ABSTRACT
Gas chromatography and gas chromatography–flame ionization detector analysis of essential oil of Coriandrum sativum fruit showed a high percentage of linalool (79.22%) γ-terpinene (6.26%), camphor (2.63%), α-pinene (2.32%), geranyl acetate (1.75%), and p-cymene (1.70%). Significant differences in insect mortality were observed within insect species, oil concentrations, and exposure time. Fumigant treatments have shown that three insect mortality rate increased with the increase in essential oils concentration. Complete insect mortality was detected at the highest concentration within 24 h of exposure for the single compound against Tribolium castaneum and Lasioderma serricorne. Probit analysis showed that LC50 values of coriander essential oil against L. serricorne than Sitophilus oryzae and T. castaneum were 5.25 µL/L air, and 145.49 µL/L air and 276.29 µL/L air, respectively. Among the test compound, linalool demonstrated strong fumigant toxicity against L. serricorne. These result could be important in order to the efficacy of essential oil of coriander and could be potential for use in the insecticidal activities.
Introduction
The most important post-harvest activity is the storage of cereals. During storage most losses occurred due to insect attacks.[Citation1] In general, the insect pests of stored cereals are usually controlled by traditional synthetic insecticides which are currently used to protect stored products and to prevent post-harvest losses. Methods used to control stored grain insect pest included physical, chemical, and biological treatments such as methyl bromide or phosphine. However, methyl bromide was banned in many countries because of its ozone-depleting properties.[Citation2] Several studies have replaced the methyl bromide fumigation for stored product and quarantine uses. It is necessary to reduce the dependence on expensive synthetic pesticides and to change the toxic fumigants by the natural substances, yet were wide acceptance by environmental pollution, health, economical, and beneficial to value.[Citation3] However, many problems are associated with these chemicals, such as toxic residues in the food, worker’s safety, insect resistance, and the cost of treatments.[Citation4] On the other hand, the use of synthetic insecticides could offset some of the problems such as environmental contamination and toxicity to living organisms,[Citation5–Citation7] indicating the necessity to product the natural pesticides that were not hazardous to the human and environment.
Recently, the research focused on plant extracts, especially essential oils, which play an important role in protecting grains against insect infections.[Citation8–Citation11] Essential oils exhibit various insecticidal properties; they may have a fumigant activity and may also act as repellents [Citation12,Citation13] Coriandrum sativum L. is one of the most useful essential oil-bearing spices as well as medicinal plants, belonging to the family Apiaceae. The fruit of coriander exhibited various remedy properties, can be used for cooking and for children’s digestive upset and diarrhoea; also it has antidiabetogenic,[Citation14] antibacterial,[Citation15,Citation16] antimicrobial, anti-inflammatory, hypolipidaemic,[Citation17,Citation18] anticancerous, antimutagenic,[Citation19] and antioxidant effects.[Citation20] The essential oil of coriander exhibited volatile toxicity to stored product insects. Indeed, coriander essential oil can play an important role in stored grain protection and reduce the risks associated with the use of synthetic insecticides and, hence, can become an interesting alternative to conventional chemical control strategies.[Citation21,Citation22] In this context, López et al.[Citation23] fractionated the essential oil of coriander and tested against three stored rice pests, Sitophilus oryzae, Rhyzopertha dominica, and Cryptolestes pusillus. The result showed the active compound of coriander essential oil against S. oryzae was linalool, while the fractions that contained mixtures of linalool, camphor, and geranyl acetate were as active against R. dominica and C. pusillus as linalool alone. In addition, it can be showed that the fraction reached of camphor (over 400 ppm) was toxic against R. dominica and C. pusillus. Indeed, Islam et al.[Citation21] showed that the coriander essential oil obtained by the fresh aerial parts had fumigant toxicity on Tribolium castaneum adults, larvae, and pupae. So, the aim of this work was to study the insecticidal activity of coriander essential oil and its major compound, linalool, against three stored product insects: T. castaneum, Lasioderma serricorne, and S. oryzae.
Materials and methods
Plant essential oil and chemical
Coriander (C. sativum) fruits were obtained from Korba region [northeast of Tunisia-Nabeul, Northwestern Tunisia; latitude 36°34″38.22″ (N); longitude 10°51″29.63″ (E); altitude 637 m]. Linalool (purity 97%) was obtained from Sigma-Aldrich (Milwaukee, WI, USA). Acetone was purchased from Merck (99.8%).
Essential oil extraction
The coriander fruit (300 g of dry matter) was hydrodistilled for 4 h using a Clevenger apparatus, and the yield of volatile oil was calculated for meal sample. The obtained essential oil was dried over anhydrous sodium sulphate, then stored at 4°C until it is tested and analysed.
Insect rearing
The rust-red flour beetle T. castaneum, S. oryzae, and the cigarette beetle L. serricorne were reared on wheat flour. The rearing conditions were darkness in 25°C ± 1°C and 65% ± 5% [relative humidity (RH)]. Adult insects of mixed sex, 7–14 days old, were used for bioassays tests.
Fumigant toxicity bioassays
To determine the fumigant toxicity of coriander fruit essential oil, 2-cm-diameter filter papers (Whatman No. 1) were impregnated with the tested essential oil doses, calculated to give equivalent fumigant concentrations. The impregnated filter paper was then attached to the screw caps of a 44 mL plexiglas bottle. Caps were screwed tightly on the vials, each of which contained separately 10 adults (1–7 days old) of each species. When no leg or antennal movements were observed, insects were considered dead. The mortality was calculated using the Abbott correction formula.[Citation24]
A second experiment was designed to assess 50% lethal doses. A series of dilutions was prepared to evaluate insects’ mortality after an initial dose-setting experiment. Essential oil amounts tested on T. castaneum, L. serricorne, and S. oryzae were 0.5, 2, 5, and 25 μL corresponding to concentrations of 12.5, 50, 125, and 625 µl/L air, respectively. Control insects were kept under the same conditions without any essential oil and each dose was replicated three times. The number of dead and alive insects in each bottle was calculated after 1, 6, 12, 18, 24, 30, 36, 40, 48, 64, and 72 h of exposure. The mortality was evaluated by direct observation of the insects every hour till total mortality. Probit analysis was used to estimate LC50 and LC95 values[Citation25].
Repellent activity
Repellency assays of C. sativum essential oils were carried out according to the experimental method described by Jilani and Saxena[Citation26] at 25°C ± 1°C and 65% ± 5% RH. Whatman filter papers (diameter 8 cm) were cut in half. Test solutions were prepared by dissolving 2.5, 5, 7.5, and 10 µL of coriander essential oil in 1 mL acetone. Each solution was applied to half a filter paper disc as uniformly as possible with a micropipette. The other half of the filter paper was treated with acetone alone as a control. The treated and control half discs were air-dried under a fan to evaporate the solvent completely. Treated and untreated halves were attached to their opposites using adhesive tape and placed in Petri dishes. Twenty adult (7–14 days old) beetles of mixed sex were released at the centre of each filter paper disc. The dishes were then covered and sealed with Parafilm. Finally, the numbers of insects present on both the treated and untreated halves were observed after 1, 3, 5, and 24 h.
Percentage repellency and median repellent dose (RD50)
The numbers of total insects (T. castaneum, L. serricorne, and S. oryzae) were registered after different periods of exposure on the treated and untreated portions of the paper. The percentage repellency (PR) was calculated using the following equation[Citation27]:
PR = [Nc – Nt/(Nc + Nt)] × 100.
where Nc is the number of insects on the untreated area after the exposure interval and Nt is the number of insects on the treated area after the exposure interval. Tests were carried out in triplicate. The mean number of insects on the treated portion of the filter paper was compared with the number on the untreated portion.
Gas chromatography–flame ionization detector (GC-FID) analysis
Essential oils were analysed by gas chromatography (GC) using a Hewlett-Packard 6890 apparatus (Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and an electronic pressure control injector. A HP-Innowax capillary column (polyethylene glycol: 30 m × 0.25 mm i.dx·0.25 mm film thickness; Agilent Technologies, Hewlett-Packard, CA, USA) was used; the flow of the carrier gas (N2, U) was 1.6 mL/min. Analyses were performed using the following temperature programme: oven isotherm at 35°C during 10 min, from 35°C to 205°C at the rate of 3°C/min, and isotherm at 205°C during 10 min. Injector and detector temperatures were held, respectively, at 250°C and 300°C. Diluted samples of 2.0 µL were injected in the split/splitless mode (60:1 split) mode.
Gas chromatography–mass spectrometry (GC-MS)
Essential oil analysis by GC-MS was performed on an Agilent 7890A GC system, coupled to an Agilent 5972C mass spectroscopy detector with electron impact ionization (70 eV). A HP-5 MS capillary column (30 m × 0.25 mm, coated with 5% phenyl methyl silicone, 95% dimethylpolysiloxane, 0.25 mm film thickness; Hewlett-Packard, CA, USA) was used. The column temperature was programmed to rise from 40°C to 240°C with a 5°C/min rate, the carrier gas was helium N60 with a 0.9 mL/min flow rate; split ratio was 100:1. Scan time and mass range were 1 s and 50–550 m/z, respectively.
The compound identification was based on mass spectra (compared with Wiley Registry 9th Edition/NIST 2011 edition mass spectral library) and by comparison of their Kovats retention indices (Ri) with either those in the literature[Citation28,Citation29] or with those of authentic compounds available in our laboratories. Kovats retention indices were determined in relation to a homologous series of n-alkanes (C8–C40) under the same conditions.
Statistical analysis
One-way ANOVA using Statistica[Citation30] was performed on the data. A Duncan test was applied to the means to detect significant differences of repellency among concentration sand oils at the 0.05% level. Data are presented in tables as means with standard errors. Probit analysis[Citation25] was conducted to estimate median repellent dose (RD50), median lethal concentrations (LC50) with their 95% fiducial limits.
Results and discussion
Essential oil composition
The essential oil yield in coriander fruit was 0.28% (w/w). Our results were thus in accordance with those of Sriti et al.,[Citation31] who found that the essential oil yield of coriander mature fruit from Tunisia was 0.32%. Ravi et al.[Citation32] also found that essential oil yield of Indian coriander fruit dropped from 0.18% to 0.39%. The essential oil volatile compounds of coriander fruit are listed in . The most constitutes in the essential oil from coriander fruits were found as follows: linalool (79.22%), γ-terpinene (6.26%), camphor (2.63%), α-pinene (2.32%), geranyl acetate, (1.75%) and p-cymene (1.70%). Chemical analysis indicated clearly that linalool was the main component of coriander essential oil. The result of our analysis was in agreement with the other literatures that reported linalool as a major constituent in the essential oil of coriander.[Citation23,Citation33–Citation35] Bhuiyan et al.[Citation35] reported that the major compounds in the essential oil of C. sativum in Bangladesh were linalool (37.7%), geranyl acetate (17.6%), and γ-terpinene (14.4%). This variation in essential oil composition can be explained by the maturity stage and environmental factors (temperature, photoperiod, and hygrometry), the analytical methods, and the major effects of geographical and ecological variations among habitats.[Citation36–Citation38]
Table 1. Essential oil composition (% w/w) of Coriandrum sativum fruit.
Fumigant activity
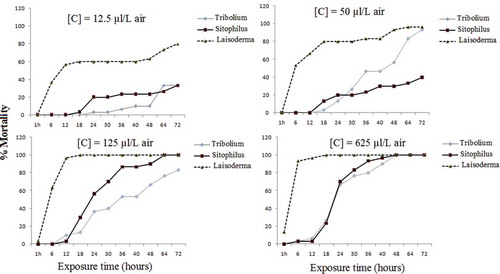
The insecticidal activity of coriander essential oil and its major compound, linalool, against T. castaneum, L. serricorne, and S. oryzae was assessed by the fumigation toxicity, and the results are shown in and . Results indicated that the coriander essential oil was relatively more toxic against T. castaneum, L. serricorne, and S. oryzae (). The increasing doses of essential oils caused a significant increase in the mortality when the T. castaneum, L. serricorne, and S. oryzae adults were exposed to these oils for 72 h. Percentage mortality of T. castaneum, S. oryzae, and L. serricorne were 66.67%, 70%, and 100%, respectively, at the 625 µL/L air dose after 24 h exposure when exposed to essential oil of coriander and the same dose caused complete mortality (100%) when exposed to linalool against T. castaneum and L. serricorne and 80% against S. oryzae. According to Lopez et al.,[Citation23] the coriander essential oil against S. oryzae, R. dominica, and C. pusillus produced on average 56%, 90%, and 100% dead insects after 24 h exposure. Indeed, these authors showed that the active compound of coriander essential oil against S. oryzae was linalool since subfraction contained mixture of linalool and camphor produced on average 63% with 1723 ppm of linalool while the mixture contained of linalool, camphor, and geraniol produced 73% dead insects with 1450 ppm. Although the fraction rich in camphor (over 400 ppm) was very toxic against R. dominica and C. pusillus. In this context, numerous studies showed that some essential oils rich in linalool and camphor compounds had high insecticidal activity against stored products pests.[Citation39–Citation41] LC50 and LC95 values of the essential oil of coriander and its major compound, linalool, tested against T. castaneum than L. serricorne and S. oryzae after 24 h exposure are summarized in . Probit analysis showed that LC50 values of these essential oils against T. castaneum than L. serricorne and S. oryzae were 276.29, 5.25, and 145.49 µL/L air for coriander essential oil, respectively. LC95 values revealed that L. serricorne was more susceptible to coriander (102.31 µL/L air) than T. castaneum and S. oryzae (4543.74 µL/L air and 10124.20 µL/L air, respectively). LC50 values of linalool against L. serricorne, T. castaneum, and S. oryzae were 31.79 µL/L air, 90.89 µL/L air, and 172.37 µL/L air, respectively.
Table 2. LC50 and LC95(µl/L air) values of coriander essential oil and linalool compound against Tribolium castaneum, Sitophilus oryzae, and Lasioderma serricorne.
Figure 1. Percentage of mortality of Tribolium castaneum, Lasioderma serricorne, and Sitophilus oryzae exposed for various periods of time to essential oil from linalool.
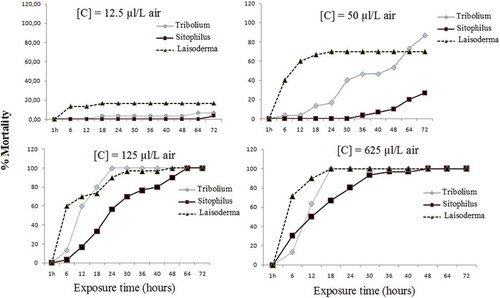
Figure 2. Percentage of mortality of Tribolium castaneum, Lasioderma serricorne, and Sitophilus oryzae exposed for various periods of time to essential oil from coriander fruit.
There are no previous citations concerning the insecticidal effects of C. sativum fruit essential oil from Tunisia on adults of T. castaneum, S. oryzae, and L. serricorne. In this context, Khani and Rahdari[Citation42] reported that coriander essential oil was more toxic against Callosobruchus maculatus adults (LC50 = 1.34 μL/L air) than Tribolium confusum (LC50 = 318.02 μL/L air). Various studies revealed that the insecticidal activity of coriander essential oil can be attributed to its major compound, linalool.[Citation21,Citation23] Recently, Chung et al.[Citation43] demonstrated that linalool has significant toxic effects against the larvae of Aedes aegypti with an LC50 value of 21.5 ppm and should play an important role as immunotoxicity on this insect. However, the differences in the response of the insect to the essential oils were related to their major constituent.[Citation22,Citation39,Citation44] The variation observed against the toxic difference seems to be reasonable because of different insect species and methodology of oil extraction, as shown in similar experiments with various stored product pests and essential oil vapours.[Citation45,Citation46]
The insecticidal activity of essential oil of many plant contributed for the monoterpenoids through their high volatility, and they have fumigant activity that might be of importance for controlling stored-product insects.[Citation8,Citation47,Citation48] The high toxicity of linalool was reported against S. oryzae and R. dominica.[Citation40] Essential oil and their compound for many plants are gaining increasing interest because of their fumigant action.[Citation49,Citation50] Indeed, essential oils are active against both adults and larvae and frequently act to inhibit reproduction.
Repellent activity tests
Repellency tests results are shown in while gives the calculated median repellent dose (RD50) values. Results indicated that the coriander essential oil showed significant pest repellent activity to T. castaneum, S. oryzae, and L. serricorne adults. Repellent action was highly dependent upon oil concentration and exposure time. Essential oil of coriander at 0.39 µL/cm2 showed the highest repellent activity against L. serricorne adults after the 1 h time interval. PR of coriander essential oil was 75% versus observed at the highest concentration (0.39 µL/cm2) after 1 h of exposure for linalool standards against T. castaneum (). Coriander essential exhibited a repellent at the highest concentration (0.39 and 0.29 µL/cm2) against three beetle species but especially after the shorter exposure times (1 and 3 h). The toxicity of C. sativum oil on T. castaneum, L. serricorne, and S. oryzae was significantly different, as inferred by the confidence intervals of RD50 (). Probit analysis showed that S. oryzae was more susceptible (RD50 = 0.084 µL/cm2) to coriander essential oil than linalool standards (RD50 = 0.206 µL/cm2).
Table 3. Percentage repellency (mean SE) of coriander essential oil and linalool standard against Tribolium castaneum, Sitophilus oryzae, and Lasioderma serricorne adults after various periods of exposure.
Table 4. RD50 values (µL/cm2) of coriander essential oil and linalool compound against adults of Tribolium castaneum, Sitophilus oryzae, and Lasioderma serricorne.
For both insect species (T. castaneum and L. serricorne), coriander essential oil was more effective compared to the linalool standards. The corresponding RD50 values were respectively 0.049 µL/cm2 for L. serricorne and 0.136 µL/cm2 for T. castaneum (). In this context, Hori[Citation51] demonstrated the highly repellent effect coriander essential oil against L. serricorne in an olfactometer repellency bioassay system. The superior insecticidal potential of coriander oil could be attributed to a complex interaction among its different constituents: linalool (79.22%); γ-terpinene (6.26%), camphor (2.63%), α-pinene (2.32%), geranyl acetate (1.75%), and p-cymene (1.70%).
Besides, it was shown that the response of essential oil against stored products pests cannot be attributed by the action of their major components only but also a heterogeneous group of complex mixtures of organic substances.[Citation52] Even minor compounds may be involved in the insecticidal activities of the corresponding oils and may have a synergistic effect. In fact, it has been demonstrated that the fumigant activities against R. dominica, T. castaneum, and S. oryzae of nine essential oil compounds revealed 1,8-cineole to be the most effective, followed by camphor and linalool.[Citation40] Indeed, the repellent effects of essential oil of many plants have been reported previously against stored products pests. For example, the essential oil of Adhatoda vasica (Acanthaceae) exhibited repellent activity against S. oryzae and Bruchus chinensis[Citation53] and Acorus calamus essential oil repelled T. castaneum.[Citation26] This confirms the findings of recent studies where reporting the ovicidal, repellent, antifeedant, sterilization, and toxic effects of essential oil and extracts of plant against the insects.[Citation9,Citation54]
Conclusion
Results of the present study indicated that C. sativum essential oil has insecticidal property against adults of T. castaneum, S. oryzae, and L. serricorne, which could be mainly attributed to monoterpenoids, compounds that possess insecticidal activity against a range of insect species. Indeed, the higher insecticidal capacity of coriander essential oil could be linked to the presence of linalool. However, the inhibitory activity of the essential oil is the result of a complex interaction among its different constituents, which might produce synergistic or additive effects, even for those present at low concentrations. The bioactivity of coriander fruit essential oil may be attributed to the high concentration of linalool, camphor, and α-pinene. In our study, the results obtained showed good potential for the use of C. sativum oil as both toxicant and repellent agents against stored product pests.
References
- Ngamo, T. S. L.; Ngatanko, I.; Ngassoum, M. B.; Mapongmestsem, P. M.; Hance, T. Insecticidal Efficiency of Essential Oils of 5 Aromatic Plants Tested Both Alone and in Combination Towards Sitophilus Oryzae (L.) (Coleoptera: Curculionidae). Research. J. Biol. Sci. 2007, 2, 75–80.
- Hansen, L. S.; Jensen, K. M. V. Effect of Temperature on Parasitism and Host-Feeding of Trichogrammaturkestanica (Hymenoptera: Trichogrammatidae) on Ephestia Kuehniella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2002, 95, 50–56. DOI: 10.1603/0022-0493-95.1.50.
- Ayvaz, A.; Albayrak, S.; Karaborklu, S. Gamma Radiation Sensitivity of the Eggs, Larvae and Pupae of Indian Meal Moth Plodiainter Punctella (Hübner) (Lepidoptera: Pyralidae). Pest Manag. Sci. 2008, 64, 505–512. DOI: 10.1002/(ISSN)1526-4998.
- Sighamony, S.; Aness, I.; Chandrakala, T.; Osmani, Z. Efficacy of Certain Indigenous Plant Products as Grain Protectans against Sitophilus Oryzae (L.) And Rhyzoperthadominica (F.). J. Stored Prod. Res. 1986, 22, 21–23. DOI: 10.1016/0022-474X(86)90042-1.
- Raizada, R. B.; Srivastava, M. K.; Kaushal, R. A.; Singh, R. P. Azadirachtin, a Neem Biopesticide: Subchronic Toxicity Assessment in Rats. Food Chem. Toxicol. 2001, 39, 477–483. DOI: 10.1016/S0278-6915(00)00153-8.
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and Oxidative Stress: A Review. Med. Sci. Monitor. 2004, 10, 141–147.
- Nakata, H.; Hirakawa, Y.; Kawazo, M.; Nakabo, T.; Arizono, K.; Abe, S. I.; Kitano, T.; Shimada, H.; Watanabe, I.; Li, W.; Ding, X. Concentrations and Compositions of Organochlorine Contaminants in Sediments, Soils, Crustaceans, Fishes and Birds Collected from Lake Tai, Hangzhou Bay and Shanghai City Region, China. Environ. Pollut. J. 2005, 133, 415–429. DOI: 10.1016/j.envpol.2004.07.003.
- Regnault-Roger, C.; Hamraoui, A. Fumigant Toxic Activity and Reproductive Inhibition Induced by Monoterpenes on Acanthoscelidesobtectus (Say) (Coleoptera), a Bruchid of Kidney Bean (Phaseolus Vulgaris L.). J. Stored Prod. Res. 1995, 31, 291–299. DOI: 10.1016/0022-474X(95)00025-3.
- Isman, M. B.;. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. DOI: 10.1146/annurev.ento.51.110104.151146.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils: A Review. Food Chem. Toxicol. 2008, 46, 446–475. DOI: 10.1016/j.fct.2007.09.106.
- Batish, D. R.; Sing, P. H.; Kohli, K. R.; Kaur, S. Eucalyptus Essential Oil as a Natural Pesticide. For. Ecol. Manage. 2008, 256, 2166–2174. DOI: 10.1016/j.foreco.2008.08.008.
- Shaaya, E.; Kostjukovski, M.; Eilberg, J.; Sukprakarn, C. Plant Oils as Fumigants and Contact Insecticides for the Control of Stored-Product Insects. J. Stored Prod. Res. 1997, 33, 7–15. DOI: 10.1016/S0022-474X(96)00032-X.
- Saim, N.; Meloan, E. C. Compounds from Leaves of Bay (Laurusnobilis L.) As Repellents for Triboliumcastaneum (Herbst) When Added to Wheat Flour. J. Stored Prod. Res. 1986, 22, 141–144. DOI: 10.1016/0022-474X(86)90007-X.
- Gallagher, A. M.; Flat, P. R. T.; Duffy, G.; Abdel-Wahab, Y. H. A. The Effects of Traditional Antidiabetic Plants on in Vitro Glucose Diffusion. Nutr. Res. 2003, 23, 413–424. DOI: 10.1016/S0271-5317(02)00533-X.
- Kubo, I.; Fujita, K.; Kubo, A.; Nihei, K.; Ogura, T. Antibacterial Activity of Coriander Volatile Compounds against Salmonella Choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. DOI: 10.1021/jf0354186.
- Matasyoh, J. C.; Maiy, O. Z. C.; Ngure, R. M.; Chepkorir, R. Chemical Composition and Antimicrobial Activity of the Essential Oil of Coriandrum Sativum. Food Chem. 2009, 113, 526–529. DOI: 10.1016/j.foodchem.2008.07.097.
- Lo Cantore, P.; Iacobelli, N. S.; Marco, A.; Capasso, F.; Senatore, F. Antibacterial Activity of Coriandrum Sativum L. And Foeniculum Vulgare Miller VarVulgare (Miller) Essential Oils. J. Agric. Food Chem. 2004, 52, 7862–7866. DOI: 10.1021/jf0493122.
- Chithra, V.; Leelamma, S. Coriandrum Sativum: Effect on Lipid Metabolism in 1,2-Dimethyl Hydrazine Induced Colon Cancer. J. Ethnopharmacol. 2000, 71, 457–463. DOI: 10.1016/S0378-8741(00)00182-3.
- Lal, A. A. S.; Tkumar, P. B. M.; Pillai, K. S. Hypolipidemic Effect of Coriandrum Sativum L. Intriton-Induced Hyperlipidemic Rats. Indian J. Exp. Biol. 2004, 42, 909–912.
- Wangensteen, H.; Samuelsen, A. B.; Malterud, K. E. Antioxidant Activity in Extracts from Coriander. Food Chem. 2004, 88, 293–297. DOI: 10.1016/j.foodchem.2004.01.047.
- Islam, M. S.; Mahbub Hasan, M.; Xiong, W.; Zhang, S. C.; Lei, C. L. Fumigant and Repellent Activities of Essential Oil from Coriandrum Sativum (L.) (Apiaceae) against Red Flour Beetle Tribolium Castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Pest. Sci (2004). 2009, 82, 171–177. DOI: 10.1007/s10340-008-0236-7.
- Khani, A.; Asghari, J. Insecticide Activity of Essential Oils of Mentha Longifolia, Pulicaria Gnaphalodes and Achillea Wilhelmsii against Two Stored Product Pests, the Flour Beetle, Tribolium Castaneum and the Cowpea Weevil, Callosobruchus Maculates. J. Insect Sci. 2012, 12, 12–73. DOI: 10.1673/031.012.7301.
- Lopez, M. D.; Jordan, M. J.; Pascual-Villalobos, M. J. Toxic Compounds in Essential Oils of Coriander, Caraway and Basil Active against Stored Rice Pests. J. Stored Prod. Res. 2008, 44, 273–278. DOI: 10.1016/j.jspr.2008.02.005.
- Abbott, W. S.;. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. DOI: 10.1093/jee/18.2.265a.
- Finney, D. J.;. Probit Analysis, 3rd ed.; Cambridge University Press: London, 1971.
- Jilani, G.; Saxena, R. C.; Rueda, B. P. Repellent and Growth Inhibition Effects of Turmeric Oil, Sweetflag Oil, Neem Oil and Margosan-O on Red Flour Beetle (Coleoptera: Tenebrionodae). J. Econ. Entomol. 1988, 81, 1226–1230. DOI: 10.1093/jee/81.4.1226.
- Nerio, L.; Olivero-Verbel, J.; Stashenko, E. Repellency Activity of Essential Oils from Seven Aromatic Plants Grown in Colombia against Sitophilus Zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 2009, 45, 212–214. DOI: 10.1016/j.jspr.2009.01.002.
- Adams, R. P.;. IIdentification of essential oil components by gas chromatography/mass spectorscopy. Allured Publishing Corporation, Carol Stream, IL, USA, 2007.
- Davies, N. W.;. Gas Chromatographic Retention Indices of Monoterpenes and Sesquiterpenes on Methyl Silicon and Carbowax 20M Phases. J. Chromatogr A. 1990, 503, 1–24. DOI: 10.1016/S0021-9673(01)81487-4.
- Stasoft. STATISTICA for Windows (Computer Program Electronic Manual); StatSoftInc; Tulsa, OK, 1998.
- Sriti, J.; Talou, T.; Aidi Wannes, W.; Cerny, M.; Marzouk, B. Essential Oil, Fatty Acid and Sterol Composition of Tunisian Coriander Fruit Different Parts. J. Sci. Food Agric. 2009, 89, 1659–1664. DOI: 10.1002/jsfa.v89:10.
- Ravi, R.; Prakash, M.; Bhatt, K. K. Aroma Characterization of Coriander (Coriandrum Sativum L.) Oil Samples. Eur. Food Res. Technol. 2006, 6, 425–427.
- Sriti, J.; Aidi Wannes, W.; Talou, T.; Vilarem, G.; Marzouk, B. Chemical Composition and Antioxidant Activities of Tunisian and Canadian Coriander (Coriandrum Sativum L.) Fruit. J. Essential Oil Res. 2011, 23, 7–15. DOI: 10.1080/10412905.2011.9700462.
- Pino, J. A.; Rosado, A.; Fuentes, V. Chemical Composition of the Seed Oil of Coriandrum Sativum L. From Cuba. J. Essential Oil Res. 1996, 8, 97–98. DOI: 10.1080/10412905.1996.9700565.
- Bhuiyan, M. N. I.; Begum, J.; Sultana, M. Chemical Composition of Leaf and Seed Essential Oil of Coriandrum Sativum L. From Bangladesh. Bangladesh J. Pharmacol. 2009, 4, 150–153. DOI: 10.3329/bjp.v4i2.2800.
- Misharina, T. A.;. Influence of the Duration and Conditions of Storage on the Composition of the Essential Oil from Coriander Seeds. Appl. Biochem. Microbiol. 2001, 37, 622–628. DOI: 10.1023/A:1012315403828.
- Smallfield, B. M.; VanKlink, J. W.; Perry, N. B.; Dodds, K. G. Coriander Spice Oil: Effects of Fruit Crushing and Distillation Time on Yield and Composition. J. Agric. Food Chem. 2001, 49, 118–123. DOI: 10.1021/jf001024s.
- Gil, A.; de la Fuente, E. B.; Lenardis, A. E. Coriander Essential Oil Composition from Two Genotypes Grown in Different Environmental Conditions. J. Agric. Food Chem. 2002, 50, 2870–2877. DOI: 10.1021/jf011128i.
- Lee, S.; Peterson, C. J.; Coats, J. R. Fumigation Toxicity of Monoterpenoids to Several Stored Product Insects. J. Stored Prod. Res. 2002, 39, 77–85. DOI: 10.1016/S0022-474X(02)00020-6.
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of Naturally Occurring Compounds of Lamiaceae and Lauraceaeto Three Stored-Product Insects. J. Stored Prod. Res. 2007, 43, 349–355. DOI: 10.1016/j.jspr.2006.09.001.
- Abdelgaleil, S. A. M.; Mohamed, M. I. E.; Badawy, M. E. I.; El-Arami, S. A. A. Fumigant and Contact Toxicities of Monoterpenes to Sitophilus Oryzae (L.) And Tribolium Castaneum (Herbst) and Their Inhibitory Effects on Acetyl Cholinesterase Activity. J. Chem. Ecol. 2009, 35, 518–525. DOI: 10.1007/s10886-009-9635-3.
- Khani, A.; Rahdari, T. Chemical Composition and Insecticidal Activity of Essential Oil from Coriandrum Sativum Seeds against Tribolium Confusum and Callosobruchus Maculates. ISRN Pharm. 2012, 1–5. DOI: 10.5402/2012/263517.
- Chung, I. M.; Ahmad, A.; Kim, E. H. Immunotoxicity Activity from the Essential Oils of Coriander (Coriandrum Sativum) Seeds. Immunopharmacol. Immunotoxicol. 2012, 34, 499–503. DOI: 10.3109/08923973.2011.637500.
- Negahban, M.; Moharramipour, S.; Sefidkon, F. Fumigant Toxicity of Essential Oil from Artemisia sieberiBesser against Three Stored-Product Insects. J. Stored Prod. Res. 2007, 43, 123–128. DOI: 10.1016/j.jspr.2006.02.002.
- Huang, Y.; Lam, S. L.; Ho, S. H. Bioactivities of Essential Oil from Elletaria Cardamomum (L.) Maton. To Sitophilus Zeamais Motschulsky and Tribolium Castaneum (Herbst). J. Stored Prod. Res. 2000, 36, 107–117. DOI: 10.1016/S0022-474X(99)00040-5.
- Sanon, A.; Garba, M.; Auger, J.; Huignard, J. Analysis of the Insecticidal Activity of Methyl Isothiocyanate on Callosobruchus Maculatus (F.) (Coleoptera: Bruchidae) and Its Parasitoid Dinarmus Basalis (Rondani) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2002, 38, 129–138. DOI: 10.1016/S0022-474X(01)00008-X.
- Konstantopoulou, I.; Vassilopoulou, L.; Mavragani-Tsipidou, P.; Scouras, Z. G. Insecticidal Effects of Essential Oils. A Study of the Effects of Essential Oils Extracted from Eleven Greek Aromatic Plants on Drosophila Auraria. Experientia. 1992, 48, 616–619. DOI: 10.1007/BF01920251.
- Ahn, Y. J.; Lee, S. B.; Lee, H. S.; Kim, G. H. Insecticidal and Acaricidal Activity of Carvacrol and β-thujaplicine Derived from Thujopsis Dolabrata Var. Hondai Sawdust. J. Chem. Ecol. 1998, 24, 81–89. DOI: 10.1023/A:1022388829078.
- Kim, S. I.; Roh, J. Y.; Kim, D. H.; Lee, H. S.; Ahn, Y. J. Insecticidal Activities of Aromatic Plant Extracts and Essential Oils against Sitophilus Oryzae and Callosobruchus Chinensis. J. Stored Prod. Res. 2003, 39, 293–303. DOI: 10.1016/S0022-474X(02)00017-6.
- Koul, O.;. Biological Activity of Volatile Di-N-Propyl Disulfide from Seeds of Neem, Azadirachta Indica (Meliaceae), to Two Species of Stored Grain Pests, Sitophilus Oryzae (L.) And Tribolium Castaneum (Herbst). J. Econ. Entomol. 2004, 97, 1142–1147. DOI: 10.1093/jee/97.3.1142.
- Hori, M.;. Repellency of Essential Oils against the Cigarette Beetle, Lasioderma Serricorne (Fabricius) (Coleoptera: Anobiidae). Appl. Entomol. Zool. 2003, 38, 467–473. DOI: 10.1303/aez.2003.467.
- Burt, S.;. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods. Int. J. Food Microbiol. 2004, 94, 223–253. DOI: 10.1016/j.ijfoodmicro.2004.03.022.
- Kokate, C. K.; D’Cruz, J. L.; Kumar, R. A.; Apte, S. S. Anti-Insect and Juvenoidal Activity of Phytochemicals Derived from AdbatodapasicaNees. Indian J. Nat. Prod. 1985, 1, 7–9.
- Nawrot, J.; Harmatha, J. Natural Products as Antifeedants against Stored Product Insects. Postharvest News Inf. 1994, 5, 17N–21N.
