ABSTRACT
The objective of this work was to characterize the traditional cheese, Bouhezza made with raw goat’s milk. There were three cheese making performed; two at the laboratory scale (FH1, FH2) and one in a farm (FH3). The evaluation consisted in the determination of physico-chemical characteristics, proteolysis, microbiological and sensory status. The microbiological characterization was performed during cheese ripening with identification of lactic acid bacteria by 16S rDNA sequencing using PCR-TTGE. The sensory analysis and volatile profile were determined by GC-MS. The pH decreased during cheese ripening from 4.69 to 3.99. The moisture in defatted cheese was 79.05% and fat-in-dry matter 34.77%, which allowed its classification according to the Codex alimentarius as ripened soft and mid-fat cheese. The urea-PAGE pattern of caseins showed a fast proteolysis, which started from the first day of ripening. The sensory evaluation showed that Bouhezza cheese was salty, spicy and intense in odour and aroma. A total, of 100 volatile compounds were identified, where the main components were carboxylic acids (14 compounds) followed by their esters (32) and alcohols (13), aldehydes (5), ketones (8), terpenes and miscellaneous compounds. This study showed that Bouhezza cheese was considered as a product of satisfactory microbiological quality and rich in LABs whose contribute to its ripening, where many of identified strains were the same as those found in other dairy products. The main sensory descriptors as flavour and odor, was lactic flavor and lactic acidic odor. The products of proteolysis gives to Bouhezza a riches in volatiles and fragrance characteristics, which reflect the uniqueness of this cheese of terroir. Finally, an identity card of the Bouhezza cheese was achieved following results of this study.
Introduction
Bouhezza is a traditional cheese manufactured from different types of milk (cow or goat) in the Chaouia regions of Eastern Algeria. Its manufacture is carried out in a permeable skin bag called “Chekoua or Jeld of Bouhezza”. This cheese is made without rennet, suffers no heat treatment; the self-regulation of manufacturing is based on the salt and the acid produced by the indigenous microflora of milk. They contribute to protect the product regarding the contamination flora and pathogenic germs, and thus keep cheese safely.
Before manufacture of Bouhezza, Rayeb and Lben are prepared. The raw milk was undergo a spontaneous fermentation during 24–36 h at ambient temperature (25–30°C) until gelation; the curd obtained is called “Rayeb”. This last is vigorously churned during 30–60 min and some warm water is added (0.1 L/liter of the mixture) which promotes gathering a butter grains and facilitate the skimming operation. After a partial skimming the product obtained, called “Lben” is used as a prime matter for Bouhezza manufacturing. Cheese manufacturing begins with the salted Lben (amount of salt was 20–25 g/L of raw material). Some quantity fill the Chekoua (half or 3/4 the volume) and followed by several additions (2 at 3 liters) at 3–4 days interval of Lben and finally whole raw milk (2 at 3 L at the same interval), two weeks before recover the ripened cheese. The Chekoua is suspended in a ventilated area or in the shade. During Bouhezza preparation (in average 2 at 3 months), the cheese is subjected to ripening in the Chekoua, where draining is occurred through the skin bag pores. Cleaning the Chekoua from the outside was done every day. At the end, the cheese was spicy with a red hot pepper to give a specific taste to Bouhezza according to the habits of consumers.
Proteolysis and lipolysis are the important processes, which take place during the ripening. This biochemical catalysis of substrates (proteins and fats) is the process that leads to the production of metabolites and aromatic compounds, which contribute to the organoleptic properties of cheeses, such as taste, aroma, appearance and texture.
Proteolysis can occurs in cheeses made with raw milk without starter culture; through indigenous milk enzymes, adventitious non-starter microflora and enzymes from secondary flora. Proteolysis contributes to textural changes of the cheese matrix, due to breakdown of the protein network.[Citation1] Traditional cheeses produced with raw milk, are known only in a limited geographical area, and are consumed locally in small quantities. Dagdemir and Ozdemir[Citation2] have reported that the enzymatic system of indigenous microflora in raw milk is much more complex than starter-bacteria used in cheese manufacture. Also, it has been established that indigenous microflora plays an important role in proteolysis.[Citation3]
Several studies have focused on the comparison of sensory characteristics of cheese varieties prepared from raw milk, pasteurized or micro-filtered milk. Beuvier et al.[Citation4] have demonstrated that microflora in the raw milk has an important role on biochemical and sensory characteristics of cheese. In addition, the enzymes of LAB (proteolytic or peptidolytic) contribute to the ripening and sensory properties of the final products.
Traditional cheeses in Algeria are few, little known and studied, but they begin to have a re-newed interest in the last two decades by the scientific community. Characterization studies on the traditional cheese Bouhezza cow’s milk were carried out by Aissaoui Zitoun et al. [Citation5; Citation6]; and on Bouhezza goat milk by Medjoudj et al.[Citation7] Characterization of the traditional cheese Mechouna by Derouiche and Zidoune.[Citation8] Other products were also of interest and were characterized, most notably; Djben and Klila[Citation9] in eastern Algeria.
The objective of this work was to characterize the Algerian traditional cheese, Bouhezza made with goat’s raw milk. Cheese production was carried out in order to characterize Bouhezza in microbiological point of view by the research and identify of the microbial ecosystem and showing its biodiversity. The identification of lactic acid bacteria, which have an influence on ripening process to highlight the eventual presence of the pathogenic and contamination flora. The physicochemical characterization allowing to classifiy the cheese, determination and highlighting the proteolysis what undergoes the cheese during the process of ripening. The aim was also to perform a sensory analysis of the final products and volatile profile as a result of enzymes activity and of indigenous microflora action giving the aromatic components, of the flavour, aroma and odor of this product of the terroir. At the end, establishing the first technical card identifying cheese Bouhezza made with goat’s raw milk.
Materials and methods
Cheese manufacturing
Three cheese making of Bouhezza (FH1, FH2, and FH3) were prepared with goat’s milk according to the survey in Chaouia region and to the traditional process described by Medjoudj et al.[Citation7] (). The manufacture requires use of fermented milk called Lben. Three liters of salted Lben (25 g of NaCl per L of Lben) were put into each Chekoua (3 Chekouates). Lben was continuously added during six weeks for FH1 and FH2, and for eight weeks for the FH3 (3 L to start on day 1, and then every three days from 2 to 2.5 L until the day 34 for the FH1 and FH2, and 56 day for FH3). After, the process was completed with the additions of whole raw milk every three days (2 to 2.5 L/Chekoua) to correct the acidity and salt level, and to provide fat. At the end (at 50th day for FH1 and FH2 and at 72nd day for FH3), a part of the cheese produced was spiced with red chili pepper.
Figure 1. Diagram of traditional cheese manufacture Bouhezza made with goat’s raw milk according to the survey in Chaouia region and the process described by Medjoudj et al.[Citation7].
![Figure 1. Diagram of traditional cheese manufacture Bouhezza made with goat’s raw milk according to the survey in Chaouia region and the process described by Medjoudj et al.[Citation7].](/cms/asset/ecf9eb60-76fc-4950-9cc6-2efda46f532d/ljfp_a_1375515_f0001_oc.jpg)
Chemical analysis
Sampling
Analyses were performed on Lben (3 samples) at the first day, 3rd and 6th weeks of Lben addition. Samples of goat’s raw milk (3 samples) and cheese at different ripening age were analyzed. The volume levied for Lben and milk was 60–70 mL in a sterile bottle (three bottles for one sampling thus about 200 mL in total by sample). To evaluate the cheese microbiological and physicochemical parameters, sampling was carried out as follows: FH1, FH2 at 1, 7, 15, 28, 34, 42, 50 and FH3 at 1, 15, 30, 45, 56, 64 and 72 days. The cheese quantity levied was 55–60 g of cheese in three sterile bottles for each sample, thus between 175–180 g per sampling. Each bottle was used to perform a group of analyzes; one for microbiological analysis which were performed on fresh samples, the second one for physico-chemical and the third bottle was stored at −20°C until analysis for the urea- PAGE, PCR-TTGE, GC-MS. For urea-PAGE and PCR-TTGE analyses, two samples of traditional cheesemaking of Bouhezza made with cow’s milk were used for comparison. These samples were obtained from Hammamet (Tebessa) and Ain Fakroun (Oum El-Bouaghi) with a ripening time more than 30 days.
Chemical analysis
Milk, Lben and cheese samples were analyzed in duplicate for moisture by the oven drying method at 102°C ± 2°C [9a], salt by Volhard method[Citation10], fat by the Van Gulik method and ash.[Citation11] The pH was measured using a digital pH meter.[Citation12] Titratable acidity according to AOAC[Citation13]; and the results were expressed as the percentage of lactic acid. Protein was determined by the Lowry method.[Citation14] All these analyses were performed in triplicate. The yield of fresh or ripe cheese was calculated for FH1 and FH2 samples.
Proteolysis analysis
Nitrogen fractions
Proteolysis was analysed by determining the water-soluble nitrogen content in acetate buffer at pH 4.6 (pH 4.6-SN) and total nitrogen, at different ripening times by the micro-Kjeldahl method.[Citation15]
Urea polyacrylamide gel electrophoresis (urea-page) of caseins
The pH 4.6-insoluble N content (pH 4.6-IN) of Bouhezza cheese was determined as described by Fallico et al.[Citation16] This fraction was analysed by urea-PAGE using a Protean II xi vertical slab gel unit (BioRad Laboratories Ltd., Watford and Herts, United Kingdom) according to the method described by Andrews.[Citation17] The gels were stained using a modified method of Blakesley and Boezi[Citation18] using the dye, Coomassie Brilliant Blue G250.
Microbiological analysis
Ten grams (10 g) of cheese were, aseptically sampled, mixed in 90 mL of sterile 2% trisodium citrate buffer, pH 7.5. Cheese, milk and Lben decimal dilutions were mixed with sterile 1/4 strength Ringer’s solution.[Citation19] Total aerobic mesophilic bacteria (TAMB) count on PCA; incubated at 30°C for 3 days (72 h). Presumptive lactococci counts were enumerated on M17 agar after incubation at 37°C for 48h. Presumptive lactobacilli were enumerated on de Man Rogosa Sharpe (MRS) agar after an anaerobic incubation at 37°C for 48h. Yeasts and moulds were enumerated on Oxytetracycline Glucose Agar after incubation at 25°C for 5 days. Total and fecal coliforms were counted on Desoxycholate agar at 0.1% at 37°C and 44°C for 48 h and 24 h, respectively. Fecal streptococci were counted in liquid medium Rothe, incubated at 37°C for 48 h and confirmed in Litsky medium at 37°C for 24 to 48 h. Sulphite-reducing Clostridium were grown on Meat-Liver media which were incubated at 37°C for 72 h. The pathogenic bacteria in the cheese samples were analyzed with Salmonella Shigella (SS) and Baird Parker agars for Salmonella and Staphylococcus aureus, respectively. An enrichissement, in the selenite of natrium and Giollitti Cantoni respectively, for 48 hours at 37°C. The respective media were inseminated from a positive tube of broth, and incubated at 37°C for 24 and 48 hours. For the control samples of cow’s milk cheese, enumerations of pathogens (Salmonella, S. aureus, Listeria and E. coli O157 H7) were performed, using the BAX® System (DuPont Qualicon, UK) after enrichment. The BAX System was used only on the samples of cow’s milk used for comparison with those of goat’milk cheese. Because no microbiological analysis have not been done on these samples.
PCR-TTGE identification
The method adopted in our work for identification of microbial biodiversity and ecosystem of Bouhezza cheese, was PCR-Temporal Temperature Gel Electrophoresis (PCR-TTGE). DNA extraction from cheese samples and amplification of the V3 region (16S rRNA gene) were performed as described by Licitra et al.[Citation20] Amplification according to the protocol proposed by Parayre et al.[Citation21], in the region of the DNA V3 gene encoding 16S ribosomal RNA (16S rDNA) by PCR using universal primers V3P3- GC-Clamp (5ʹGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCGGGGGGGCCTCGGGAGGCAGCAG-3ʹ) and V3P2 (5ʹ-ATTACCGCGGCTG CTGG-3 ‘) (Sigma, Italy). These primers give the PCR products about 233 base pairs. A DCode universal mutation detection system was used to separate the V3 region PCR product from total genomic DNA. The reaction mixture of the primers was composed of 50 µL into each PCR tube with 40 µL of PCR Mix and 10 µL of the extract of bacterial DNA obtained from the cheese matrix.
Thermocycler cyclist Thermal cycler (BioRad Laboratories, Hercules, CA) amplifies samples by incubation at 94°C/2min, followed by 35 cycles. A cycle was the succession of three steps: 30 s denaturation at 95°C, 60 s hybridization at 63°C and elongation for 1 min at 72°C following a final extension of 5 min at 72°C and at the end stabilization of the temperature reaction at 4°C.
Temporal temperature gel electrophoresis (TTGE) analyzes were performed with the universal mutation detection system (BIO-RAD Laboratories, Hercules, CA, USA). The gel used was composed of 10% acrylamide, 40% bisacrylamide (37.5: 1) and 7 M urea (10.5 g) for the Resolving gel, and 8% acrylamide for the Stacking gel.
Ten μL of the amplifier (PCR product) of each sample were mixed with 5μL of loading buffer (0.05% of bromophenol blue, 0.05% of xylene cyanol and 70% of glycerol). The mixture of each sample was then deposited in a well of the Stacking gel. The marker was used with 12 strains (Lactobacillus plantarum CNRZ211T, Lactobacillus fermentum CNRZ209T, Enterecoccus faecium LMG 8149, Lactobacillus helveticus CNRZ 137, Enterecoccus faecalis CNRZ137 13; Lactococcus lactis CNRZ105T, Streptococcus thermophilus CIP102303T, Corynebacterium moorparkenese CIP107183T, Lactobacillus paracasei LMG9192; Arthrobacter nicotianae CIP82.107T, Brevibacterium casei CIP102111T, Propionibacterium cyclohexanicum TL1365T), 30 μL of marker were deposited per well (the marker was obtained from INRA-Agrocompus laboratories in Rennes, France).
Migration was performed on a urea polyacrylamide gel under a tension of 41 V for 16 h with a temperature gradient of 63°C to 70°C (rate of 0.4°C h−1) for bacteria low-level GC. After staining with ethidium bromide (0.6 μg of ethydium bromide per mL of buffer TAE 1.25X) for 15 min. The gel was then immersed in distilled water for 30 min. The bands were finally visualized and photographed on a UV transillumination table (E-Box 1000/26M, Euroclone, Siziano, Italy) and analysed using Gel Compare software.
The bands were analysed using the TTGE species database developed by Parayre et al.[Citation21] using the BioNumerics program, version 4.6 (Applied Maths, Kortrijk, Belgium). The data base compares the bands to the 56 most common species in cheeses and dairy products belonging to the 18 genera (Arthrobacter, Bacillus, Bifidobacterium, Brachybacterium, Brevibacterium, Corynebacterium, Enterobacterium, Kocuria, Microbacterium, Propionibacterium, Staphylococcus, Hafnia, Escherichia, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc and Streptococcus). In order to specifically identify the main microorganism detected by TTGE profiles, a species-specific PCR assay was performed using specific primers. Nineteen colonies were isolated from M17 plate at 37°C of Bouhezza cheese sample at 7 days. The Lactococcus lactis specific assay was carried out using 1RL and LacreR primers with conditions recommended by Ogier et al.[Citation22]
Volatile analysis
Bouhezza cheese prepared in the goat-skin bag were sliced into small granules and, placed immediately in glass bottles in a freezer at −20°C. Volatiles were determined by solid-phase microextraction (SPME) method using gas chromatography-mass spectrometry system (Shimadzu Corporation, Kyoto, Japan), as described in Sulejmani et al.[Citation23] Concentrations were calculated by the comparison of the internal standard peak area and the unknown compounds. Each compound was expressed in a microgram per kg of cheese.
Sensory analysis
This analysis provides sensory description of three spiced samples, two from the experimental cheese making in the laboratory (FH1 and FH2 removed at 50 d of ripening) and the third Farmhouse 1 (FH3 sampled at 72 d). According to Bérodier et al.[Citation24], the sensory characteristics of the cheese can obtained and clarified through tasting and visual observations. A panel of ten trained persons (students of final year of Engineer in Agri-Food Sciences in INATAA (Institute of Nutrition, Food and Agro-Food Technologies) University of Constantine 1). The subjects were trained in sensory evaluation before test. They studied the sensory analysis and organoleptic properties of foods (courses are given for students during their education).The group evaluated the spicy cheeses for texture, odour, aroma, flavour and persistence taste in mouth. According to the guidelines of the V09-001 standard (AFNOR)[Citation25], the tests were conducted. Sensory evaluation was carried out with scoring test, on a point scale from 1 (low) to 7 (high).
Statistical analysis
Analysis of chemical parameters at different stage of ripening was examined by ANOVA (Costat 6.400, CoHort Software 1998–2008) followed by the least significant difference (LSD) test . The relationship between variables in volatiles was assessed by the principal component analysis (PCA). The data of sensory description were analyzed, with the Student test at threshold of 5%. Both tests were performed using XLSTAT[Citation26] version 2009.1.02 (Copyright Addinsoft 1995–2009) for Windows.
Results and discussions
Chemical composition
Physico-chemical characterization of the raw material
The pH of milk was 6.3 ± 0.19 corresponding to 1.85 ± 0.02 g/L of lactic acid. The Lben used in this manufacturing was more acid taste (pH 3.8 ± 0.70 and 8.65 ± 0.06% of lactic acid) than that used by Medjoudj et al.[Citation7] which was pH 4.70. The total solids of milk (13.05 ± 0.75%) were close to values reported in the literature (12.5–13.0%). The rate of total dry solid of Lben was 7.44 ± 0.04%, chlorides content of milk and Lben were 0.24 ± 0.04% and 0.2 ± 0.02%, respectively.
Fat in cheese plays a decisive role on the organoleptic quality, especially on the taste and flavor. The level of fat obtained for Lben was 2.1%, which was lower than that cited in literature (4.03%).
According to the literature, goat milk contains between 2.8 and 3.5% of protein. It depends on the feed supply, the period of lactation and the health of the animal. The milk used contains 3.87 ± 0.06% of protein, this content increased slightly in the Lben (4.25 ± 0.07%). The ash level of milk was 0.42 ± 0.0% which increased in the Lben to 2.94 ± 0.14%, due to the converting of milk into Lben, where minerals are released into the curd (Rayeb) after coagulation.
Physico-chemical properties bouhezza cheese
During cheese manufacturing, total solid, fat, protein contents and titratable acidity increased. The same trend was reported for Tulum[Citation27], Xinotyri[Citation28] and Bouhezza cow’s milk[Citation5] cheeses. The pH of the cheeses decreased during ripening, its values were 4.69 for day 7 and 3.99 for day 50. No significant difference was observed in pH value of FH1 and FH2 cheeses. The same notes were observed for goat cheese Bouhezza.[Citation7] The levels of titratable acidity ranged from 0.85 to 1.59% and no significant difference was recorded between the three-cheese makings. The dry matter (DM) content increased and reached 30.60% at the end of ripening. Difference in DM between the FH1 and FH2, and FH2 and FH3 cheese making were significant. The rate of fat and protein also increased proportionally with DM. This increase was due to the water drainage through the pores of Chekoua. The salt and ash content exhibited the same changes during ripening and no statistical differences were observed (P > 0.05).
The different characteristics of FH1 and FH2 compared to FH3 are probably due to the difference in the origin of raw milk and the difference in the goats’ grazing. The codex alimentarius standard (2006) issued a classification table in which the cheeses were classified according to three criteria. The first one is the firmness expressed as moisture content in the defatted cheese (MDC) with an interval between 69 to 51% of the extra soft to hard paste (soft paste = MDC> 67%), Bouhezza has a MDC of 79.05%. The second criterion was that of the fat relative to the dry matter (FDM), the ranges of values for classifying the cheeses according to content of fat in the dry matter, the value of FDM obtained for Bouhezza was an average of 34.77% (mid-fat = FDM between 25–45%). The third criterion was that of cheese ripening characteristics (on the surface, inside the paste, ripened on surface or mass with molds, and fresh cheese). According to the Codex Alimentarius (2006), these results allowed their classification among the ripened cheeses soft, mid-fat. Medjoudj et al.[Citation7] classified Bouhezza goat’s milk cheese, as ripened cheese soft, mid-fat. The dry cheese yields of FH1 and FH2 were 40.62% and 40.67% and, 15.09 and 13.9% for fresh cheese yield, respectively.
Proteolysis
Protein fractions
Protein fractions in Bouhezza goat’s cheese are shown in . The total protein level was 15.65% in the samples of the final product. The proteolysis rate expressed by water-soluble nitrogen (WSN or SN at pH 4.4) in total nitrogen was 13.04% as the average of the three cheese making trial. These results were slightly lower than that of Medjoudj et al.[Citation7] in Bouhezza goat’s cheese with 14.44% at 72 d. However, lesser than that of Tulum cheese ripened in goat-skin bag.[Citation29] The obtained data was similar to those reported by Aissaoui Zitoun et al.[Citation6] and higher than in Xinotyri cheese from goat’s milk obtained at 45 and 60 days of ripening.[Citation30]
Table 1. Chemical composition and pH of traditional Algerian Bouhezza goat’milk cheese. Total Nitrogen (TN) and Water Soluble Nitrogen (WSN/TN %) expressed as average ± standard deviation. Acidity was expressed as % of lactic acid.
Urea-page electrophoresis of insoluble fraction of proteins in bouhezza cheese
Urea-PAGE profiles () showed two main bands at different electrophoretic mobility, corresponded to native caseins. The casein group with low mobility corresponds to β-casein, while the once with higher mobility was αs-casein.[Citation31] Three peptides with a low electrophoretic mobility were detected, given the general profile of the caseins. These were identified in Bouhezza cow cheese[Citation6] and goat’s cheese[Citation7] with β-CN f29-209 (γ1-CN), β-CN f106-209 (γ2-CN), β-CN f108-209 (γ3-CN). These peptides appear as a result of the early action milk indigenous enzyme as plasmin on β-casein.
Figure 2. Urea-PAGE of the pH 4.6 insoluble N from Bouhezza cheese samples made with goat milk derived from two different experimental cheese making (FH1- FH2): 1 cheese after 1 day of ripening, 2.cheese after 14d, 3.cheese after28d, 4.cheese after 42d, 5.cheese after 50d without pepper and 6.cheese after 50 d with pepper. Experimental cheeses were compared with Bouhezza cheese samples made with goat milk derived from traditional cheese making (FH3: 1 cheese after 1 day of ripening, 2.cheese after 15d, 3. Cheese after 30d, 4.cheese after 45d, 5.cheese after 64d without pepper and 6.cheese after72d with pepper) and with two Bouhezza cheese samples >30d made with cow milk (Teb-Ain).
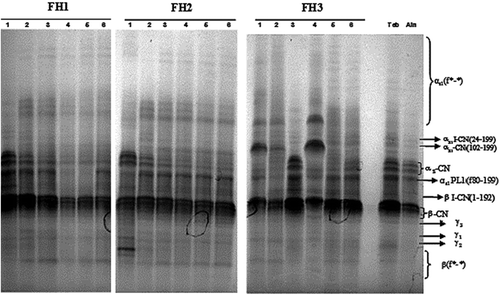
Some peptides moving slower than γ-CN, (βf*−*) were detected at each stage of ripening. Fallico et al.[Citation16] reported that these fragments were probably produced from β-casein by the action of plasmin or microbial proteinases with a trypsin-like activity. A fragment between αs-casein and β-casein bands was also detected. Some authors identified this band as αs1 (f80-199) (αs-PL1), may be allowed in Bouhezza cheese from a further action of plasmin on αs-casein.[Citation32] The bands found just above αs-casein, these had the position of αs- casein (f24-199) and αs-casein (f102-199), fragments produced probably by cathepsin D. The latter, have similarity of chymosin, which is the most active acid proteinase in cheese on αs-casein stimulated with increasing salt content in cheese.[Citation33] Although the manufacture of Bouhezza cheese does not contain the addition of rennet, detection of these peptides could be due to an enzymatic activity of cathepsin D on caseins, in particular αs1-casein. Few differences were detected in comparing casein profiles of cheeses, but in the αs-casein region resulted in another bands in the experimental samples (FH1-FH2). Furthermore, casein bands in FH3 at 30 d resulted in a little difference from the others. The addition of raw milk allowed further enzymatic activity resulting in increasing of hydrolysis as demonstrated by the disappearance of the peptide positioned just under β-CN f106-209 (γ2 -CN) band.
Microbiological analysis
summarized the microbiological data. The counts for TAMB in milk and Lben varied from 9.81 to 9.65 log cfu/mL, but decreased in cheese from 9.23 log cfu/g (7days) to 7.51 log cfu/g (at the end of ripening process). Lactic acid bacteria (LAB) in milk presented an average of 8.40 log cfu/mL (lactococci) and 8.51 log cfu/mL (lactobacilli). They increased after processing of milk into Lben with the level of 9.99 and 8.68 log cfu/g, respectively. Ouadghiri et al.[Citation34] observed the same counts of lactic acid bacteria with 7 log cfu/mL in raw milk, which amounted to 9 log cfu/mL in Moroccan fermented skim milk (Lben). Those results can be explains by the low pH and high content of lactic acid thus the LABs increased in Lben because acidity is one of the favourable factor for growth of these bacteria.
Table 2. Results of the count of microbial flora in the raw material as log cfu/mL (milk and Lben) and evolution of the microbial population in cheese Bouhezza goat’s milk during ripening, in log cfu/g of three samples, F1, F2 and F3.
Lactic flora showed some fluctuations during manufacturing with a large load of the LAB about 7 to 9 log cfu/g consisting essentially strains: Lc. lactis subsp. cremoris and S. gallolyticus subsp. macedonicus. Lactobacilli are present with a low load of 6 to 8 log cfu/g containing Lb. plantarum, Lb. johnsonni, Lb. gasseri, Lb. fermentum, Lb. helveticus, Lb. acidophilus and Lb. crispatus.
LAB in Bouhezza cheese were stable with slight decreasing from 1rst to 42nd day
from 9.54 to 8.03 log ufc/g and from 8.37 to 7.52 log ufc/g respectively for lactococci and lactobacilli. These counts decreased to 7.67 and 7.31 log cfu/g respectively at the last 15 days of ripening for the two genus. The decreasing of LABs at the end production of cheese was probably due to lyse of bacteria cells and their disappearing when releasing their enzymes into the cheese matrix during ripening.
Total and fecal coliforms in milk and Lben, total coliform (TC) were 1.4 log cfu/mL (and Fecal coliform (FC) were not found with exception for one sample at the first production where it was found 3.15 and 1.55 log cfu/mL of TC and FC, respectively. TC in milk, increased slightly in the Lben to 2.50 log cfu/mL and 2.68 log cfu/mL and then decrease slightly in the non-spicy cheese 2.68 log cfu/g. The FC were absent in milk and present at very low levels in the Lben (). Aissaoui Zitoun et al.[Citation6] found a higher numbers of TC and FC in cows Lben, which decreased in spicy and non-spicy Bouhezza.
The presence of fecal coliforms in Lben and cheese and their absence in milk can be explained by the fact that milking is carried out under hygienic conditions and irreproachable cleanliness. However, during the preparation of the Lben, there is addition of non-sterile lukewarm tap water before skimming, which might contaminate of faecal coliforms and thus contaminate the Lben and consequently the cheese. The successive additions of Lben to which water was added, can probably be a source of coliforms. During the manufacture of Bouhezza, the number of CF increases from the beginning of manufacture of 1.03 log cfu/g on the first day to 3.24 log cfu/g on the 21st day. Then there was a gradual decrease to 1.22 log cfu/g at end of ripening. This final value was below the health quality standards (Official Journal N ° 35 of 27th May 1998 of the People’s Democratic Republic of Algeria) which require a CT number of <30 cfu/g for soft cheeses, which considers Bouhezza as a satisfactory microbiological quality product, thus can be safely consumed. Also for the raw material used; compared with CF standards in raw milk (<3.104 cfu/mL) and Lben (acidified milk <90 cfu/mL), they are aligned as a satisfactory mirobiological quality products. A precision can be brought, to explain the presence of TC in the samples of Bouhezza; those of the laboratory’s experimental manufacture, the presence was in the Lben and in cheese Bouhezza, only at the first day for the two manufacturing and in the 7days sample in the F1 with 90 cfu/g. Then they disappeared of all samples until final product. On the other hand, for the traditional farm manufacture F3 the conditions of preparation which were non-rigorous there is always a risk of contamination. Either, by the tap water added during churning or through the various manipulations during manufacture were probably the cause of the presence of CF in the Lben, and in cheese from the beginning to the end of manufacture.
Enumerations of fungal flora of cheese ranged from 3 to 6 log cfu/g. Thus, Yeasts and Moulds were 5.17 and 6.36 log cfu/mL in milk and Lben respectively. In Bouhezza cheese, the fungal flora was stable during 4 weeks with levels between 3 and 4 log cfu/g. This flora reached 6.45 log cfu/g at the 4th week, then decreased during the three following weeks and finally reached 6.53 log cfu/g at the 8th week. This increasing was probably due to the favourable acidic conditions (low pH and high acidity) and salt contents for yeasts. The level in Tulum at 60 days was 5.79 and 6.95 log cfu/g at 90 days.[Citation27] Faecal Streptococci are absent in raw milk but present in Lben, there was 1.70 log cfu/mL. At the first week, there was 2.04 and 2.48 log cfu/mL in F1 and F2, respectively. Moreover, they disappeared in the following stages of manufacture. The pathogenic microorganisms were not present in the raw material (milk and Lben), nor in cheese.
PCR-TTGE identification
The TTGE electrophoretic profil () showed eight bands corresponding to species of lactic acid bacteria, found in Bouhezza cheese samples made using goat’s raw milk. These species identified, according to Parayre et al.[Citation21], as; Lb. plantarum/Lb . johnsonni/Lb. gasseri (band a), Lb. fermentum (band b), Lb. helveticus/Lb. acidophilus/Lb. cripatus (band j); appear from 28 until 50 d for FH1 and at 14 d for FH2. S. gallolyticus subsp. macedonicus (band n) and Lc. lactis subsp. cremoris (band p) were present in Lben (1d) and in all cheese samples, including control cheese sample made with cow’s milk, from Ain Fakroun (). On the other hand, S. xylosus (band h) was present only in FH3 after 64 and 72 days of ripening. Lb. buchneri (band r) and the band v correspond to B. breve/Hafnia alvei are present in control cheese sample (from Ain Fakroun).
Figure 3. TTGE (Temporal Temperature Gradient Electrophoresis) of Bouhezza cheese made with goat milk after DNA extraction of samples derived from two cheese making in Laboratory (FH1); 1. Cheese after 1day ripening, 2. After 14 days ripening, 3. Cheese after 28d ripening, 4.after 42 d ripening, 5. After 50 d ripening without Red pepper and 6. After 50 days of ripening with red pepper. FH2; 7.after 1 day ripening; 8.after 14d ripening and 9.after 50d ripening without red pepper. The two experimental cheese making are compared to the traditional cheese making at farm:FH3; 10.after 64days ripening without red pepper and 11.after 72d ripening with red pepper, and two samples farm making teb+RP;12.cheese >30d ripening wih red pepper; AinFak:13.>30d ripening with red pepper. M: genomic DNA marker.
Band: Specie assegnate to database: A. Lb. plantarum/Lb. johnsonni/Lb. gasseri; b. Lb. Fermentum; h S. xylosus; j. Lb. helveticus/Lb. acidophilus/Lb. crispatus; n. S. gallolyticus subsp. macedonicus; p. Lc. lactis subsp. cremoris; r. Lb. buchneri; v. B. breve/Hafnia alvei.
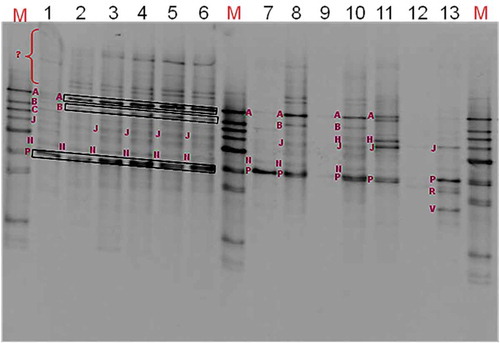
The variety of goat milk used in cheese making, the difference in animal diet, and possibly the climate of the region have given this difference in the bacterial composition between experimental (FH1, FH2) and farm (FH3). No band was shown in samples 9 and 12. The detection of three (possibly five) unknown bands with a lower percentage of G + C in the FH1 and FH2 samples was noted.
Among the lactic acid bacteria found in cheese samples from goat’s milk, the genus Lactobacillus was predominant, due to their resistance to acidity and salt in cheese. The same species were found in Lebanese Darfiyeh cheese made with goat’s raw milk; such as Lb. plantarum and Lc. lactis subsp. cremoris.[Citation35] Aissaoui Zitoun et al.[Citation6] reported that the major species found in Bouhezza cheese made with cow’s milk, Lc. lactis, Lb. plantarum, Leuconostoc cremoris/Leu. mesenteroides. Lc. lactis was the major species identified by TTGE and is the one of the most common species found in cheese ecosystems.[Citation36]
Enzymatic system of LABs play a vital role during cheese manufacture, because amino acids resulting from proteolysis of casein are the major precursors of specific flavor compounds, such as various alcohols, aldehydes, acids, esters, and sulfur compounds.[Citation37] Microbial diversity has a great influence on hygienic and sanitary quality, and thus on the nutritional, organoleptic and sensorial aspects. Fermented dairy products generally contain complex microbiota, particularly when they are made from raw milk[Citation21] such as Bouhezza. The cheese making from raw milk has shown its beneficial effect on several levels. The acidity developed during the lactic fermentation with the amount of lactic acid released in the cheese mass, which has an antiseptic effect as well as its advantages in the intestinal absorption of the calcium. Then the residual lactose that is degraded to give the molecules of the flavor and the cheese taste. The proteolysis and lipolysis that occur during ripening process are also advantages, on the cheese texture, products of hydrolyze released, which are peptides and essential fatty acids. These latters will in turn be hydrolyzed by the enzymes such as peptidases and lipases of the original bacterias (LABs), that results in a variety of flavoring molecules giving diversified more intense and more pronounced sensory and aromatic profiles, to the cheese. Biochemical events where the microbial enzymatic system involved during ripening are very important for the development of the various volatile aromatic products until the end of manufacture. Some of the flavoring components released have antiseptic and antimicrobial effects. The secreting of bacteriocins by the LABs in the matrix of the cheese will complete the sanitization or protection of the cheese with regard to the contamination germs and the pathogenic germs.
Volatile composition
Esters
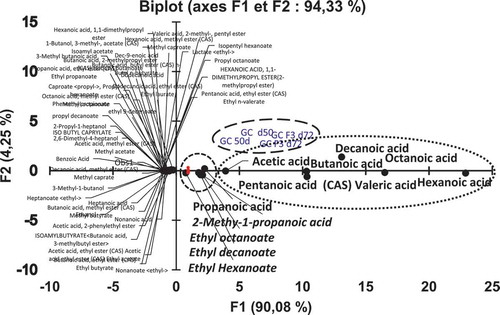
The major esters found in Bouhezza cheese () were ethyl esters (14) (ethyl acetate, butanoate, hexanoate, octanoate and decanoate and 2-phenethyl acetate), which were determined at 72 d. Others esters were methyl (5), propyl (7), butyl (3), isoamyl and isopentyl esters (2). Their concentrations changed during ripening.
Table 3. Esters (mean ±sd. µg.Kg−1 of Cheese) in the Bouhezza cheese making with goat milk.
The highest levels of some esters (ethyl butanoate, hexanoate, octanoate, decanoate and acetate) were determined after 72 d. The levels of these compounds were higher in the FH3 sample than those found in FH1 and FH2 at 50 d. While, propyl and butyl esters were low levels at d 50, but increased at d 72. As reported by Hayaloglu et al.[Citation38], the most important esters in the goat’s cheese were ethyl hexanoate, ethyl octanoate and ethyl decanoate in Turkish cheeses of the goat’s milk Gokceada and Saanen. Ethyl acetate formed from lactose by lactic bacteria (LAB); citrate and lactate metabolism, or as a product of the catabolism of amino acids is an important flavor compound in many cheeses.[Citation39]
Carboxylic acids
The carboxylic acids characterized in this study were more concentrated in cheese samples (). The most abundant carboxylic acids at 50 or at 72 d were acetic, propanoic, butanoic, pentanoic, hexanoic, octanoic and decanoic acids. However, 3-methylbutanoic, heptanoic, nonanoic, dec-9-enoic, benzoic and dodecanoic acids have lower levels in Bouhezza cheese. At the 50 d, the increase of the short-chain carboxylic acids concentrations (363.95 to 2,614.67 µg. kg−1) was very important, slightly more than those observed at 72 d.
Table 4. Carboxylic acids (mean ±sd. µg.Kg−1 of Cheese) in the Bouhezza cheese making with goat milk.
Among carboxylic acids, hexanoic was present at the highest level, followed by octanoic, pentanoic, decanoic, butanoic and acetic acids. Medjoudj et al.[Citation7] reported the same level and order of carboxylic acids in Bouhezza cheese made with goat’s milk, ripened at 72 d. These acids were the main acids presented in many goat cheeses made using raw or pasteurized milk.[Citation7,Citation38,Citation40–Citation42] This high level is probably due to the contribution of fat brought by the adding of the whole raw milk during the manufacturing of Bouhezza from 34 and 56d. The short and medium-chain fatty acids (FAs), released into the cheese by lipolysis, contribute directly to cheese flavor.[Citation43] They are also precursors of molecules of flavor and aroma compounds.[Citation44,Citation45] FAs were identified in soft goat cheese, and characterized as goaty aroma.[Citation40]
Alcohols
Thirteen alcohols were identified in the cheese () during 50 d of ripening and only five alcohols were found in farm sample ripened at 72 d. Ethanol, 1-octanol and phenethyl alcohols presented a higher level in cheese at 72 d. In contrast, 3-methyl-butanol and 2-nonanol were higher at 50 d than at 72 d.
Table 5. Alcohols (mean ±sd. µg.Kg−1 of heese) in the Bouhezza cheese making with goat milk.
Ethanol has a limited aromatic role in cheeses, but it is the precursor of several esters and the conditions in the cheese can promote the rapid reduction of aldehydes and ketones to their primary and secondary alcohols.[Citation46] The concentrations of 3-methyl-1-butanol decreased from 50 d to 72 d. Some compounds were present at d 50 of ripening and absent at 72 d, such as 2.6-dimethyl-4-heptanol, 2-propyl-1 heptanol, 4-methyl-2-pentanol, 1-hexanol, 2-methyl-2-propanol, 2,3-butanediol, linalool L and 2-furanemethanol.
Benzyl alcohol was not present in all cheese samples at 50 and 72 d. The whole raw milk added at d 34 and 56 of manufacture; have probably, an effect on aromatic alcohols profile in the final cheese. The most abundant alcohols found, were phenethyl acohol followed by ethanol and 3-methy-1-butanol. In Darfiyeh cheese, the most abundant were 1-phenylethanol, octanol, 3-methylbutanol and 1-propanol.[Citation47]
Aldehydes
Five aldehydes were identified in cheese samples at 72 d, and four at 50 d (). The highest component was 3-methyl-1-butanal at 72 d (not detected at 50 d). The level of Hexanal was higher at 72 d than at 50 d, followed by furfural, nonanal and benzaldehyde. The levels of these compounds were found higher in FH3 than FH1 and FH2. Hayaloglu et al.[Citation38] reported that hexanal was abundant in all the samples of Gokceada and Turkish Saanen. The 3-Methyl-1-butanal was identified in Darfiyeh cheese as the major aldehyde found and it plays an important role in the flavor’s development because of its low perception threshold. It provides to cheese; malt, oil or aroma butter.[Citation47]
Table 6. Aldehyds and Ketones (mean ±sd. µg.Kg−1 of Cheese) in the Bouhezza cheese making with goat milk.
Ketones
In FH1 and FH2, eight ketones were characterized and only six ones in FH3 (), -octanone and 3-hydroxy-2-butanone, were detected only at 50 d. Ketones levels were higher at 72 d than in samples at 50 d of ripening. Poveda et al.[Citation45] reported that ketones contribute to the aromatic properties of goat’s cheese such as 2-heptanone (mushroom), 2-nonanone (sour), 3-hydroxy-2-butanone (buttery). According to Le Quéré et al.[Citation40], 3-hydroxy-2-butanone was identified in goat cheese varieties and in artisanal fresh goat’s cheese.[Citation42] When the fatty acids are released in cheese, by lipids degradation the corresponding methyl-ketones are also released.[Citation44] Fatty acids are oxidized into α-keto-acids.[Citation45]
Terpenes
Fifteen terpenes were characterized in cheese samples at 50 d and eleven terpenes appeared at 72 d (). dl-Limonene was the highest compounds in both samples (at 50 and 72 d), followed by α-pinene, gamma-terpinene and then β-myrcene. Levels of sabinene, β-pinene, delta-3-carene and para-cymene were higher at 72 d than in 50 d. Terpinyl acetate were present at 50 d and absent in the sample farm at 72 d. The group of terpenes is widely varied in Bouhezza cheese goat’s milk, as reported by Medjoudj et al.,[Citation7] which obtained all of these compounds, with a level close to the farm sample at 72 d.
Table 7. Terpenes and Miscellaneous compound (mean ±sd. µg.Kg−1 of Cheese) in the Bouhezza cheese making with goat milk.
Table 8. Technical data sheet (card) of traditional cheese Bouhezza made with goat’s raw milk.
Miscellaneous compounds
Thirteen miscellaneous compounds were found in Bouhezza goat’s cheese at 50 d and twelve at 72 d (). Toluene and 1.2-Dimethyl benzene were found with high levels in both samples of cheese. Methyl groups such as 3-Methyl-hexane and 2-Methylhexane are present at d 50 with higher levels than at 72 d, unlike toluene, which was more concentrated at 72 d. Relative to toluene; Hayaloglu et al.[Citation38] reported that they have obtained similar results with the Gokceada goat’s milk cheese at 90 d. The levels of compounds found in farmhouse production were close to those reported by Medjoudj et al.[Citation7] for Bouhezza goat cheese at 72 d. It is possible that these similarities are due to the region where these cheeses were produced (wilaya of Tebessa), thus the same vegetation, climate and environmental conditions for the goats producing the raw matter.
PCA of volatiles
Two principal components are used in most cases of PCA analysis, which was sufficient to explain a great proportion of the variation into the original parameters. A bi-plot of sample scores was shown in , where the most important loadings and the percentage accounted by the two-first principal components (PC1 and PC2) after analysis. The variability of 90.08% and 4.25% were explained respectively by PC1 and PC2. The total variance explained 94.33% of the GC-MS data. A bi-plot showed that carboxylic acids contribute strongly to the cheese flavour such as octanoic, decanoic, hexanoic, acetic, butanoic and pentanoic acids which are grouped in the positive side of the PC1 axis. They represent the most important acids with high levels in the samples of Bouhezza cheese goat’s milk (50 and 72 d) (). The ethyl esters of octanoate (caprylate), decanoate, hexanoate, propanoic acid and 2-methyl-1-propanoic acid are also grouped in the positive side of the PC1 axis and in the negative side of the PC2 axis. The contribution of these esters are second order to the cheese flavour than the acids. The rest of compounds are grouped together around the center of bi-plot. The most of them are in the negative side of PC1 and on the line zero of the PC2 and then the contribution to the principal component is smaller than the two groups of the acids and their esters.
Sensorial description of spicy bouhezza cheese
The spicy Bouhezza goat milk cheese has been described as white paste cheese, with medium consistency and some red-orange spots due to the red hot pepper. Texture is a spreadable paste, smooth, firm and sticky. Odor is quite strong with acidic lactic or more intense. In addition the Lben and goat odor was perceived in the FH1 and FH2.
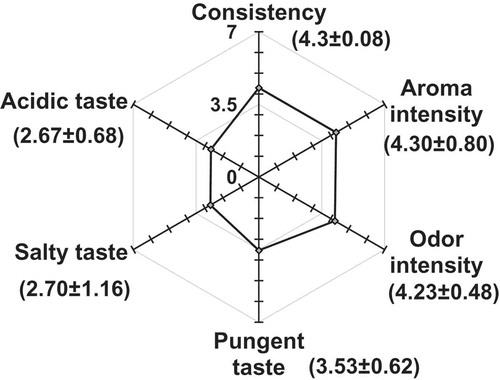
The conclusion for assessment of flavors and trigeminal sensations is that Bouhezza cheese was moderately spicy and slightly salty with acid taste. The assessment of the overall persistence of Bouhezza goat cheese was intense in flavor (90% subjects), pleasant and very typical (30%) and rich aroma (70%). It was persistent in the mouth for longer than 30 seconds. According to the , sensory profile of Bouhezza is a soft cheese, with fairly medium to high-pronounced acidity. The cheese has a medium salty taste, characterized by its slightly spicy taste due to the addition of red pepper, intense in odor and aroma.
Figure 5. Mean results of sensorial description of Bouhezza goat’s cheese spiced at 50 and 72 d of ripening (mean±sd).
The test of the comparison between FH1 and FH2 showed that there is no significant difference (P > 0.05). The intensity and persistence of taste make difficult the distinction between the two cheeses. The Student test comparing two means of two samples matched between F1 and F2 showed no significant difference between their sensory tests (P > 0.05). However, the t-test between FH1 and FH3 manufacturing and between FH2 and FH3 at the 5% level showed a significant difference. FH3 was different from FH1 and FH2 (P < 0.05). This is probably due to the difference of environmental conditions during production. In addition, the nature and the quality of goat milk used in manufacture was obtained from localities situated at the great distance (about 200 km) with a different vegetation; therefore, a different feed goats. The difference between the aromatic compounds was showed between experimental and farm productions.
Conclusion
Bouhezza cheese is prepared with salted Lben in a goatskin, with regular additions of Lben and raw milk at the end production during 2–3 months of cheese making. Lactic acid bacteria were the major microorganisms of the Bouhezza cheese microflora. The count of indigenous LAB, lactococcus and lactobacilli were stable at the first stage with slight decreasing till 42nd day and decreased at the end of ripening. The counts for lactococci were higher than lactobacilli during ripening. The main species identified were Lb. plantarum, Lb. fermentum, Lb. helveticus Lb. acidophilus and Lc. lactis subsp. cremoris. Fungal flora as yeasts and moulds increased from the 1rst to the last day of cheese making (from 4.44 to 6.53 log ufc/g). The count of CT and CF showed that the cheese is a reliable product and of satisfactory microbiological hygienic quality with the absence of pathogenic flora. The protein profile was determined by urea-PAGE and the protein fractions involved in the cheese during maturation. The sensory analysis described Bouhezza as having two principal descriptors; aroma and odor, as lactic flavor and acidic lactic odor. The phenomenon such as proteolysis and lipolysis involved during ripening, where the breakdown of proteins and fats liberated their hydrolysis products which were the precursors of small size molecules which have contributed to the taste and flavor characteristics of this cheese made with goat’ raw milk. The results of this study allowed establishing the first technical card of traditional goat’s raw milk Bouhezza cheese. Further studies are needed to determine the main species having a crucial role in ripening, with those present in the skin-bag, which contribute to biochemical process involved in cheese. In addition, probiotic and species having antimicrobial role during ripening needs to be also researched. At the end, the synthesis of results was collected in the , which represent an identity card of the Bouhezza cheese achieved following results of this study.
Acknowledgments
The authors thank the responsible of CoRFiLac Laboratory (Ragusa, Italy) to have allowed carrying out the analysis of Urea-PAGE, TTGE-PCR and, Microbiological analysis using system BAX, out in their labs. The Professor Licitra G. Director of the CoRFiLac; Carpino S. research director, and Pediliggieri C. for her help in the performed analysis.
References
- Sousa, M. J.; Ardo, Y.; McSweeney, P. L. H. Advances in the Study of Proteolysis during Cheese Ripening. Int. Dairy J.2001, 11, 327–345.
- Dagdemir, E.; Ozdemir, S. Technological Characterization of the Natural Lactic Acid Bacteria of Artisanal Turkish White Pickled Cheese. Int. J. Dairy Technol.2008, 6, 133–140.
- Fox, P. F.;. Proteolysis during Cheese Manufacture and Ripening. J. Dairy Sci.1989, 72, 1379–1400.
- Beuvier, E.; Berthaud, K.; Cegarra, S.; Dasen, A.; Pochet, S.; Buchin, S.; Duboz, G. Ripening and Quality of Swiss-Type Cheese Made from Raw, Pasteurized or Microfiltered Milk. Int. Dairy J.1997, 7, 311–323.
- Aissaoui Zitoun, O.; Benatallah, L.; Ghennam, E.-H.; Zidoune, M. N. Manufacture and Characteristics of the Traditional Algerian Ripened Bouhezza Cheese. J. Food Agric. Environ.2011, 9, 96–100.
- Aissaoui Zitoun, O.; Pediliggieri, C.; Benatallah, L.; Lortal, S.; Licitra, G.; Zidoune, M. N.; Carpino, S. Bouhezza, a Traditional Algerian Raw Milk Cheese, Made and Ripened in Goatskin Bags. J. Food, Agric. Environ.2012, 10, 289–295.
- Medjoudj, H.; Zidoune, M. N.; Hayaloglu, A. A. Proteolysis and Volatile Profile in the Algerian Traditional Bouhezza Cheese Made Using Raw Goat’s Milk. Int. J. Food Properties.2016, SN - 1532-2386. DOI: 10.1080/10942912.2016.1222588.
- Derouiche, M.; Zidoune, M. N. Caractérisation D’un Fromage Traditionnel, Le Michouna De La Région De Tébessa; Livestock Research for Rural Development: Algérie, 2015; Vol. 27, Article #229. Retrieved April 5, 2016, from http://www.lrrd.org/lrrd27/11/meri27229.html
- Leksir, C.; Chemmam, M. Contribution À La Caractérisation Du Klida, Un Fromage Traditionnel De L’est De l’Algérie; Livestock Research for Rural Development; Fundación CIPAV: Cali, Colombia; 2015; Vol. 27, Article #83. Retrieved June 22, 2015, from http://www.lrrd.org/lrrd27/5/chem27083.html
- A.O.A.C. Association of Official Agricultural Chemists. Official Methods of Analysis, 17thed.; USA: Washington, DC, 2000.
- AFNOR. Recueils Des Normes Françaises, Contrôle De La Qualité Des Produits Alimentaires, Lait Et Produits Laitiers, Analyses Physico-Chimique. (A) (9a) Norme NF V04-282 (1985) - Fromages Et Fromages Fondus: Détermination De La Matière Sèche (Méthode De Référence). P.300-303. (B) Norme NF V04-287 (1972) Fromages: Détermination De La Teneur En Matière Grasse, Méthode Acido - Butyrométrique De Van Gulik. P. 323-327. (C) Norme NF V04-287 (1990) Lait: Détermination De La Teneur En Matière Grasse. Méthode Acido-Butyrométrique. P195-211. In: Recueil De Normes Françaises. Contrôle De La Qualité De Produits Alimentaires: Laits Et Produits Laitiers. Analyses physicochimiques.Afnor-dgccrf. (4ème Ed.); La Défense: Paris, 1993; pp 562.
- AFNOR. Collection of French Standards. Product Quality Control Food: Milk and Dairy Products. Physicochemical Analysis. Afnor-Dgccrf, 4thed.; La Défense: Paris, 1985; pp 562.
- A.O.A.C. Official Methods of Analysis., 16thed.; Association of official Chemists International: Airlington, VA, 1995; Vol. II.
- Lowry, O. H.; Rosebrough, N. J.; Farr, A. L.; Randall, R. J. Protein Measurement with the Folin Phenol Reagent. J. Biologie Chem.1951, 193 (1), 265–275.
- International Dairy Federation, (IDF). Standard Method 20B: Milk. Determination of Nitrogen Content, Part 1 and 2; International Dairy Federation: Brussels, Belgium, 1993.
- Fallico, V.; McSweeney, P. L. H.; Siebert, K. J.; Horne, J.; Carpino, S.; Licitra, G. Chemometric Analysis of Proteolysis during Ripening of Ragusano Cheese. J. Dairy Sci.2004, 87, 3138–3152.
- Andrews, A. T.;. Proteinases in Normal Bovine Milk and Their Action on the Caseins. J. Dairy Res.1983, 50, 45–55.
- Blakesley, R. W.; Boezi, J. A. A New Staining Technique for Proteins in Polyacrylamide Gels Using Comassie Brillant Blue G250. Anal. Biochem.1977, 82, 580–581.
- Guiraud, J. P.;. Microbiologie Alimentaire, Édition DUNOD; Tec et Doc Lavoisier: Paris, 2003; pp 652.
- Licitra, G.; Ogier, J. C.; Parayre, S.; Pediliggieri, C.; Carnemolla, T. M.; Falentin, H.; Madec, M. N.; Carpino, S.; Lortal, S. Variability of the Bacterial Biofilms of the “Tina”Wood Vat Used in the Ragusano Cheese Making Process. Appl. Environ. Microbiol.2007, 73, 6980–6987.
- Parayre, S.; Falentin, H.; Madec, M. N.; Sivieri, K.; Le Dizes, A. S.; Sohier, D.; Lortal, S. Easy DNA Extraction Method and Optimisation of PCR-Temporal Temperature Gel Electrophoresis to Identify the Predominant High and Low GC-content Bacteria from Dairy Products. J. Microbiol. Methods.2007, 69, 431–441.
- Ogier, J. C.; Lafarge, V.; Girard, V.; Rault, A.; Maladen, V.; Gruss, A.; Leveau, J. Y.; Delacroix-Buchet, A. Molecular Fingerprinting of Dairy Microbial Ecosystems by Use of Temporal Temperature and Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol.2004, 70, 5628–5643.
- Sulejmani, E.; Rafajlovska, V.; Guneser, O.; Karagul-Yuceer, Y.; Hayaloglu, A. A. Volatile Compounds and Proteolysis in Traditional Beaten (Bieno Sirenje) Ewe’s Milk Cheese. Int. J. Dairy Technol.2014, 67, 584–593.
- Bérodier, F.; Lavanchy, P.; Zannoni, M.; Casals, J.; Herrero, L.; Adamo, C. Guide Dévaluation Olfacto-Gustative Des Fromages À Pâte Dure Et Semi-Dure. Lebensm. Wiss. Technol.1997, 30, 653–664.
- AFNOR, Collection of French Standards. General Standards for Methodology. Afnor-Dgccrf, 5thed.; La Défense, AFNOR: Paris, 1995; pp 400.
- XLSTAT, 2009 version 2009.1.02for Windows. Copyright Addinsoft 1995-2009 https://www.xlstat.com. (accessed on 18 august 2016).
- Hayaloglu, A. A.; Cakmakci, S.; Brechany, E. Y.; Deegan, K. C.; McSweeney, P. L. H. Microbiology, Biochemistry, and Volatile Composition of Tulum Cheese Ripened in Goat’s Skin or Plastic Bags. J. Dairy Sci.2007a, 90, 1102–1121.
- Bontinis, T. G.; Mallatou, H.; Alichanidis, E.; Kakouri, A.; Samelis, J. Physicochemical, Microbiological and Sensory Changes during Ripening and Storage of Xinotyri, a Traditional Greek Cheese from Raw Goat’s Milk. Int. J. Dairy Technol.2008, 61 (3), 229–236.
- Hayaloglu, A. A.; Fox Patrick, F.; Mehmet, G.; Songul, C. Cheeses of Turkey: 1. Varieties Ripened in Goat-Skin Bags. Lait.2007, 87, 79–95.
- Bontinis, T. G.; Mallatou, H.; Pappa, E. C.; Massouras, T.; Alichanidis, E. Study of Proteolysis, Lipolysis and Volatile Profile of a Traditional Greek Goat Cheese (Xinotyri) during Ripening, Small Ruminant Research; Elsevier B.V.; 2012; Vol. 105, pp 193–201.
- Sousa, M. J.; Malcata, F. X. Proteolysis of Ovine and Caprine Caseins in Solution by Enzymatic Extracts from Flowers of Cynara Cardunculus; Elsevier Science Inc: 1998; pp p10.
- Addeo, F.; Garro, G.; Intorcia, N.; Pellegrino, L.; Resmini, P.; Chianese, L. Gel Electrophoresis and Immunoblotting for the Detection of Casein Proteolysis in Cheese. J. Dairy Res.1995, 62, 207–309.
- Fox, P. F.; McSweeney, P. L. H. Rennets: Their Role in Milk Coagulation and Cheese Ripening. In Microbiology and Biochemistry of Cheese and Fermented Milk, 2nded.; Law, B. A.; Ed.; Chapman and Hall: London, UK; 1997; pp 1–40, 363.
- Ouadghiri, M.; Vancanneyt, M.; Vandamme, P.; Naser, S.; Gevers, D.; Lefebvre, K.; Swings, J.; Amar, M. Identification of Lactic Acid Bacteria in Moroccan Raw Milk and Traditionally Fermented Skimmed Milk ‘Lben’. J. Appl. Microbiol.; The Society for Applied Microbiology, 2008, 106(2), 486–95 ISSN 1364–5072.
- Serhan, M.; Catherine, C.-G.; Frederic, B.; Anne-Marie, R.-J.; Chadi, H.; Jacques, F. Bacterial Diversity of Darfiyeh, a Lebanese Artisanal Raw Goat’s Milk Cheese. Food Microbiol.2009, 26, 645–652.
- Ogier, J.-C.; Lafarge, V.; Girard, V.; Rault, A.; Maladen, V.; Gruss, A.; Leveau, J.-Y.; Delacroix-Buchet, A. Molecular Fingerprinting of Dairy Microbial Ecosystems by Use of Temporal Temperature and Denaturing Gradient Gel Electrophoresis, Applied and Environmental Microbiology; American Society for Microbiology.; 2004; Vol. 70.9, pp 5628–5643.
- Smit, G.; Smit, B. A.; Engels, W. J. M. Flavour Formation by Lactic Acid Bacteria and Biochemical Flavour Profiling of Cheese Products. FEMS Microbiol. Rev.2005, 29, 591–610.
- Hayaloglu, A. A.; Tolu, C.; Yasar, K.; Sahingil, D. Volatiles and Sensory Evaluation of Goat Milk Cheese Gokceada as Affected by Goat Breeds (Gokceada and Turkish Saanen) and Starter Culture Systems during Ripening. J. Dairy Sci.2013, 96, 2765–2780.
- McSweeney, P. L. H.; Sousa, M. J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Lait.2000, 80 (2000), 293–324. © INRA, EDP Sciences.
- Le Quéré, J.-L.; Pierre, A.; Riaublanc, A.; Demaizières, D. Characterization of Aroma Compounds in the Volatile Fraction of Soft Goat Cheese during Ripening. Lait.1998, 78, 279–290.
- Carunchia Whetstine, M. E.; Karagul-Yuceer, Y.; Avsar, Y. K.; Drake, M. A. Identification and Quantification of Character Aroma Components in Fresh Chevre-Style Goat Cheese. J. Food Sci.2003, 68, 2441–2447.
- Guillen, M. D.; Ibargoitia María, L.; Sopelana, P.; Palencia, G. Components Detected by Headspace-Solid Phase Microextraction in Artisanal Fresh Goat’s Cheese Smoked Using Dry Prickly Pear (Opuntia Ficus Indica). Lait.2004, 84, 385–397.
- Collins, Y. F.; McSweeney, P. L. H.; Wilkinson, M. G. Lipolysis and Free Fatty Acid Catabolism in Cheese: A Review of Current Knowledge. Int. Dairy J.2003, 13, 841–866.
- Molimard, P.; Spinnler, H. E. Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. Dairy Food. J. Dairy Sci.1996, 79, 169–184.
- Poveda, J. M.; Sanchez-Palomo, E.; Perez-Coello, M. S.; Cabezas, L. Volatile Composition, Olfactometry Profile and Sensory Evaluation of Semi-Hard Spanish Goat Cheeses. Dairy Sci. Technol.2008, 88, 355–367.
- Castillo, I.; Calvo, M. V.; Alonso, L.; Juarez, M.; Fontech, J. Changes in Lipolysis and Volatile Fraction of a Goat Cheese Manufactured Employing a Hygienized Rennet Paste and a Defined Strain Starter. Food Chem.2007, 100, 590–598.
- Serhan, M.; Linder, M.; Hosri, C.; Fanni, J. Changes in Proteolysis and Volatile Fraction during Ripening of Darfiyeh, a Lebanese Artisanal Raw Goat’s Milk Cheese. Small Rumin. Res.2010, 90, 75–82.