ABSTRACT
Effects of stewing time (1, 2, and 3 h) on the levels of taste-active and volatile compounds were measured. The flavor characteristics of the stewed yellow-feather chicken meat were assessed with sensory evaluation and electronic nose. Results showed that increasing stewing time significantly decreased the contents of taste components such as free amino acids, 5′-nucleotides, minerals. Inosine 5′-monophosphate and chloride were the major umami-related compounds in stewed meat and decreased significantly during stewing. The taste-active values of the equivalent umami concentration decreased from 283.2 to 38.7 after 3 h of stewing. In contrast, increasing stewing time improved aroma levels. The volatile compounds mainly included pentanal, hexanal, heptanal, octanal, (E)-2-octenal, nonanal, (Z)-4-decenal, decanal, (E,E)-2,4-decadienal, 1-pentanol, and 1-octen-3-ol. With increased stewing time, aldehydes significantly decreased (P < 0.05), whereas alcohols significantly increased (P < 0.05). The high-intensity aroma after 2 h of stewing could be attributed to 1-pentanol and 1-octen-3-ol. The aroma scores of the chicken meat were at maximum after stewing for 3 h. The overall flavor characteristics tended to stabilize after 2 h of stewing. In general, stewing improved the aroma but decreased the taste components in the chicken meat, especially within the first 2 h. The data herein not only provides insight into the changes in odor and taste of chicken meat during cooking, but also guidelines for improving the stewing process.
Introduction
In 2015, China produced 4445 kilotons of yellow-feather chickens, which was 31.8% of total broiler production. In one year, consumption of meat from the yellow-feather chicken breed increased by 4.5%.[Citation1] These yellow-feather broiler chickens have a long feeding cycle, which exceeds 90 days, and are mainly processed into boiled chicken meat or chicken soup. Chicken soup made with this type of chicken is preferred by Chinese consumers because of its umami and aroma.[Citation2]
Flavor consists of odor and taste and is an important quality for consumers. The aroma of cooked meat is derived from lipid degradation, the Maillard reaction, and the interaction between these two processes.[Citation3,Citation4] Taste-active compounds include nucleotides, free amino acids (FAAs), organic acids, sugars, and inorganic ions. Limited research has shown that the odor components of boiled chicken include 2-furfurylthiol, hexanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, (E,E)-2,4-decadienal, and (E,Z)-2,4-decadienal.[Citation5,Citation6] Of these volatile compounds, 2-furfurylthiol is generated through a Maillard reaction between ribose and cysteine[Citation4], while the aldehydes mentioned above are generated from lipid oxidation.[Citation7] The Maillard reaction and lipid degradation are also involved in flavor formation of pot-stewed chicken meat products.[Citation8,Citation9] However, our previous research has shown that lipid degradation plays an important role in flavor formation of chicken soup.[Citation7] Therefore, it is possible that lipid degradation may be the predominant flavor-forming reaction in stewed chicken meat. One aim of this work was to determine whether lipid degradation or other thermal reactions contribute to the flavor of stewed chicken meat cooked by long-term boiling of yellow-feathered chickens.
Jayasena et al.[Citation10] have reported that Korean yellow-feather chickens supply higher levels of reducing sugars, FAAs, inosine 5′-monophosphate (IMP), betaine, and carnitine than white commercial broilers. Furthermore, Rotola-Pukkila et al.[Citation11] have reported that the levels of amino acids and IMP in pork meat remain constant but that 5ʹ-adenosine monophosphate (AMP) increases significantly with prolonged stewing time. However, our team has reported that FAAs, 5ʹ-nucleotides, and minerals in Chinese yellow-feathered chicken broth increase remarkably with increased stewing time.[Citation7] Similar results are reported by other authors.[Citation12,Citation13] These FAAs, 5ʹ-nucleotides, and minerals migrate from the chicken meat into the broth. Thus, the levels of these taste components should be changing in chicken meat during stewing. However, relevant research on this idea was limited. The methods to assess overall flavor, such as electronic tongue, electronic nose (E-nose), and taste activity value (TAV), have been extensively used to assess other meats.[Citation14–Citation16] However, in these reports, the boiling times were all less than 3 h. Yellow-feather chicken meat requires longer cooking times to reach acceptable textures because the long feeding period induces changes in the connective tissue.[Citation17,Citation18] According to our preliminary studies (data not shown), stewing can lead to the poor texture of chicken meat and the chewiness of chicken meat was unacceptable if the cooking time is more than 3 h. Therefore, 3 h of stewing was used as the longest stewing time in the present experiment. Furthermore, most manufacturers cook chicken broth for 1 or 2 h in industrial production. Thus, cooking for 1, 2, and 3 h was used for preparing the chicken soup. However, little is known about the flavor changes that occur during long-term cooking of the meat of this breed.
This study was designed to assess the flavor-related compounds and overall flavor profiles of yellow-feather chicken meat after stewing for 3 h. The aims were to identify the key volatile compounds and how they arose during stewing. The information obtained provides insight into the unique flavor of stewed meat, particularly the meat of breeds not widely studied, as well as guidelines to improve stewing.
Materials and methods
Sample preparation
Live, female yellow-feather chickens, fed a commercial diet for 300 days in local commercial hatches (Sungo Co., Ltd., Xinyang, Henan, China), were slaughtered by and obtained from Henan Sungo Agricultural and Livestock Co., Ltd. (Xinyang, Henan, China). The neck and feet were removed. The body weights were 800–1000 g. Forty-eight chickens were evenly divided into four groups (12 chickens for raw meat and 36 chickens for three stewing experiments of 1, 2, or 3 h). The chickens were evenly cut into two pieces and were cooked in 4 L stainless steel pots (ST22J1, Supor, Hubei, China) with purified water (Yibao, Shenzheng, China) in a volume that was twice as heavy as the chicken meat.
Chicken meat was placed into the water at a temperature of 95–99°C. Cooking was timed once the water temperature returned to 95–99°C. An electromagnetic cooktop (C21-SC011, Joyoung, Shandong, China) set at 300 W for 20 min, 120 W for 20 min, and 300 W for 20 min was used to maintain this temperature during stewing. The cycle was repeated twice to heat for 2 h and thrice to heat for 3 h. The temperatures of the breast and thigh meat were between 30°C and 35°C at the start, reached 93–95°C after stewing for 25 min, and remained between 93°C and 98°C for the rest of stewing.
The carcass was removed from the broth after stewing and cooled down to room temperature without aid. The breast and thigh of raw or cooked chicken meat were split, and the visible skin, fat, and connective tissues were removed. From each of the chicken carcasses, the breast and thigh meat, in natural proportion, were mixed and minced using a mini chopper (J10, Aux, Guangzhou, China) for analysis of the taste and volatile compounds using E-nose and, for the cooked samples, sensory evaluation.
FAA assay
FAAs were extracted and analyzed using a method reported by Kim et al.[Citation19] with some modifications. Approximately 4 g of chicken meat was homogenized in a 50 mL centrifuge tube that contained 20 mL of 3% (w/v) sulfosalicylic acid. The meat mixture was homogenized for 60 s (20 s × 3) at 10,000 rpm using an Ultra-turrax homogenizer (T-25, IKA, Staufen, Germany). The mixture was centrifuged (12,096 × g, 15 min; Avanti J-26XP, Beckman, Shanghai, China). The supernatant was removed and shaken with 2 mL of n-hexane. The aqueous phase (2 mL) was filtered through a 0.45 μm membrane prior to FAA analysis. FAAs in meat were analyzed using an automatic amino acid analyzer (L-8900; Hitachi, Tokyo, Japan). The identity and quantity of each amino acid were determined by comparison with the retention times and peak areas of each amino acid standard.
5′-Nucleotide assay
5′-Nucleotides were extracted[Citation20] and analyzed using a high-performance liquid chromatography system (E2695 Waters Ltd., Massachusetts, USA) equipped with an X Bridge C18 (5 μm; 4.6 × 250 mm) chromatographic column and 2998 photodiode array detector. The eluents used were (A) KH2PO3 (0.05 M, pH 4.5) and (B) methanol. The flow rate was 1.0 mL·min−1. A gradient elution mode was used: 98% A for 14 min, then reduced to 85% A over 7 min, and finally 98% A for 9 min. The UV detector wavelength was 254 nm. Each nucleotide was identified and quantified by comparing the peak area of the nucleotide with that of the external standard. The 5ʹ-nucleotides analyzed were guanosine 5ʹ-monophosphate (GMP), IMP, AMP, hypoxanthine (Hx), and inosine (I).
Mineral assays
The elements Ca, K, Mg, P, and Na were measured in the chicken meat using an inductively coupled plasma optical emission spectrometer (VISTA-MPX, Agilent Ltd., California, USA) according to the method described by Jin et al.[Citation15] Chloride was measured according to the Carpentier–Vohlard method.[Citation21]
Equivalent umami concentration
Equivalent umami concentration (EUC) is defined as the concentration of monosodium glutamate (MSG, g 100 g−1) that is equivalent to the umami intensity of the umami amino acids [aspartic acid (Asp) or glutamic acid (Glu)] and 5′-nucleotides [AMP, GMP, and IMP].[Citation14] EUC is calculated using the following equation[Citation22]:
where EUC is expressed as g MSG per 100 g; αi is Asp or Glu concentration (g 100 g−1); αj is IMP, GMP, or AMP concentration (g 100 g−1); βi is the relative umami concentration (RUC) for Glu (1) and Asp (0.077) to MSG; βj is the RUC of IMP (1), GMP (2.3), and AMP (0.18); and 1218 is the synergistic constant based on the concentration of g 100 g−1 used.
Taste activity value
TAV reflects the contribution of a single compound to taste characteristics. The substance contributes less to the taste when TAV is less than 1 and significantly contributes to the taste when TAV exceeds 1. TAV is calculated as the ratio between its concentration in the chicken meat and its taste threshold value, which is generally obtained from previous reports.[Citation14]
Volatile compounds analysis
GC-MS analysis was performed using a Thermo GC/MS system that consisted of a TRACE GC ULTRA gas chromatograph equipped with a TRIPLUS auto sampler and a DSQ II mass selective detector (Thermo Scientific, Waltham, MA, USA). Approximately 5 g of chicken meat was placed in a 20 mL vial (Supelco, Bellefonte, USA), which was then capped tightly. The volatile compounds in chicken meat were extracted with a headspace solid-phase microextraction fiber (75 μm, carboxen/polydimethylsiloxane; Supelco, Bellefonte, USA) and collected for 30 min at 50°C. The fiber was then inserted into the GC injector port and desorbed for 3 min at 250°C. The volatile compounds were separated on a TR-5 MS capillary column (30 m length × 0.25 mm i.d. × 025 μm film thickness; Thermo Scientific, Waltham, MA, USA). The oven temperature was maintained for 3 min at 40°C, increased at 3°C/min to 70°C, then at 5°C/min to 180°C, then at 10°C/min to 280°C, and finally maintained for 5 min at 280°C. The carrier gas was helium at a constant flow velocity of 1 mL/min. The mass-selective detector was operated in the electron impact mode (70 eV) and full scan mode (35–550 m/z range). The retention time for each compound was converted to the linear retention index (LRI) using n-alkanes (C7–C30) (Sigma, Shanghai, China).
The volatile compounds were identified by comparing their mass spectra with those in the NIST 08 library (National Institute of Standards and Technology, Gaithersburg, MD, USA), the LRI values with those reported in the literature, and the data listed in authentic online databases (http://www.flavornet.org, http://www.odour.org.uk). The odor activity values (OAVs; ratio of the concentration of the compound to the odor threshold reported in the literature for the aqueous phase) for the volatile compounds were calculated to evaluate their sensory significance. A compound with an OAV ≥1 was defined as a contributor to the aroma.[Citation23]
Electronic nose assay
A FOX 4000 E-nose system (Alpha MOS, Toulouse, France) equipped with 18 metal oxide semiconductor sensors was used for the assay. Meat (2.0 g) was placed into a 20 mL precision thread vial for headspace (HS) equilibration at 70°C for 5 min. Then, 1000 μL of HS gas was injected in the sample for 1 s. Sensor signals were recorded after 120 s. The response values of the E-nose were recorded and analyzed using principal component analysis (PCA). E-nose detection was repeated four times four each treatment.
Sensory evaluation
The sensory analysis included three types of flavor characteristics (umami, saltiness, and aroma) and was performed following the method of Krasnow et al.,[Citation24] with some modifications. The meat samples were maintained between 54°C and 60°C during testing by immersing the sample jars in a controlled-temperature water bath (HH-42, Lihua, Jiangsu, China). The samples were then randomly distributed to eight trained panelists from the National Center of Meat Quality and Safety Control (four females and four males). These panelists were trained for a period of 2 months in 1 h sessions that occurred 12 times a month (36 h in total). Each panelist evaluated approximately 5 g of sample per treatment. The semantic differential scales were performed based on flavor strength, which was expressed as a score from −3 to 3. Each sample was evaluated once.
Statistical analysis
The effects of stewing on the taste components, volatile compounds, and overall flavor profiles of chicken meat were determined through one-way ANOVA with SAS software (SAS Institute Inc., USA). Means were compared using Duncan multiple-range test. The significance of difference was set as P < 0.05. Data from E-nose were analyzed using PCA software from StatSoft (version 7.0, Tulsa, Oklahoma, USA). Four replicate determinations were carried out.
Results and discussion
Changes in FAA content with different stewing times
Stewing significantly decreased the concentrations of FAAs (). In raw meat, the total FAA concentration (258.52 ± 3.20, ) was similar to the value reported for Japanese yellow-feather chicken meat (226 mg/100 g)[Citation25], and was more than reported for duck breast meat (171 mg/100 g).[Citation26] Each hour of stewing resulted in a significant decrease in FAA content. These decreases in FAAs in the chicken meat could be due to the migration of the original FAA from the chicken meat into the broth and/or to the degradation of proteins and small peptides.[Citation27] Krasnow et al.[Citation24] reported that there was no significant breakdown of proteins into their constituent amino acids in boiled chicken meat even after cooking for 4 h. Thus, the decreased FAA content in the chicken meat observed herein can be attributed to enhanced migration from the boiled meat into the broth, as evidenced by the increases of FAAs in the chicken broth.[Citation7] Rotola-Pukkila et al.[Citation11] have reported that the FAAs in pork meat remain constant during cooking at 80°C for 120 min. This discrepancy could be due to differences in the intensity of the stewing.
Table 1. Changes in the contents of free amino acids and the TAVs of chicken meat at different stewing times.
In raw and stewed meat, lysine, threonine, and glutamic acid were the most abundant amino acids (). Glutamic acid and threonine, which accounted for approximately 20–25% of the total amino acids, were the major flavor amino acids in the chicken meat. Serine and alanine also likely contribute flavor. Although proline and lysine constituted approximately 35–50% of the total amino acids, these FAAs have no taste.[Citation28] The concentration of glutamic acid was reported to be over 30 mg 100 g−1 in Korean yellow-feather chicken legs[Citation10], similar to the value found here for a mixture of breast and leg meat. Similar findings have been observed in chicken meat and chicken broth.[Citation22,Citation25]
The TAVs of each FAA, except for glutamic acid, were all less than one in raw chicken meat () and decreased as stewing time increased. Although the TAVs of serine, glycine, and alanine were all less than one, they can interact synergistically with IMP to strongly enhance the umami taste.[Citation29] Additionally, arginine can cooperate with NaCl and glutamate to contribute a pleasant overall taste.[Citation30]
Changes in 5′-nucleotides in meat
Stewing time significantly affected the levels of the 5′-nucleotides (). IMP, GMP, and Hx contents significantly decreased as stewing time increased. Similar levels have been reported for Korean and Japanese yellow-feather chickens meat.[Citation2,Citation10,Citation31] The decreases in IMP, GMP, and Hx could result from both their migration from the meat into the broth and the thermal degradation of IMP to inosine during stewing.[Citation32] The decrease in the IMP in the chicken meat during stewing were consistent with results in goat meat after cooking[Citation33] and was accompanied by a remarkable increase in the IMP content in the chicken broth.[Citation7] Rotola-Pukkila et al.[Citation11] have reported that IMP in pork meat remained constant after cooking at 80°C for 120 min. This discrepancy could be due to differences in the intensity of the stewing.
Table 2. Analysis of the 5′-nucleotide concentrations and the TAVs of chicken meat at different stewing times.
IMP was the main nucleotide component in the chicken meat. Adenosine 5ʹ-triphosphate (ATP) and adenosine 5ʹ-diphosphate are the major nucleotides in live chickens, and the majority of ATP decomposes to IMP after slaughter.[Citation30] The IMP concentration of Korean yellow-feather chickens was between 244.47 and 403.81 mg/100 g in the breast and between 125.68 and 157.22 mg/100 g in the leg.[Citation10,Citation31] These values were higher than those of commercial broilers (94.4 mg/100 g). Similar results were reported for Hinai–Jidori chickens (Japanese yellow-feather chicken) and in Wenchang and Xianju chickens (Chinese yellow-feather chickens).[Citation2,Citation25] In the present experiment, meat from the breast and leg from Chinese yellow-feather chickens were mixed. Thus, the IMP concentration was in agreement with previous reports.
The GMP and Hx concentrations in cooked chicken meat were close to the values in cooked meat of Yangtze Coilia ectenes[Citation30] and decreased after stewing for 3 h. These decreases in the GMP and Hx in the chicken meat were accompanied by a remarkable increase in the content of GMP and Hx in the chicken soup during the stewing process.[Citation7] Interestingly, AMP levels significantly increased after stewing for 1 h and remained constant over the following hours. Increases in AMP are observed due to ATP metabolites increasing during the early heating period.[Citation34] Inosine (I) concentration significantly fluctuated during stewing. Given that I is a thermal degradation product of IMP[Citation35], I concentration increased concomitant with IMP degradation. Since I can be transferred from meat to the water, the I concentration in chicken meat fluctuated during stewing.
Flavorful 5′-nucleotides, including AMP, IMP, and GMP, contribute to the umami taste.[Citation33,Citation36] The TAV of IMP was greater than 1 in both the raw and stewed meat samples (1, 2, and 3 h; ). IMP was the most important flavor-providing 5′-nucleotide in meat and is known to impart a pleasant taste and contribute to the umami taste.[Citation15] The TAV of GMP exceeded 1 in raw meat and decreased to less than 1 after 1 h of stewing. GMP contributes to a meaty flavor and is a stronger flavor enhancer than MSG[Citation15], thus implying that GMP is a potential umami nucleotide in raw and stewed meat. Although the TAV of AMP in all experimental groups was less than 1, a synergistic interaction between AMP and IMP in eliciting umami taste should be considered in addition to the synergistic interactions between the 5′-nucleotides and the FAAs.[Citation37]
The EUC value, which reflects the umami taste, of chicken meat (8.50 MSG g/100 g) was higher than that reported for Chinese mitten crab (2.48–2.97 g MSG/100 g). [Citation14] Moreover, the TAV of EUC significantly decreased as stewing time increased (). This result might explain the decrease in umami sensory score as stewing time increased ().
Table 3. Changes in the mineral and chloride contents of chicken meat after stewing.
Table 4. Changes in the concentrations of odorous compounds and the OAVs of chicken meat during stewing.
Table 5. Sensory evaluation of chicken meat after stewing.
Changes in mineral levels in meat
Inorganic ions were significantly affected by the stewing time (P < 0.05, ). Ca2+, K+, Mg2+, Na+, and PO43− concentrations in raw meat were close to the values reported by Gall et al.[Citation38] K+ and PO43− were the main mineral components in chicken meat, and their percentages were similar to those in squid.[Citation15] The levels of Ca2+, K+, Mg2+, Na+, and PO43− significantly decreased (P < 0.05) after 3 h of stewing. These findings were in agreement with those of Kadim et al.[Citation39] Since minerals are generally stable during cooking[Citation40], these decreases in the chicken meat were attributed to the migration of the minerals from the boiled chicken meat into the broth, as evidenced by the increased amount of inorganic ions in the chicken broth.[Citation7]
Of these minerals, only the TAV of PO43− exceeded 1 in raw and stewed meats (). The TAV of K+ exceeded 1 in raw meat but was less than 1 after 1 h of stewing. Thus, PO43− likely has a vital role in the taste profile as it has been reported to reduce bitterness and increase the intensities of MSG-like tastes.[Citation41] Although the TAVs of K+, Mg2+, Na+, and Ca2+ were less than 1, these minerals may affect flavor in other ways. For example, Mg2+ can be perceived as an increase in saltiness.[Citation41] Na+ can strengthen the umami taste of meat by combining with 5′-nucleotides and FAAs.[Citation42] Any effect of minerals on taste would become weaker as the inorganic ion content decreased during stewing.
Chloride, which contributes to saltiness[Citation43], remarkably decreased () after stewing for 3 h and correlated with the decrease in the perceived saltiness (). These results implied that chloride should play a vital role in saltiness. Cl– reduces the perceived sourness while enhancing sweetness and MSG-like tastes[Citation41] and can enhance umami flavor of meat by combining with 5′-nucleotides and FAAs.[Citation42] Thus, the decrease in chloride would not only reduced taste intensity but also weaken the umami perception. Combined with the effects of the other minerals on taste profiles, these changes could be another reason for the decreasing taste scores as stewing time increased ().
Changes in volatile components
There were 56–58 volatile compounds identified in chicken meat after 1, 2, or 3 h of stewing (). These compounds represented eight chemical classes, including aldehydes, ketones, alcohols, hydrocarbons, furans, sulfur-containing compounds, nitrogen-containing compounds, and other compounds. As stewing time increased, alcohols, ketones, and hydrocarbons significantly increased, whereas aldehydes and sulfur-containing compounds significantly decreased. Esters significantly decreased, then significantly increased at longer stewing times. 2-n-Butylfuran increased significantly within 2 h of stewing. Nitrogen-containing compounds were only detected at 3 h of stewing.
In boiled meat, thermal oxidation of the acyl chains of lipids forms lipid degradation products, which are the dominant volatile compounds in meat products.[Citation44] These compounds substantially increased because of the ongoing lipid oxidation during stewing.[Citation44] In addition, stewing caused the loss of odor compounds through water evaporation. Thus, the increases and decreases in the volatile compounds of chicken meat can be attributed to the lipid breakdown, migration to broth and evaporation during stewing, which were in agreement with the changes in the volatile compound levels in chicken broth.[Citation7]
Earlier studies have reported that hexanal, octanal, nonanal, (E,E)-2,4-nonadienal, (E,Z)-2,4-decadienal, (E,E)-2,4-decadienal, trans-4,5-epoxy-(E)-2-decenal, a few sulfur-containing compounds (such as 2-furfurylthiol), and furan derivatives are the primary odor compounds in boiled chicken meat.[Citation5,Citation6] Although sulfur-containing compounds and furan derivatives were not identified in the present research, most of the previously reported aldehydes were detected. These discrepancies may have resulted from the differences in the intensity of the heating process and the raw material (genotype, sex, and diet). Moreover, previous reports indicated that 2-furfurylthiol is generated from a Maillard reaction between ribose and cysteine[Citation4], and aldehydes are generated from lipid oxidation. However, due to the absence of sulfur-containing compounds and most furan derivatives in our stewed chicken, the present data suggested that lipid oxidation could play a more important role in flavor formation of stewed Chinese yellow-feathered chicken meat.
The aldehydes, given their low odor threshold and high concentration, are likely the most important odor compounds in chicken meat. Among these aldehydes, pentanal, hexanal, heptanal, octanal, (E)-2-octenal, nonanal, (Z)-4-decenal, decanal, and (E,E)-2,4-decadienal were the primary active compounds (OAV ≥10; ). Furthermore, hexanal, heptanal, octanal, and (Z)-4-decenal likely play vital roles in meat odor because their TAVs exceeded 100 during the stewing process. Previous research showed that aldehydes may be decomposition products of lipid oxidation. For example, hexanal and heptanal are generated mainly from oxidation of linoleic and arachidonic acid[Citation45], while octanal and nonanal are generated from oleic acid oxidation.[Citation46]
Alcohols exhibit a high odor threshold and thus have a low effect on the aroma of samples. However, 1-pentanol and 1-octen-3-ol were detected at high concentrations and had large OAVs (1-pentanol, OAVs in the 16–500 range; 1-octen-3-ol, OAVs ≥100). 1-pentanol, which has a pleasant odor, has been found in cooked foal meat and is generated from the oxidation of C18:2n-6.[Citation47,Citation48] The compound 1-octen-3-ol is an odor contributor to the fatty characteristics of meat flavors in boiled chicken meat.[Citation8] This compound is formed by the auto-oxidation of linoleic acid or other polyunsaturated fatty acids.[Citation47] Sensory panelists reported that chicken meat showed a more intense flavor after 3 h of stewing compared with that after 2 h of stewing. This result may reflect the significant increase of 1-pentanol levels after 3 h of stewing.
Furans, which are vital heterocyclic compounds in meat flavor, might also affect the odor of chicken meat. 2-n-Butylfuran increased significantly within 2 h of stewing but was not identified after 3 h of stewing. At the same time, its OAV increased from 4 to 15. Thus, 2-n-butylfuran might play a role in the flavor of meat stewed for 2 h. 2-n-Butylfuran exists widely in chicken and other meat flavor.[Citation16,Citation49] Furan might originate from the dehydration of carbohydrates, Amadori rearrangement reaction, and/or oxidation of fatty acids.[Citation50] Ketones, hydrocarbons, esters, sulfur-containing compounds, and nitrogen-containing compounds might have insignificant effects on aroma perception given their low concentrations and high thresholds. Therefore, the effects of these compounds on chicken meat flavor characteristics were not taken into further consideration.
E-nose analysis
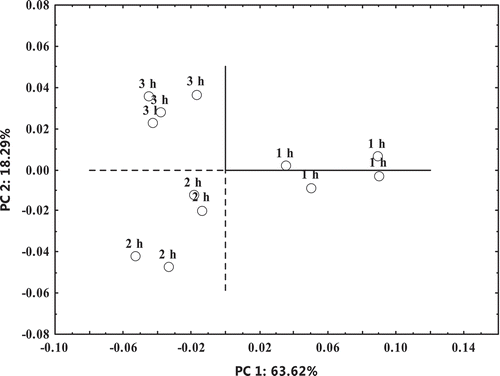
A two-dimensional PCA was performed to analyze the data acquired from the E-nose (). The result indicated good discrimination among the three treatment groups as the contributions of PC1 and PC2 explained more than 80% of the total variance between meat samples stewed for 1, 2 or 3 h. The major variations that resulted from PC1 allowed for the distinction between the 1 h treatment group and the others, whereas the 2 h and 3 h groups were divided into different areas by PC2. This result indicated that the overall odor discrepancy between the 2 and 1 h groups was more than that between the 3 and 2 h groups. In other words, the major difference in overall odor profiles occurred within the first 2 h of the stewing process, with small additional differences at the later stewing period. Similar to the GC-MS data, these results suggested that aldehydes, alcohols, and furans accounted for the major differences in overall odor profiles.
Sensory evaluation
The aroma and taste profiles of the stewed chicken meat were evaluated by a sensory panel consisting of eight trained individuals (). As stewing time increased, the umami and saltiness ratings decreased significantly, whereas aroma ratings increased significantly. As mentioned, the decrease in saltiness of the meat might result from the substantial loss of chloride, while the decrease in umami perception might be due to the decreased EUC and umami-related compounds. These results suggested that stewing for more than 2 h negatively affected the taste perception of chicken meat.
The increased aroma score indicated that stewing increased meat aroma (). As seen in the levels of the volatile components, 1-pentanol was important in maintaining a pleasant odor after stewing for 3 h. Aldehyde and furan concentrations were lower than those in the 2 h group. The difference in aroma ratings between the 1 h treatment group (0.75 ± 0.46) and the 2 and 3 h treatment groups (1.50 ± 0.53 and 1.75 ± 0.46, respectively) was in accordance with the result of the E-nose data. This, coupled with the considerable loss of taste compounds (FAAs, 5′-nucleotides, and minerals) observed within 2 h of stewing, indicates that the major differences in overall flavor characteristics occurred within 2 h of stewing. Since Chinese yellow-feather chicken meat is greatly enjoyed for its umami flavor and aroma, it is important to optimize stewing time for a balanced umami taste and aroma. Our results suggested that acceptable umami taste and aroma levels are attained after 2 h of stewing.
Conclusion
Stewing time had significant effects on the taste-active and volatile compounds found in chicken meat. Stewing improved the aroma profiles but decreased the content of taste components. The main umami-related compounds in chicken meat were IMP and chloride, and the primary volatile compounds included pentanal, hexanal, heptanal, octanal, (E)-2-octenal, nonanal, (Z)-4-decenal, decanal, (E,E)-2,4-decadienal, 1-pentanol, and 1-octen-3-ol, derived from lipid oxidation. The compound 1-pentanol was responsible for maintaining the high intensity aroma after 3 h of stewing because of the significant decreases in the aldehydes. The overall flavor changes tended to stabilize after 2 h of stewing. Thus, stewing for 2 h should be considered an appropriate cooking time to balance umami taste and aroma in stewed chicken meat.
Additional information
Funding
References
- Zheng, M. Q.; Gong, G. F.; Gao, H. J.; Yao, W. Y.; Lv, S. Y.; Tian, L. J.; Wen, J. Report of Chinese Broiler Industry Development in 2015; China Poultry: 2016; Vol. 38, 67–70pp. ( In Chinese)
- Tang, H.; Gong, Y.; Wu, C.; Jiang, J.; Wang, Y.; Li, K. Variation of Meat Quality Traits among Five Genotypes of Chicken. Poultry Science 2009, 88, 2212–2218.
- Jayasena, D. D.; Ahn, D. U.; Nam, K. C.; Jo, C. Factors Affecting Cooked Chicken Meat Flavour: A Review. World’s Poultry Science Journal 2013, 69, 515–526.
- Jayasena, D. D.; Ahn, D. U.; Nam, K. C.; Jo, C. Flavour Chemistry of Chicken Meat: A Review. Asian-Australasian Journal of Animal Sciences 2013, 26, 732–742.
- Kerler, J.; Grosch, W. Character Impact Odorants of Boiled Chicken: Changes during Refrigerated Storage and Reheating. Zeitschrift Für Lebensmitteluntersuchung und-Forschung A 1997, 205, 232–238.
- Gasser, U.; Grosch, W. Primary Odorants of Chicken Broth. Zeitschrift Für Lebensmittel-Untersuchung Und Forschung 1990, 190, 3–8.
- Qi, J.; Liu, D. Y.; Zhou, G. H.; Xu, X. L. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. Journal of Food Science 2017, 82, 2031–2040.
- Sun, L.; Chen, J.; Li, M.; Liu, Y.; Zhao, G. Effect of Star Anise (Illicium Verum) on the Volatile Compounds of Stewed Chicken. Journal of Food Process Engineering 2014, 37, 131–145.
- Duan, Y.; Zheng, F.; Chen, H.; Huang, M.; Xie, J.; Chen, F.; Sun, B. Analysis of Volatiles in Dezhou Braised Chicken by Comprehensive Two-Dimensional Gas Chromatography/High Resolution-Time of Flight Mass Spectrometry. LWT-Food Science and Technology 2015, 60, 1235–1242.
- Jayasena, D. D.; Jung, S.; Kim, H. J.; Yong, H. I.; Nam, K. C.; Jo, C. Taste-Active Compound Levels in Korean Native Chicken Meat: The Effects of Bird Age and the Cooking Process. Poultry Science 2015, 94, 1964–1972.
- Rotola-Pukkila, M. K.; Pihlajaviita, S. T.; Kaimainen, M. T.; Hopia, A. I. Concentration of Umami Compounds in Pork Meat and Cooking Juice with Different Cooking Times and Temperatures. Journal of Food Science 2015, 80, C2711–C2716.
- Chang, Y. N.; Zhao, G. M.; Liu, Y. X.; Li, M. Y.; Huang, X. Q.; Sun, L. X. Changes of Free Amino Acids in Chicken and Its Broth during Cooking; Science and Technology of Food Industry: 2014; Vol. 35, 333–337pp. (In Chinese)
- Zhang, J.; Chen, D. D.; Qi, K. Z.; Xu, Y. Q. Determinaton of Mineral Elements in Chicken Soup by ICP-AES Method. Guangdong Trace Elements Science 2011, 18, 47–51. In Chinese
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir Sinensis). Journal of Food Science 2016, 81, S1–S14.
- Jin, Y.; Zhang, Y.; Jin, Y.; Deng, Y.; Zhao, Y. Impact of High Hydrostatic Pressure on Non-Volatile and Volatile Compounds of Squid Muscles. Food Chemistry 2016, 194, 12–19.
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Lin, L. Determination of the Effects of Different Washing Processes on Aroma Characteristics in Silver Carp Mince by MMSE–GC–MS, E-Nose and Sensory Evaluation. Food Chemistry 2016, 207, 205–213.
- Wattanachant, S.; Benjakul, S.; Ledward, D. A. Microstructure and Thermal Characteristics of Thai Indigenous and Broiler Chicken Muscles. Poultry Science 2005, 84, 328–336.
- Wattanachant, S.; Benjakul, S.; Ledward, D. A. Composition, Color, and Texture of Thai Indigenous and Broiler Chicken Muscles. Poultry Science 2004, 83, 123–128.
- Kim, S. K.; Takeuchi, T.; Yokoyama, M.; Murata, Y.; Kaneniwa, M.; Sakakura, Y. Effect of Dietary Taurine Levels on Growth and Feeding Behavior of Juvenile Japanese Flounder Paralichthys Olivaceus. Aquaculture 2005, 250, 765–774.
- Chen, D. W.; Zhang, M. Non-Volatile Taste Active Compounds in the Meat of Chinese Mitten Crab (Eriocheir Sinensis). Food Chemistry 2007, 104, 1200–1205.
- ISO 1841–1:1996 (E). Meat and Meat Products-Determination of Chloride Content, 1st; International Standard: Geneva; 1996.
- Chiang, P. D.; Yen, C. T.; Mau, J. L. Non-Volatile Taste Components of Various Broth Cubes. Food Chemistry 2007, 101, 932–937.
- Chen, G.; Song, H.; Ma, C. Aroma-Active Compounds of Beijing Roast Duck. Flavour and Fragrance Journal 2009, 24, 186–191.
- Krasnow, M.; Bunch, T.; Shoemaker, C.; Loss, C. R. Effects of Cooking Temperatures on the Physicochemical Properties and Consumer Acceptance of Chicken Stock. Journal of Food Science 2012, 77, S19–S23.
- Rikimaru, K.; Takahashi, H. Evaluation of the Meat from Hinai-Jidori Chickens and Broilers: Analysis of General Biochemical Components, Free Amino Acids, Inosine 5ʹ-Monophosphate, and Fatty Acids. The Journal of Applied Poultry Research 2010, 19, 327–333.
- Wang, D.; Deng, S.; Zhang, M.; Geng, Z.; Sun, C.; Bian, H.; Xu, W.; Zhu, Y.; Liu, F.; Wu, H. The Effect of Adenosine 5ʹ-Monophosphate (AMP) on Tenderness, Microstructure and Chemical-Physical Index of Duck Breast Meat. Journal of the Science of Food and Agriculture 2016, 96, 1467–1473.
- Zhang, J.; Yao, Y.; Ye, X.; Fang, Z.; Chen, J.; Wu, D.; Liu, D.; Hu, Y. Effect of Cooking Temperatures on Protein Hydrolysates and Sensory Quality in Crucian Carp (Carassius Auratus) Soup. Journal of Food Science and Technology 2013, 50, 542–548.
- Dajanta, K.; Apichartsrangkoon, A.; Chukeatirote, E.; Frazier, R. A. Free-Amino Acid Profiles of Thua Nao, a Thai Fermented Soybean. Food Chemistry 2011, 125, 342–347.
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste Enhancements between Various Amino Acids and IMP. Chemical Senses 2002, 27, 739–745.
- Zheng, J. Y.; Tao, N. P.; Gong, J.; Gu, S. Q.; Xu, C. H. Comparison of Non-Volatile Taste-Active Compounds between the Cooked Meats of Pre-And Post-Spawning Yangtze Coilia Ectenes. Fisheries Science 2015, 81, 559–568.
- Jayasena, D. D.; Kim, S. H.; Lee, H. J.; Jung, S.; Lee, J. H.; Park, H. B.; Jo, C. Comparison of the Amounts of Taste-Related Compounds in Raw and Cooked Meats from Broilers and Korean Native Chickens. Poultry Science 2014, 93, 3163–3170.
- Jayasena, D. D.; Jung, S.; Alahakoon, A. U.; Nam, K. C.; Lee, J. H.; Jo, C. Bioactive and Taste-Related Compounds in Defatted Freeze-Dried Chicken Soup Made from Two Different Chicken Breeds Obtained at Retail. The Journal of Poultry Science 2015, 52, 156–165.
- Madruga, M.; Elmore, J.; Oruna-Concha, M.; Balagiannis, D.; Mottram, D. Determination of Some Water-Soluble Aroma Precursors in Goat Meat and Their Enrolment on Flavour Profile of Goat Meat. Food Chemistry 2010, 123, 513–520.
- Macy, R. L.; Naumann, H. D.; Bailey, M. E. Water-Soluble Flavor and Odor Precursors of Meat. 4. Influence of Cooking on Nucleosides and Bases of Beef Steaks and Roasts and Their Relationship to Flavor, Aroma and Juiciness. Journal of Food Science 1970, 35, 81–83.
- Vani, N.; Modi, V.; Kavitha, S.; Sachindra, N.; Mahendrakar, N. Degradation of Inosine-5ʹ-Monophosphate (IMP) in Aqueous and in Layering Chicken Muscle Fibre Systems: Effect of pH and Temperature. LWT-Food Science and Technology 2006, 39, 627–632.
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of Specific Taste-Active Components on Meat Flavor as Affected by Intrinsic and Extrinsic Factors: An Overview. European Food Research and Technology 2015, 241, 157–171.
- Fuke, S.; Ueda, Y. Interactions between Umami and Other Flavor Characteristics. Trends in Food Science & Technology 1996, 7, 407–411.
- Gall, K. L.; Otwell, W. S.; Koburgier, J. A.; Appledorf, H. Effects of Four Cooking Methods on the Proximate, Mineral and Fatty Acid Composition of Fish Fillets. Journal of Food Science 1983, 48, 1068–1074.
- Kadim, I. T.; Al-Ani, M. R.; Al-Maqbaly, R. S.; Mansour, M. H.; Mahgoub, O.; Johnson, E. H. Proximate, Amino Acid, Fatty Acid and Mineral Composition of Raw and Cooked Camel (Camelus Dromedarius) Meat. British Food Journal 2011, 113, 482–493.
- Rickman, J. C.; Bruhn, C. M.; Barrett, D. M. Nutritional Comparison of Fresh, Frozen, and Canned Fruits and Vegetables. II. Vitamin A and Carotenoids, Vitamin E, Minerals and Fiber. Journal of the Science of Food and Agriculture 2007, 87, 1185–1196.
- Schlichtherle-Cerny, H.; Grosch, W. Evaluation of Taste Compounds of Stewed Beef Juice. Zeitschrift Für Lebensmitteluntersuchung und-Forschung A 1998, 207, 369–376.
- Chi, A. Y.; Ji, H. W.; Gao, J. L.; Lu, H. Y.; Lan, W. B.; Meng, L. Y. Effects of Different Heating Treatments on Taste-Active Components of Litopenaeus Vannamei. Modern Food Science and Technology 2012, 28, 776–779.
- Batenburg, M.; Rob, V. D. V. Saltiness Enhancement by Savory Aroma Compounds. Journal of Food Science 2011, 76, S280–288.
- Mottram, D. S.;. The Chemistry of Meat Flavour. In Flavour of Meat and Meat Products; Shahidi, F.; ed.; Blackie Academic and Professional: Glasgow, UK, 1998; 9–10.
- Ruiz, J.; Garcı́A, C.; Muriel, E.; Andrés, A. I.; Ventanas, J. Influence of Sensory Characteristics on the Acceptability of Dry-Cured Ham. Meat Science 2002, 61, 347–354.
- Tanimoto, S.; Kitabayashi, K.; Fukusima, C.; Sugiyama, S.; Hashimoto, T. Effect of Storage Period before Reheating on the Volatile Compound Composition and Lipid Oxidation of Steamed Meat of Yellowtail Seriola Quinqueradiata. Fisheries Science 2015, 81, 1145–1155.
- Lorenzo, J. M.; Domínguez, R. Cooking Losses, Lipid Oxidation and Formation of Volatile Compounds in Foal Meat as Affected by Cooking Procedure. Flavour and Fragrance Journal 2014, 29, 240–248.
- Van Ba, H.; Amna, T.; Hwang, I. Significant Influence of Particular Unsaturated Fatty Acids and pH on the Volatile Compounds in Meat-Like Model Systems. Meat Science 2013, 94, 480–488.
- Wilson, R. A.; Katz, I. Review of Literature on Chicken Flavor and Report of Isolation of Several New Chicken Flavor Components from Aqueous Cooked Chicken Broth. Journal of Agricultural and Food Chemistry 1972, 20, 741–747.
- Fay, L. B.; Brevard, H. Contribution of Mass Spectrometry to the Study of the Maillard Reaction in Food. Mass Spectrometry Reviews 2005, 24, 487–507.