ABSTRACT
In order to find out the key factors to affect the texture variation and the formation mechanism of different cultivars lotus rhizomes after thermal processing, representative lotus rhizomes harvested in year round with 2 months interval, including Lulinhu, Miancheng, and Elian 5, were chosen to calculate the cause of thermal process texture. The texture (hardness), content of different fractions of alcohol insoluble residue (AIR), and microstructure of the cell wall components of the lotus rhizomes exhibited changes during processing. The changes of three pectin fractions in the three lotus rhizomes cultivars were compared by combining the effect of thermal treatment on the lotus rhizome AIR pectin content degree of esterification (DE) and analysing the texture and microstructure, the cell wall materials were found to be the crucial factor in this process. Another six cultivars lotus rhizomes have been employed to replicate the above mentioned results, the total data were used to calculate the chelator soluble fraction (CSF) to water soluble fraction (WSF) ratio value (0.25), which determines the texture of the lotus rhizomes after thermal-processing. The CSF/WSF ratio values (0.25) will give an index for the lotus rhizome processing food industry.
Introductin
Lotus rhizomes (Nelumbo nucifera Gaertn.) belong to the nymphaeaceae family of perennial aquatic vegetables. Long known as the Thousand Lake Province, Hubei Province has a temperate climate and abundant rainfall, providing unique advantages for the growth of lotus rhizomes. Hubei is also an area in which lotus rhizomes originated.[Citation1] Currently, the lotus rhizome varieties in Hubei province include the Lulinhu lotus rhizome, Miancheng lotus rhizome, Bahe lotus rhizome, Xiaogan West Lake lotus rhizome, Wuzhi No. 1, Wuzhi No. 2, Wuzhi No. 4, Hubei No. 4, and Hubei No. 5.[Citation2] Based on the cooked texture, lotus rhizomes can be divided into mealy and crisp types; mealy lotus rhizomes are soft and glutinous, very sticky, and mainly cooked by stewing and steaming; crisp lotus rhizomes are crisp and tender, not very sticky, and suitable for stir-frying, cold dressing with sauces, and making lotus rhizome drinks.[Citation3] A model food is desired for the study of textural complexity,[Citation4] so lotus rhizome is the best choice.
After thermal processing, the water soluble materials in the cell walls and within the cells of fruits and vegetables will dissolve.[Citation5] As a result, the bond strength between cells decreases, the gap between cells increases, and the cell walls dissolve and expand or partially dissolve, leading to decreased mechanical strength and thus softening of the tissues of the fruits and vegetables. Many reports have been published regarding the correlation between texture changes and pectin components in the processing of fruits and vegetables, including in degradation and changes in the pectin in the processes of growth and maturation,[Citation6] thermal treatment,[Citation7] and high-pressure treatment.[Citation8] These are important aspects of food processing.[Citation9] Njoroge et al.[Citation10] found that the cotyledon of hard-to-cook kidney beans cannot be softened after thermal treatment in contrast to that of the easy-to-cook kidney beans. Studies have shown that differences in the structure and solubility of pectin lead to differences in the textures of kidney beans after thermal treatment. Macromolecules such as cellulose and semi-cellulose are cross-linked to the pectin materials, resulting in damage to the pectin structures, degradation of vegetable cells, and softening of the texture. For the cell wall materials (CWM), the degradation of the Na2CO3 soluble fraction (NSF) and the hemicellulose fraction (HF) are closely related to the maturation and softening of fruits.[Citation11]
The present study compared the effect of thermal treatment on the texture, microstructure, and content of the CWM of different lotus rhizome varieties in different harvest time. And many kind of lotus rhizome harvested in different time were employed to evaluate the relationship between the CSF/WSF value and the cooked textures, to find a mark used to identify the cooked texture of an unknown lotus rhizome cultivar. The results will provide theoretical guidance for the breeding, cultivation, comprehensive development, utilisation, and industrial production of lotus rhizomes with different edibility and processing characteristics.
Materials and methods
Materials
Lulinhu lotus rhizome, Miancheng lotus rhizome, and Elian 5 were used. The materials were harvested in June, August, October, and December of 2014 and February and April of 2015 in Lilinhu Village, Pengchang Town, Xiantao City, Hubei Province, China. Elian 6, Elian 7, Elian 8, Elian 9, Saizhengzhu and Baiyu lotus rhizome cultivars harvested in April, 2015 from Wuhan Vegetable Research Institute, wuhan, Hubei Province, China.
The lotus rhizomes were shipped to lab after harvested and then cut into slices with a thickness of 1.5 cm. The hardness of fresh samples were determined by using the fresh lotus rhizome slices, and the samples that were thermally treated at 100°C for one hour and cooled to room temperature were used to measure the hardness of thermally processed lotus rhizome slices.
Reagents and equipments
Ethanol, methanol, chloroform, sodium acetate, acetic acid, sodium carbonate, sodium borohydride, potassium hydroxide, sodium hydroxide, sulfuric acid, borax, m-benzene phenol, monosodium phosphate, disodium phosphate, and sodium chloride were all analytical grade reagents and purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Diaminocyclohexane tetraacetic acid (CDTA), acetylacetone, and alcohol oxidase were purchase from Sigma Co. Ltd.
The equipment included a vacuum freeze dryer (LGJ-30F from Beijing Songyuan Huaxing Technology Develop Co., Ltd), texture analyser (TA. XT. Plus from STABLE MICRO.SYS, UK), dialysis tubing (3500D from Sigma), a rotate evaporator (R-210 from BUCHI, Switzerland), and a visible spectrophotometer (722 from Puruisi Equipment Co., Ltd, Tianjing, China).
Hardness measurement
The methods reported previously were followed.[Citation12] Metering mode: texture profile analysis (TPA); probe: P6; plotting parameters: 1.00 mm/sec; testing speed: 1.00 mm/sec; post-test speed: 1.00 mm/sec; compression ratio: 30%; time between two compressions: 2 s.
Scanning Electron Microscopy (SEM)
Structure observation was carried out using a JSM-6390 scanning electron microscope according to the methods reported previously,[Citation13] magnification of 450 × were used in most of the micrographs in the present study. At least three samples for each treatment, which showed similar images, were used for results.
Separation of cell wall components
The methods reported previously were followed.[Citation14] The alcohol insoluble residue (AIR) was obtained after removing starch from lotus rhizomes. The water soluble fraction (WSF), chelator soluble fraction (CSF), NSF, HF, and residue were then extracted in separate steps.
Determination of the amount of galacturonic acid
The methods reported previously were followed.[Citation14] The standard curve was plotted by measuring D-galacturonic acid using these methods. The standard curve is represented by y = 0.0084 x - 0.0599 (R2 = 0.998).
Determination of the degree of esterification of pectin
The methods reported previously were followed.[Citation15] The standard curve was plotted by measuring the amount of methanol. The standard curve is represented by y = 0.0301x + 0.192 (R2 = 0.998).
Statistics data processing
All data were measured in triplicates. The values shown in the figures were corresponding to the average value and statistical significance of the analysis of each parameter. Average values and statistical significance according to LSD test (p < 0.05) were calculated using the package data processing system (DPS 7.05) (Zhangjiang University, Hangzhou, China). The figures were drawn by using OriginPro 8.0 (Northampton, MA, USA).
Results and discussion
Hardness of thermally processed lotus rhizome of different cultivars at different harvesting time
The lotus rhizome in different cultivars featured mealy or crispy texture after thermal processing, but the change of the texture of cooked lotus rhizome was not fully measured before. Chiang & Luo[Citation16] reported that during the pressurised cooking process, pectin loss increased, causing an increase in solid loss and softening of the texture, but the native texture attribute and the cultivars of the lotus rhizome used is not clear. In this work the data of the hardness of the cooked lotus rhizome in three cultivars, representing mealy, crispy and medium type texture lotus rhizome in year-round harvesting time were shown in . As the lotus rhizome starts growing from May, the first harvesting time is in June, so the data of the texture for the cooked lotus rhizome were from June, and end in April of the next year. The data showed that during the growing time the hardness of the fresh lotus rhizome increased and reached the peak in October and then deceased to the value as that in June, there is not a significant difference in hardness of fresh lotus rhizomes between different cultivars except that in December and February of the next year. Meanwhile, the texture of the cooked lotus rhizome was significantly lower than that of the fresh samples, respectively. During the year around the hardness of the cooked lotus rhizome increased, and reach the peak in October and then decreased and kept the same level in the main harvesting time from December to April of the next year. During the main harvesting time the cooked Miancheng lotus rhizome showed the lowest hardness, followed by Cooked Elian 5 lotus rhizome, and the cooked Luinhu lotus rhizome with the highest hardness. A significant difference in hardness was observed for the different lotus rhizome cultivars after thermally processed (P < 0.05). But in the main harvesting time from December to April of the next year, for a cultivar there was significant different in texture of the cooked lotus rhizome, cooked Miancheng lotus rhizome, with mealy texture, was of the lowest hardness followed by cooked Elian 5, and cooked Lulinhu, with crispy texture, was of the highest hardness. During the year round, cooked Elian 5 harvested before November showed the crispy but after November it showed mealy texture, but for cooked Lulihu lotus rhizome it showed crispy texture year around. Meanwhile for cooked Miancheng showed lowest hardness year around, which indicated that it was of mealy textures. The three typical texture lotus rhizome cultivars were used to evaluate the changed of hardness of fresh and cooked tissue. The results agreed with the practices calculated by consumers even there are few reports on the texture evaluation of the cooked lotus rhizome.
Figure 1. Hardness of thermally-processed lotus rhizomes harvested at different times (From June, 2014 to April, 2015, Different lowercase letters indicated the significant difference of hardness of fresh lotus rhizome (P < 0.05). Different capital letters indicated the significant difference of hardness of cooked lotus rhizome (P < 0.05)).
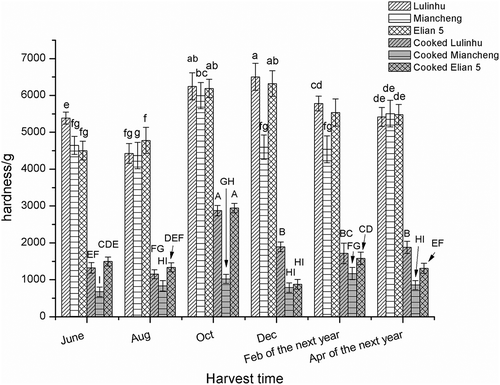
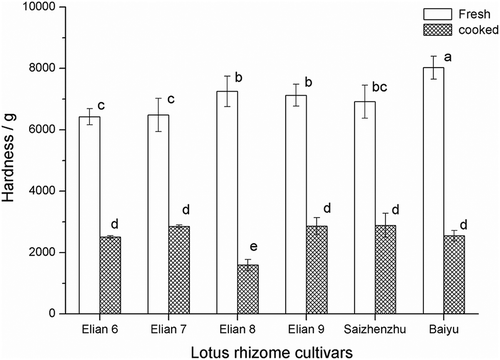
As there are different in cooked texture of lotus rhizome cultivars, in this work more lotus rhizome cultivars were employed to evaluate the varieties of the hardness of cooked lotus rhizomes. Another six cultivars were harvested in April, 2015 to measure the hardness of fresh and cooked lotus rhizomes. Among them Elian 8 was generally recognised as a cultivar with mealy texture of cooked lotus rhizome, the others were considered as crispy texture. The data showed in indicated that there was still significant difference (p < 0.05) on hardness of the fresh lotus rhizome used, for the texture of the cooked lotus rhizome there was also no significant difference except the Elian 8, which featured the lowest hardness indicating mealy property. From the organoleptic characteristic we know that Elian 8 and Miancheng lotus rhizome showed the mealy properties after cooking, but the others except Elian 5 remained the crispy properties even after cooking, for Elian 5, it showed the texture properties depending on the harvesting time, in the end of the main harvesting time it usually showed the mealy attributes, in the other time it showed the crispy properties after cooking.
SEM of the fresh and cooked lotus rhizome of lulinhu, elian 5 and miancheng
In order to demonstrate the mechanism of changes in hardness of the cooked lotus rhizome, three cultivars lotus rhizomes, Lulinhu, Miacheng, and Elian 5 harvested in December 2014, were used to analyse the changes on the cell wall structure before and after cooking as they are the typical crispy, mealy and medium mealy lotus rhizome cultivars, respectively. The results were shown in , for the fresh rhizomes, the cell wall thickness can be ranked as follows, Lulinhu>Elian 5>Miancheng. The cell wall of Lulinhu lotus rhizome became thin after cooking, but still remained relative integrity. However for Miancheng lotus rhizome the cell wall almost degraded, for Elian 5, it kept better than Miancheng lotus rhizome did, the SEM results perhaps clarify the changes of the texture during cooking in different cultivars, the more degradation of the cell wall of the lotus rhizome during cooking, the more mealy the thermally cooked tissue.
Figure 3. Microstructure of the lotus rhizomes before and after thermal treatment shown at a magnification of 450 × . A, B, C is the fresh samples, a, b, c is the cooked samples. From left to right they are Lulinhu, Miancheng, and Elian 5 lotus rhizome cultivar.
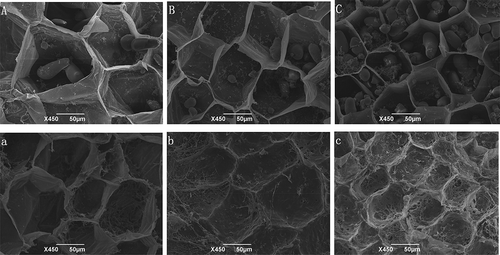
During thermal processing, the pectin between cells degraded, the original pectin degraded into soluble pectin, resulting in damage to cellular structures and a decrease in electron density between cells. In addition, due to the dissolution of pectin between the cellulose microfibers and the cellulose, the ultrastructure exhibited almost complete dissolution of the middle lamella, and loosening and softening of microfiber structure, drift of the primary wall due to the dissolution of the middle lamella, and even local degradation of parts of the primary cell were observed, resulting in thinning of the walls and a decrease in the hardness of the flesh.[Citation17] After thermal processing, just a little damage to the cell wall was observed in cooked Lulinhu lotus rhizome, and the structure was relatively intact. In contrast, the cell walls in cooked Miancheng lotus rhizome collapsed after thermal processing, and damage to the structure was severe. It was thus confirmed that the hardness value was high for cooked Lulinhu lotus rhizome but low for cooked Miancheng lotus rhizome. These results were consistent with the data reported previously.[Citation18]
Content of cell wall components the fresh lotus rhizome of different cultivars and at different harvesting time
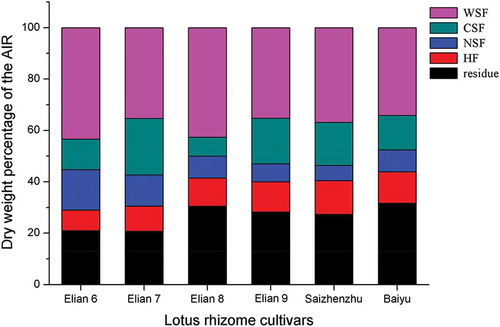
The percentage of cell wall components in the dry AIR was determined for the lotus rhizomes harvested in June, August, October, December, February and April of the next year, and the results were shown in . The results showed the changes in the content of the cell wall components of lotus rhizomes harvested at six different times. The annual mean of the percentages of the WSF (by weight) of samples Lulinhu, Miancheng, and Elian 5 in the dry AIR are 36.2%, 44.5%, and 37.9%, respectively; those of the CSF are 15.1%, 8.7%, and 12.4%, respectively; those of the NSF are 17.7%, 17.3%, and 16.0%, respectively; those of the HF are 14.2%, 15.5%, and 15.1%, respectively; and those of the residue are 16.7%, 14.0%, and 18.5%, respectively.
Figure 4. Percentage of cell wall components of the dry weight of the AIR in different lotus rhizome cultivars at different harvest time.
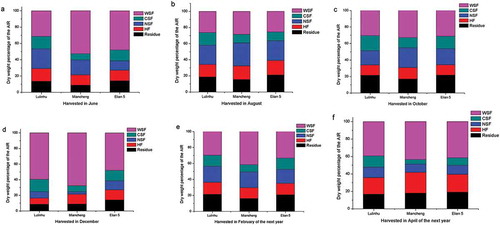
The WSF is an important component of the total pectin and is closely related to the degradation of pectin and the softening of fruits and vegetables.[Citation19] Texture was strongly correlated with water-soluble pectin.[Citation20] The CSF of pectin forms an ionic bond with poly (α-D-galactose-ranosyluronic acid) cross-linked by calcium ions, which comprises of the main chain. The changes in the CSF and NSF affected the binding force between cells and altered the cell inter-layers and thus diminished the texture of fruits and vegetables. The HF extracted by strong basic solutions was used to measure the dissolution and transformation of cell wall components.[Citation11,Citation21] The average of the ratio of the CSF and WSF components in the cell wall pectin over the entire year was compared; the highest value was observed for Lulinhu lotus rhizome (0.45 ± 0.14), followed by Miancheng lotus rhizome (0.22 ± 0.12) and Elian 5 lotus rhizome (0.35 ± 0.11). In for one kind of lotus rhizome cultivar the relative percentage of WSF, CSF, NSF, HF and residue changed depending harvest time, and from and , we can concluded that there was a good relationship between the hardness of cooked lotus rhizome tissue and changes of the relative percentage of WSF, CSF, NSF, HF and residue in them. High WSF and low CSF relative percentage of lotus rhizomes at different harvest time meant mealy properties. and showed that at the same harvest time the relative percentage of WSF, CSF, NSF, HF and residue changed depending cultivars, there was a good relationship between the hardness of cooked lotus rhizome and the changes of the relative percentage of WSF, CSF, NSF, HF and residue in them just referring to the cultivars.
Evaluation of the relationship between the ratio of the CSF/WSF and the hardness of the cooked lotus rhizome
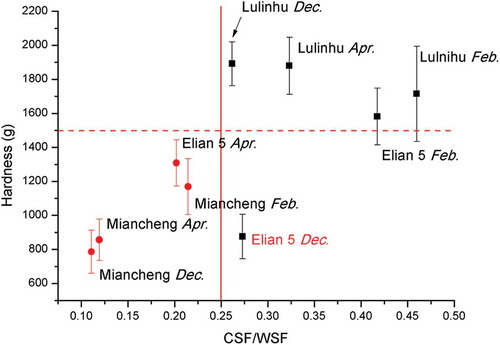
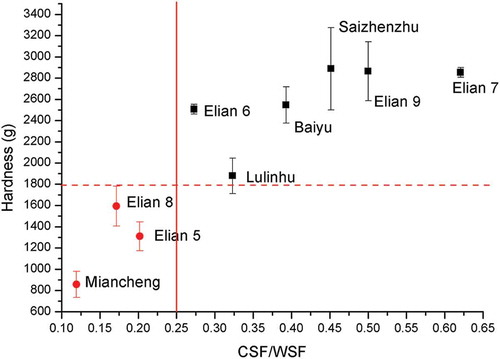
The lotus rhizomes were harvested year-round for consumer except May as this month is the germination season of lotus rhizome and from December to April of the next year is the main harvesting period with the highest yield. In , the CSF/WSF ratio of three lotus rhizomes cultivar in different harvesting time and the hardness values were list, the cooked lotus rhizome showed the mealy properties, if the CSF/WSF ratio is lower than 0.25, or crispy if the CSF/WSF ratio is higher than 0.25. Miancheng lotus rhizome is a wild type one with mealy properties after cooking, From , we know that Miancheng lotus rhizome harvested in December, February, and April showed mealy properties after cooking, this result was consistent to practice calculated by consumer. And for the medium type crispy lotus rhizome Elian 5, the cooked properties changed depending the harvesting time, from December to April of the next year, the cooked properties changed from crispy to mealy. As we know that Lulinhu lotus rhizome, a local cultivar, kept crispy cooked properties throughout the harvesting time. In the cooked Lulinhu lotus rhizome showed a relative higher hardness, indicating crispy properties, with a higher CSF/WSF ratio (≥0.25) in the studied harvesting time. But there was a exception Elian 5 harvested in December, cooked sample showed mealy properties but the CSF/WSF is 0.27 higher than 0.25, further work was needed to confirm it. In , the relationship between the hardness of the cooked lotus rhizome and the ratio CSF/WSF of different cultivars harvested in April, 2015 was shown, for the mealy property lotus rhizome cultivars (the hardness is lower than 1500 g) the ratio CSF/WSF is lower than 0.25, For the crispy property lotus rhizome cultivars the ratio CSF/WSF is higher than 0.25. So the ratio CSF/WSF 0.25 is a best mark to determine the cooked property of lotus rhizome.
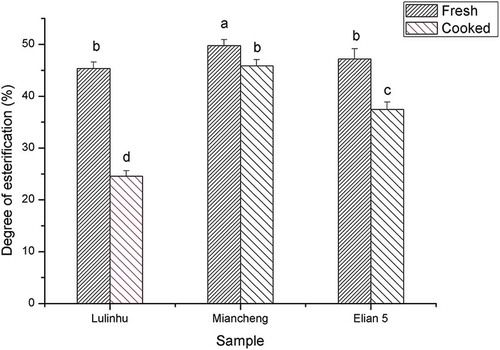
Effect of thermal processing on the pectin content and the degree of esterification of the lotus rhizome
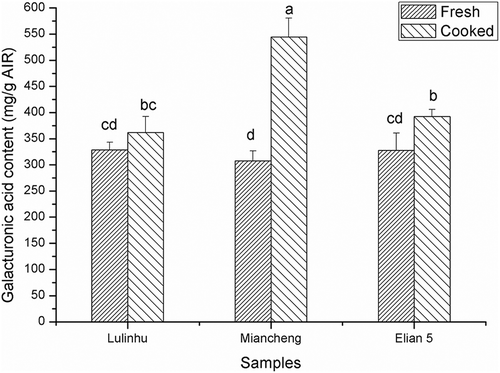
showed that after thermal processing, the galacturonic acid content increased significantly (P < 0.05), and the values of samples Lulinhu, Miancheng, and Elian 5 were 361.76 ± 30.54 mg/g AIR, 544.59 ± 35.79 mg/g AIR, and 392.46 ± 13.23 mg/g AIR, respectively. Due to the high temperature, the binding force between pectin and the cell walls decreased, resulting in an increased extraction rate of pectin. Therefore, the increase in the galacturonic acid content might be another reason for the decrease in hardness of lotus rhizomes after thermal processing. The galacturonic acid content was lowest in Lulinhu lotus rhizome, and correspondingly, the hardness value was highest for Lulinhu lotus rhizome after thermal processing. In contrast, the galacturonic acid content was highest in Miancheng lotus rhizome, and correspondingly, the hardness was lowest for Miancheng lotus rhizome after thermal processing. These results were consistent with the negative correlation between the galacturonic acid content in the AIR and the sensory hardness and crispness obtained Billy et al.,[Citation22] who investigated the correlation between pectin and the texture in apples.
Figure 8. Effect of the thermal processing on the galacturonic acid content of the AIR. Different lowercase letters indicated the significant difference of hardness of fresh and cooked lotus rhizome (P < 0.05).
The degree of esterification (DE) refers to the degree of methyl esterification of galacturonic acid on the main chains of pectin, which can reveal the breaking of ester bonds in pectin. The DE affects the physiochemical properties of pectin and thus the texture of vegetable tissues.[Citation23] showed that the DE values of fresh samples of lilinhu, Miacheng, and Elian 5 were 45.37 ± 1.22%, 49.77 ± 1.17%, and 47.21 ± 1.96%, respectively, which classified these samples as low methoxyl pectins. Compared to the DE values of the fresh samples, the DE values for all samples were significantly decreased after thermal processing (P < 0.05). This result was likely explained by the fact that during thermal processing, methyl ester bonds were hydrolysed, and pectin was cross-linked to other cell wall polysaccharides, resulting in a decrease in the extraction rate of pectin. The interaction between pectin and pectin methylesterase (PME) decreases the DE. The DE values for samples Lulinhu, Miacheng, and Elian 5 after thermal treatment were 24.54 ± 1.07%, 45.88 ± 1.16%, and 37.44 ± 1.43%, respectively. The magnitude of the DE values for the samples exhibited the following order (lowest to highest): Lulinhu followed by Elian 5 and then Miancheng. Among these samples, the DE value was lowest for Lulinhu. An increased number of hydroxide radicals are present in the pectin molecule, and therefore, it was easier for pectin to form cross-linked structures with the metal ions in the tissue. The greater hardness value of Lulinhu and the lower hardness value of Miancheng after thermal treatment can be explained by the combination of the increased degree of esterification and galactonic acid content in Miancheng.
Conclusion
The integrity of cell wall is an important factor affecting the texture of lotus rhizomes, and the pectin in cell wall is the key component of cell wall strength. The SEM results showed that during thermal processing, the more degradation of the cell wall of the lotus rhizome, the mealier the tissue was. The ratio CSF/WSF 0.25 is an excellent mark to determine the property of the cooked lotus rhizome. In addition, the DE values for all samples were significantly decreased (P < 0.05) while the galacturonic acid content were significantly increased (P < 0.05) after thermal processing. Hydroxide radicals can neutralise the anions in pectin and thus are more beneficial for cross-linking between the pectin molecules, which is another reason that the texture was well maintained after the thermal processing.
Acknowledgements
The research was supported by The Major Projects of Technical Innovation in Hubei (2017ABA152) and The National “Twelfth Five-Year” Plan for Science & Technology Support (2012BAD27B03).
References
- Xu, J. R.; Wang, Q. Z.; He, J. J.; Zhou, M. Q.; Hu, Z. L. Study on Storage and Physiology of Enlarged Rhizome of Lotus. Amino Acids Biotic Res. 2003, 25(2), 4–7.
- Liu, Y. M.; Wei, Y. X.; Li, F.; Huang, X. F.; Ke, W. D. The Evolution of Lotus Rhizome Varieties in Huibei Province and Their Cultivation and Utilization Characteristics. Hubei Agric. Sci. 2006, 45(3), 342–344.
- Li, J.; Yan, S. L.; Wang, Q. Z.; Qian, W. W. Comparative Study on Cooking Characteristics of Lotus Roots of Two Different Textures. J. Changjiang Veg. 2011, 16, 128–132.
- Larsen, D. S.; Tang, J. Y.; Ferguson, L.; Morgenstern, M. P.; James, B. J. Textural Complexity Is a Food Property – Shown Using Model Foods. Int. J. Food Properties. 2016, 19(7), 1544–1555. DOI: 10.1080/10942912.2015.1027402.
- Stollesmits, T.; Beekhuizen, J. G.; Cvan, D.; Agj, V.; Recourt, K. Cell Wall Dissolution during Industrial Processing of Green Beans (Phaseolus Vulgaris L.). J. Agric. Food Chem. 1995, 43(9), 2480–2486. DOI: 10.1021/jf00057a030.
- Segonne, S. M.; Bruneau, M.; Celton, J. M.; Gall, S. L.; Francin-Allami, M.; Juchaux, M. Multiscale Investigation of Mealiness in Apple: An Atypical Role for a Pectin Methylesterase during Fruit Maturation. BMC Plant Biol. 2014, 14(1), 1–18. DOI: 10.1186/s12870-014-0375-3.
- Xu, C. C.; Yu, C.; Li, Y. F. Effect of Blanching Pretreatment on Carrot Texture Attribute, Rheological Behavior, and Cell Structure during Cooking Process. LWT Food Sci. Technol. 2015, 62, 48–52. DOI: 10.1016/j.lwt.2015.01.033.
- Roeck, A. D.; Mols, J.; Duvetter, T.; Loey, A. V.; Hendrickx, M. Carrot Texture Degradation Kinetics and Pectin Changes during Thermal Versushigh-Pressure/High-Temperature Processing: A Comparative Study. Food Chem. 2010, 120, 1104–1112. DOI: 10.1016/j.foodchem.2009.11.060.
- Balica, I.; Ejsmentewicz, T.; Sanhueza, D.; Silva, C.; Peredo, T.; Olmedo, P.; Barros, M.; Verdonk, J. C.; Paredes, R.; Meneses, C.; Prietoc, H.; Orellana, A. Biochemical and Physiological Study of the Firmness of Table Grape Berries. Postharvest Biol. Technol. 2014, 93, 15–23. DOI: 10.1016/j.postharvbio.2014.02.001.
- Njoroge, D. M.; Kinyanjui, P. K.; Makokha, A. O.; Christiaens, S.; Shpigelman, A.; Sila, D. N.; Hendrickx, M. E. Extraction and Characterization of Pectic Polysaccharides from Easy- and Hard-To-Cook Common Beans (Phaseolus Vulgaris). Food Res. Int. 2014, 64, 314–322. DOI: 10.1016/j.foodres.2014.06.044.
- Kan, J.; Liu, T.; Jin, C. H.; Xie, H. Y. Degradation of Cell Wall Polysaccharides and Related Enzyme Activities during Non-Melting-Flesh Peach Fruit Softening. Food Sci. 2011, 32(4), 268–27.
- Du, S. L.; Studies on the Differences and Influencing Factors of Cell Wall Components of Lotus Rhizomes with Crispy and Mealy Texture. Masters Degree Thesis, Huazhong Agricultural University, Wuhan, 2013.
- Delgado, A. E.; Rubiolo, A. C. Microstructural Changes in Strawberry after Freezing and Thawing Processes. LWT Food Sci. Technol. 2005, 38(2), 135–142. DOI: 10.1016/j.lwt.2004.04.015.
- Moore, J. P.; Nguema-Ona, E.; Fangel, J. U.; Willats, W. G. T.; Hugo, A.; Vivier, M. A. Profiling the Main Cell Wall Polysaccharides of Grapevine Leaves Using High-Throughput and Fractionation Methods. Carbohydr. Polym. 2014, 99(2), 190–198. DOI: 10.1016/j.carbpol.2013.08.013.
- Yao, J.; Change and Mechanism of Asparagus Lettuce Texture under High Pressure Processing. Ph. D. Dissertation, China Agricultural University, Beijing, 2014.
- Chiang, P. Y.; Luo, Y. Y. Effects of Pressurized Cooking on the Relationship between the Chemical Compositions and Texture Changes of Lotus Root (Nelumbo Nucifera Gaertn.). Food Chem. 2007, 105, 480–484. DOI: 10.1016/j.foodchem.2007.04.003.
- Zhang, P. L.; Chen, F. S.; Yang, H. S.; Li, L. T.; Gong, B. W.; Wang, L. L. Research Advances on Cell Wall Disassembly in Fruit Ripening and Softening. Food Sci. Technol. 2010, 35(11), 62–66.
- Cheng, J. S.; Shen, H. L.; Yang, X. Y.; Li, M. G.; Yu, Y.; Lian, X. H.; Sun, X. B. Tissue Structure Observation and Cell Wall Substrates Content Determination in Different Fruit Firmness of Pepper (Capsicum Annuum L.) Lines. Acta Agric. Bor. Sin. 2008, 17(1), 150–156.
- Lecain, S.; Ng, A.; Parker, M. L.; Smith, A. C.; Waldron, K. W. Modification of Cell-Wall Polymers of Onion Waste- Part I. Effect of Pressure-Cooking. Carbohydr. Polym. 1999, 38, 59–67. DOI: 10.1016/S0144-8617(98)00080-0.
- Missang, C. E.; Maingonnat, J. F.; Renards, C. M. G. C.; Audergon, J. M. Apricot Cell Wall Composition: Relation with the Intra-Fruit Texture Heterogeneity and Impact of Cooking. Food Chem. 2012, 113, 45–54. DOI: 10.1016/j.foodchem.2011.12.059.
- Mafra, I.; Lanza, B.; Reis, A.; Marsilio, V.; Campestre, C.; Angelis, M. D.; Coimbra, M. A. Effect of Ripening on Texture, Microstructure and Cell Wall Polysaccharide Composition of Olive Fruit (Olea Europaea). Physiol. Plant. 2001, 111(4), 439–447. DOI: 10.1034/j.1399-3054.2001.1110403.x.
- Billy, L.; Mehinagic, E.; Royer, G.; Renard, M. G. C. C.; Arvisenet, G.; Prost, C.; Jourjon, F. Relationship between Texture and Pectin Composition of Two Apple Cultivars during Storage. Postharvest Biol. Technol. 2008, 47(3), 315–324. DOI: 10.1016/j.postharvbio.2007.07.011.
- Ying, S. S.; Study on the Extraction and Modification of Pectin from Pitaya Peel. Masters Degree Thesis, Zhejiang University, Hangzhou, 2014.