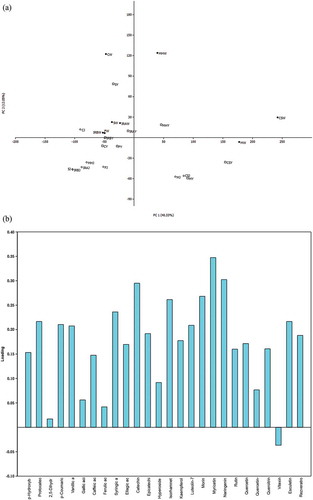
Similarly to phenolic acids, flavonoid, coumarin and stilbene compounds were more abundant in the samples and by-products of the red wines (). Among the flavonoids detected, the most dominant in the red grape products were the flavanols catechin and epicatechin, followed by the flavonol myricetin. However, the differences in-between the red varieties were obvious. Particularly, while epicatechin was dominant in the Cabernet Sauvignon related juice, young wine and wine (6.45, 15.5 and 10.8 mg/L, respectively), and completely missing in the Merlot samples, catechin was the primary compound in all of the Merlot samples (7.38, 7.70 and 6.84 mg/L, respectively). Furthermore, due to a considerably lower content of these compounds in the Frankovka samples, this variety could be separated from the red wine group based on its flavonoid profile. In addition, epicatechin and catechin were also found to be a distinctive quality of Muscat Hamburg rosé young wine and wine. Sporadic occurrence of these compounds in white grape products could be assigned to the process of proanthocyanidins decomposition. Catechin and its epimer epicatechin are the basic monomeric units of proanthocyanidin polymers. These polymers are localised in the grape skin and seeds and their content in grape products is highly dependent on the grape pressing technique, time period of skin contact or storage conditions, which are entirely different in vinification of red and white grapes.[Citation26–Citation28] Furthermore, catechin and epicatechin are highly oxidisable agents that could be involved in redox reactions resulting in the formation of quinones and yellow pigments, which together direct browning sensitivity of the white wine. Monitoring their content in white wine could be useful tool for the vintners when predicting the stability of wine.[Citation27,Citation29] Regarding the flavonol myricetin, Principal Component Analysis (PCA, ) applied to the data presented in and , showed that this compound, found only in Cabernet Sauvignon and Merlot varieties, has the greatest loadings on the first principal compound (PC1, 40.33% of total variance), separating them in the positive part of PC1. However, except for some grape juices grouping in the negative part of both PC1 and PC2, there were no obvious other groupings according to the analysis presented.
Figure 1. Score plot (a) and loading plot (b) of the Principal Component Analysis (PCA) of determined phenolics (anthocyanins not included) in grape juice, young wine and wine: distribution of samples in the two-dimensional system defined by the first two principal components(Legend: J(◊) - juice, Y(◽) - young wine, W(∙) - wine, CS - Cabernet Sauvignon, F - Frankovka, M - Merlot, MH - Muscat Hamburg, C – Chardonnay, IRA - Italian Riesling (winery Agner), S – Sila, IRB – Italian Riesling (winery Bajilo) variety).
The main dietary sources of resveratrol, the component belonging to the stilbene class of polyphenolics, are the grape seeds and skin, and consequently the wine. Certainly, the higher concentration of resveratrol in the red, compared to the white wines, the increase of its content in the examined red wine samples (from 0.80 in Merlot juice to 2.42 mg/L in wine), or its absence in grape juices and later appearance in white wines, all represent a direct consequence of the winemaking process. Namely, in the process of making the red wine, the must, the grape skin and often the seeds, are in a direct contact during the entire fermentation process. When making the white wine, their contact is limited since most of the skin and seeds are removed before fermentation, thus limiting the resveratrol occurrence in the final wine product. Furthermore, it is obvious that the applied winemaking technology could determine not only the content of resveratrol, but also other polyphenolics, since the observed concentrations in products of the same variety (Italian Riesling) were different in samples from two different wineries. Anyway, the data obtained are in a good correlation with the previously reviewed levels of resveratrol in red (1.90 mg/L), rosé (0.41 mg/L) and white wines (0.13 mg/L).[Citation30] Taking into account that a wide range of the observed activities, such as antioxidant, cardioprotective, anticancer, antidiabetic, neuroprotective and anti-aging ones, are attributed to resveratrol, its presence in wine could be strongly associated with a health-promoting impact of moderate wine consumption.[Citation30]
Anthocyanidins in V. vinifera varieties occur mainly in the grape skin as glycosides and acylglucosides of the following five compounds: malvidin, petunidin, peonidin, delphinidin and cyanidin.[Citation31,Citation32] Thus, the presented analysis was directed toward the quantification of their 3-O-glucosides in red and rosé wine related products, and the results were shown in . In general, malvidin 3-O-glucoside was the dominant anthocyanin in all samples, with some unexpected variations. To be specific, a great decrease of its amount, as well as the amount of other anthocyanins, which were observed in the Cabernet Sauvignon juice and wine, implicating that factors in the final phases of the Cabernet Sauvignon production like pH, storage temperature, light, oxygen, solvents, the presence of enzymes, some flavonoids, proteins or metallic ions, greatly affect their stability.[Citation33] This trend could be of certain value for the winemakers, since it can be used as one of the parameters in wine quality control. Nevertheless, the results of the presented study on anthocyanin composition of the Merlot and Cabernet Sauvignon varieties are somewhat in accordance with the analysis of the same varieties cultivated in Macedonia,[Citation32] both pointing out that the overall content of polyphenolics strongly depends on several factors such as grape variety, wine age, winemaking technique or storage conditions.
In general, determined in-depth polyphenolic profile could be of high value for wine producers from the Fruška Gora region (Serbia) for several reasons: it is the first comprehensive analysis of the polyphenolic profile of grape products grown in the region, including the analysis of the autochthon hybrid variety of Sila; it is a helpful starting point for the long-term monitoring of winemaking process and wine quality; it supports the sustained efforts of local vintners to establish a competitive position and a recognisable mark on the European and world-wide wine market.
Biological activity
In order to determine the antioxidant activity of grape juices, young wines and wines, several assays were undertaken. Specifically, the commonly used tests for assessment of antioxidant potency based on a single-proton/electron transfer activity, such as DPPH• neutralisation and reducing power (FRAP test), were carried out. Additionally, we found out that the evaluation of lipid peroxidation inhibition and nitric oxide (•NO) scavenging capacity could be particularly valuable for characterisation of the biological potential of the examined samples. It is documented that vasorelaxant action of the grape and wine polyphenols in blood vessels, followed by the related positive influence on cardiovascular system, is a consequence of their impact on •NO concentration, mainly by endothelial NO synthase induction.[Citation34] Knowing that the polyphenols are also powerful scavengers of free radicals, their overall impact on •NO levels and the related health benefits should be considered regarding both activities. Certainly, the higher radical neutralisation capacity of any food or beverage during the oxidative stress, which leads to various serious diseases, including cardiovascular and neurodegenerative disorders, ischemic stroke and cancer, significantly contributes to their health-promoting properties. Furthermore, oxidation of lipids, especially lipoproteins such as LDL, plays a crucial role in development of atherosclerosis, since macrophages, which take up and accumulate oxidised LDL, consequently transform to foam cells which form fatty streaks, the first indicator of atherosclerosis.[Citation5,Citation35] Therefore, this confirmation that particular foodstuff stops lipid peroxidation is highly valuable.
The primarily investigated biological activity of wine is certainly antioxidant, which is documented in numerous articles. Considering the varieties examined in this work, most authors referred to Cabernet Sauvignon, Merlot and Chardonnay.[Citation4–Citation8,Citation24,Citation25,Citation31–Citation36] Data presented in show that all of the tested samples express antioxidant power, but of different strength. The antioxidant activity determined is particularly important for the characterisation of some varieties, such as Muscat Hamburg, Frankovka or Italian Riesling, since the data on their antioxidant activities are limited,[Citation37,Citation38] or the autochthon variety Sila, whose antioxidant potency is herewith examined for the first time.
Table 4. Antioxidant and neuroprotective activities* of grape juice, young wine, wine and standard compounds.
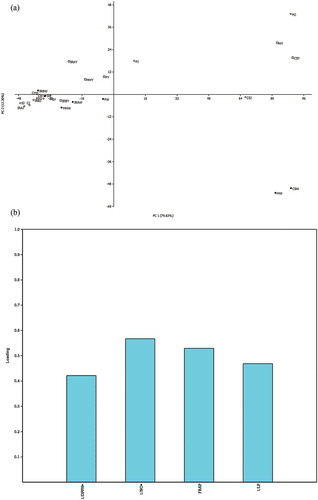
In general, the antioxidant activity could be manifested through a wide variety of actions. Different research groups use different experimental conditions for its evaluation. Therefore, ranging and comparing the overall antioxidant potential of the samples is not entirely straightforward. PCA applied to our set of data () showed that young wines (Merlot, Cabernet Sauvignon) and juices (Frankovka, Merlot) separate in positive part of PC 1 and PC 2 (79.36% and 12.30% of total variance, respectably), and also pointed out to the listed samples as the most potent products. These findings on the activities of grape juices, including the higher reducing capacity (FRAP test) of red than the white ones, are in accordance with the data previously published.[Citation24] In addition, they support the suggestion of the researchers cited that the consumption of non-alcoholic grape derivatives, as a rich source of antioxidant agents, is agreeable with the guidelines of the International Organisation of Vine and Wine (OIV). Several other obvious differences could be noticed in inhibition of lipid peroxidation: our data showed a high potential of the red grapes related products, contrary to the white ones, where only the Charrdonay variety expressed some activity. On the other hand, it is of great importance to consider the concentration of active compounds in the samples examined. For example, in the assay related to neutralisation of DPPH•, there was no statistically important difference between the activity of Cabernet Sauvignon juice and Merlot wine, but a much lower volume of juice than wine (1.10 and 11.0 µL, respectively) is needed to achieve the same level of neutralisation (50% of DPPH•). In that sense, the wine activity of Merlot and Frankovka could be compared to the activity of same varieties examined by Šeruga et al.[Citation38]: 7.6 and 8.5 µL against 11.0 and 5.88 µL, respectively, needed to achieve IC50 of DPPH• neutralisation. Regarding the activity against •NO, Merlot wine was the most potent sample (IC50 = 0.13 mg/mL, 4.11 µL equivalents), followed by Cabernet Sauvignon wine, young wine and Frankovka wine, which achieved IC50 at the statistically same concentration (0.20 mg/mL), but with different equivalents of volume: 5.91, 4.49 and 8.46 µL, respectively. All of the samples showed a much better activity than the standard antioxidant PG (IC50 = 16.3 mg/mL) or BHT, which did not express •NO inhibition, probably due to low solubility in aqueous buffers. The noticeable activity observed on neutralisation of •NO, supports the previous implications that the effects of grape products as vasorelaxants or antioxidants are probably the result of several different actions of bioactive constituents. Even though the actual antioxidant potential can only be confirmed by in vivo tests, the in vitro assays applied are valuable because they can predict a potential activity of the samples investigated.
Figure 2. Score plot (a) and loading plot (b) of the Principal Component Analysis (PCA) of grape juice, young wine and wine antioxidant activity regarding volume equivalents needed to achieve IC50: distribution of samples in the two-dimensional system defined by the first two principal components (Legend: J(◊) - juice, Y(◽) - young wine, W(∙) - wine, CS - Cabernet Sauvignon, F - Frankovka, M - Merlot, MH - Muscat Hamburg, C – Chardonnay, IRA - Italian Riesling (winery Agner), S – Sila, IRB – Italian Riesling (winery Bajilo) variety).
Up to now, in vitro anti-acetylcholineserase (anti-AChE) activity of wines has never been used for estimation of their neuroprotective activity, although it is well-known that some constituents of grapes, such as resveratrol, could prevent the increase of AChE activity in vivo.[Citation39] AChE is the enzyme responsible for acetylcholine degradation in brain, whose level, if decreased, could impair learning, memory, behaviour and emotional responses, which are all increased in neurodegenerative diseases, such Alzheimer’s. Thus, we optimised the method previously described by Ellman, Courtney, Andres and Featherstone[Citation21] and carried it out in order to estimate the anti-AChE activity of wine and other grape-related products. The anti-AChE potency was considerable and ranged widely among the samples; IC50 varied from 0.33 to 9.20 mg/mL or from 6.52 to 39.6 μL (Merlot-juice and Italian Riesling Bajilo-juice, respectively), which present the volume equivalents needed to achieve the IC50. Unfortunately, the samples of the autochthon variety of Sila showed some anti-AChE activity, but not the concentration-dependent one, and thus it was not taken into account. However, further investigation is certainly required to identify the exact compounds of the examined samples which are responsible for substantial in vitro anti-AChE activity.
Anyway, several general conclusions about the tested biological activities could be highlighted: the overall antioxidant dominance of the red grape products is confirmed, the Merlot variety being the leading one; antioxidant activity of some previously unexplored or insufficiently explored varieties (Sila, Muscat Hamburg, Italian Riesling) was determined, and it is implicated that the grape juices and young wines have the potential to be regarded as a promising source of natural antioxidants. Furthermore, the optimised in vitro assay for the assessment of AChE inhibition was established, pointing out the grape juices and red wines as samples with the most promising neuroprotective potential.
Conclusion
The present article reports novel and valuable data about the quality of three red wines (from Cabernet Sauvignon, Frankovka and Merlot varieties), one rosé wine (from Muscat Hamburg variety) and three white wines (from Italian Riesling, Chardonnay and Sila varieties), all produced in the Fruška Gora vineyards, Serbia, including an in-depth polyphenolic profile characterisation and determination of biological activities, such as antioxidant and neuroprotective ones. Besides wines, the related grape products, grape juices and young wines, were included in this study in order to valorise their use in diet. LC-MS/MS analysis showed that the derivatives of hydroxybenzoic and hydroxycinnamic acids, particularly ellagic and caffeic acids were, in general, more abundant in the samples of wines, principally in the red ones. Among the investigated flavonoids, flavanols catechin and epicatechin were the most dominant, but their higher presence was obvious in the red grapes related products. In general, malvidin 3-O-glucoside was the dominant anthocyanin in all of the samples, while the concentration of stilbene resveratrol was somewhat higher in red wines. Overall, the results confirmed that the polyphenolic profile of grape juices, young wines and wines depends on several factors such as grape variety, geographical region, wine age, winemaking technique or storage conditions. As for the biological activities, some general conclusions could be made: overall antioxidant dominance of the red grape products was confirmed, with the Merlot variety as a leading one, data on antioxidant activity of some previously un- or insufficiently explored varieties (Sila, Muscat Hamburg, Italian Riesling) were collected, and it is implicated that the grape juices and young wines also have a potential to be regarded as a promising source of natural antioxidants. Furthermore, the optimised in vitro assay for assessment of AChE inhibition was applied, and the results pointed out to grape juices and red wines as samples having the most promising neuroprotective potential. Taking everything into account, data on bioactivity of grape varieties grown in the Fruška Gora region are highly limited, which makes the results in this work truly valuable. The results obtained are of high value for wine producers from the Fruška Gora region as a helpful starting point in long-term monitoring of the winemaking process and wine quality. Clearly, with the goal to establish a competitive position and a recognisable mark on the global wine market.
Acknowledgment
We sincerely thank to Podrum Bajilo, Vinum, Vinarija Šukac, Vinarija Došen and Vinarija Agner, wineries from the Fruška Gora region, for providing the samples, the Provincial Secretariat for Science and Technological Development of the Autonomous Province of Vojvodina, Serbia (Grant No. 114-451-1752/2014-02), and the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 172058) who supported this research work. We sincerely thank MSc. Gordana Vlahović for editorial assistance.
References
- Renaud, S.; De Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet. 1992, 339, 1523–1526.
- Renaud, S. C.; Guéguen, R.; Schenker, J.; d’Houtaud, A. Alcohol and Mortality in Middle-Aged Men from Eastern France. Epidemiology. 1998, 9, 184–188.
- Estruch, R. Wine and Cardiovascular Disease. Food Res. Int. 2000, 33, 219–226.
- Iriti, M.; Faoro, F. Bioactivity of Grape Chemicals for Human Health. Nat. Prod. Commun. 2009, 4, 611–634.
- Guilford, J. M.; Pezzuto, J. M. Wine and Health: A Review. Am. J. Enol. Vitic. 2011, 62, 471–486.
- Paixão, N.; Perestrelo, R.; Marques, J. C.; Câmara, J. S. Relationship between Antioxidant Capacity and Total Phenolic Content of Red, Rosé and White Wines. Food Chem. 2007, 105, 204–214.
- Gris, E. F.; Mattivi, F.; Ferreira, E. A.; Vrhovsek, U.; Filho, D. W.; Pedrosa, R. C.; Bordignon-Luiz, M. T. Phenolic Profile and Effect of Regular Consumption of Brazilian Red Wines on in Vivo Antioxidant Activity. J. Food Compos. Anal. 2013, 31, 31–40.
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J. O.; Dommes, J. Antioxidant Capacity and Phenolic Composition of Red Wines from Various Grape Varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50.
- Havsteen, B. H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202.
- Fang, F.; Li, J.-M.; Zhang, P.; Tang, K.; Wang, W.; Pan, Q.-H.; Huang, W.-D. Effects of Grape Variety, Harvest Date, Fermentation Vessel and Wine Ageing on Flavonoid Concentration in Red Wines. Food Res. Int. 2008, 41, 53–60.
- Kennedy, J. Grape and Wine Phenolics: Observations and Recent Findings. Ciencia E Investigación Agraria. 2008, 35, 77–90.
- Reid, L. M.; O’Donnell, C. P.; Downey, G. Recent Technological Advances for the Determination of Food Authenticity. Trends Food Sci. Technol. 2006, 17, 344–353.
- Li, S.-F.; He, F.; Zhu, B.-Q.; Wang, J.; Duan, C.-Q. Comparison of Phenolic and Chromatic Characteristics of Dry Red Wines Made from Native Chinese Grape Species and Vitis Vinifera. Int. J. Food Prop. 2017, 20, 2134–2146.
- Alvarez-Casas, M.; Pajaro, M.; Lores, M.; Garcia-Jares, C. Polyphenolic Composition and Antioxidant Activity of Galician Monovarietal Wines from Native and Experimental Non-Native White Grape Varieties. Int. J. Food Prop. 2016, 19, 2307–2321.
- Vlastelica, R. Serbian Wine Routes; Službeni Glasnik: Belgrade, Serbia, 2009.
- Cvejić, J.; Puškaš, V.; Miljić, U.; Torović, L.; Rakić, D. Varietal Phenolic Composition of Probus, Rumenika and Frankovka Red Wines from Fruška Gora (Serbia) and Changes in Main Compounds during Maceration. Eur. Food Res. Technol. 2016, 242, 1319–1329.
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative Determination of Plant Phenolics in Urtica Dioica Extracts by High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometric Detection. Food Chem. 2014, 143, 48–53.
- Compendium of International Methods of Analysis - OIV, HPLC Determination of Nine Major Anthocyanins in Red and Rose Wine (MA-AS315-11); International Organization of Vine and Wine: Paris, France, 2013.
- Beara, I. N.; Lesjak, M. M.; Jovin, E. D.; Balog, K. J.; Anackov, G. T.; Orcić, D. Z.; Mimica-Dukić, N. M. Plantain (Plantago L.) Species as Novel Sources of Flavonoid Antioxidants. J. Agric. Food Chem. 2009, 57, 9268–9273.
- Lesjak, M. M.; Beara, I. N.; Orčić, D. Z.; Ristić, J. D.; Anačkov, G. T.; Božin, B. N.; Mimica-Dukić, N. M. Chemical Characterisation and Biological Effects of Juniperus Foetidissima Willd. 1806. LWT Food Sci. Technol. 2013, 53, 530–539.
- Ellman, G. L.; Courtney, K. D.; Andres, V.; Featherstone, R. M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95.
- Hammer, Ø.; Harper, D. A. T.; Ryan, P. D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electronica. 2001, 4, 9pp.
- García-Niño, W. R.; Zazueta, C. Ellagic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Liver Protection. Pharmacol. Res. 2015, 97, 84–103.
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J. A. Phenolic Compounds and Antioxidant Activity of Spanish Commercial Grape Juices. J. Food Compos. Anal. 2015, 38, 19–26.
- Tauchen, J.; Marsik, P.; Kvasnicova, M.; Maghradze, D.; Kokoska, L.; Vanek, T.; Landa, P. In Vitro Antioxidant Activity and Phenolic Composition of Georgian, Central and West European Wines. J. Food Compos. Anal. 2015, 41, 113–121.
- Lee, J. M. Degradation Kinetics of Grape Skin and Seed Proanthocyanidins in a Model Wine System. Food Chem. 2010, 123, 51–56.
- Fracassetti, D.; Lawrence, N.; Tredoux, A. G. J.; Tirelli, A.; Nieuwoudt, H. H.; Du Toit, W. J. Quantification of Glutathione, Catechin and Caffeic Acid in Grape Juice and Wine by a Novel Ultra-Performance Liquid Chromatography Method. Food Chem. 2011, 128, 1136–1142.
- Dias, D. A.; Smith, T. A.; Ghiggino, K. P.; Scollary, G. R. The Role of Light, Temperature and Wine Bottle Colour on Pigment Enhancement in White Wine. Food Chem. 2012, 135, 2934–2941.
- Chinnici, F.; Sonni, F.; Natali, N.; Riponi, C. Oxidative Evolution of (+)-Catechin in Model White Wine Solutions Containing Sulfur Dioxide, Ascorbic Acid or Gallotannins. Food Res. Int. 2013, 51, 59–65.
- Fernández-Mar, M. I.; Mateos, R.; García-Parrilla, M. C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813.
- Clifford, M. N. Anthocyanins—Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1063–1072.
- Ivanova-Petropulos, V.; Hermosín-Gutiérrez, I.; Boros, B.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Dörnyei, A.; Kilár, F. Phenolic Compounds and Antioxidant Activity of Macedonian Red Wines. J. Food Compos. Anal. 2015, 41, 1–14.
- Castañeda-Ovando, A.; Pacheco-Hernández, M. D. L.; Páez-Hernández, M. E.; Rodríguez, J. A.; Galán-Vidal, C. A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871.
- Räthel, T. R.; Samtleben, R.; Vollmar, A. M.; Dirsch, V. M. Activation of Endothelial Nitric Oxide Synthase by Red Wine Polyphenols: Impact of Grape Cultivars, Growing Area and the Vinification Process. J. Hypertens. 2007, 25, 541–549.
- Leifert, W. R.; Abeywardena, M. Y. Cardioprotective Actions of Grape Polyphenols. Nutr. Res. 2008, 28, 729–737.
- Sartor, S.; Caliari, V.; Malinovski, L. I.; Toaldo, I. M.; Bordignon-Luiz, M. T. Bioactive Profiling of Polyphenolics and Oenological Properties of Red Wines from Italian Grapes (Vitis Vinifera L.) Cultivated in a Selected Subtropical Region. Int. J. Food Prop. 2017. DOI:10.1080/10942912.2017.1344992.
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic Compounds and Antioxidant Properties of Selected China Wines. Food Chem. 2009, 112, 454–460.
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of Polyphenols Content and Antioxidant Activity of Some Red Wines by Differential Pulse Voltammetry, HPLC and Spectrophotometric Methods. Food Chem. 2011, 124, 1208–1216.
- Schmatz, R.; Melazzo Mazzanti, C.; Spanevello, R.; Stefanello, N.; Gutierres, J.; Corrêa, M.; Melgarejo Da Rosa, M.; Rubin, M. A. Resveratrol Prevents Memory Deficits and the Increase in Acetylcholinesterase Activity in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2009, 610, 42–48.
