ABSTRACT
Dual and triple combinations of high oleic acid sunflower oil (SO), SO, and blend oil (BO) were used to produce carnauba wax (CW) oleogels (OGs). Fatty acid composition, solid fat content (SFC), and rheological and textural properties of these OGs were determined and compared to laboratory-manufactured margarine. Fatty acid composition, SFC, rheological properties, and firmness value of the OGs were significantly influenced by oil combinations. The storage modulus (G’) value was higher than the loss modulus (G’’) value in all the OGs. It indicated they are solid-like characteristics. Positive correlation between hardness and AF parameter was found.
Introduction
In the food industry, the usage of hardened oils for different purposes is worldwide. Hardened oils play a crucial role in the textural and sensory properties of food products[Citation1]; therefore, they are important for consumer acceptability of the final products. Hardened oils are produced by transforming the liquid oil into the solid form. The most common methods used for this transformation are hydrogenation, inter-esterification, and fractionation.[Citation2] The common side effect of solidification method is the formation of a high amount of saturated fatty acids (SFA) or trans fats. For instance, trans fatty acids may occur in the partial hydrogenation process used in margarine production.[Citation3] For this reason, both academic and industrial researchers have made an effort to develop different methods for converting liquid oils to the solid form by minimizing the amount of trans fats and SFA.[Citation4]
Margarines containing water, milk, additives, and oils are widely consumed hardened oils. The emulsion can be consumed directly or used as an ingredient in different processed foods such as in bakery products.[Citation5] The prevalent vegetable-based oils used in margarine production are cotton oil, canola oil, and sunflower oil (SO), and the palm oil fractions such as palm stearin, palm olein, or palm kernel produced by the fractionation of palm oil.[Citation6] Consumers avoid consuming margarine or margarine-including products because of the concerns of trans fat and SFA, which have negative effects on health.
The consumption of saturated and trans fatty acids increases the risk of cardiovascular diseases. These fatty acids increase the amount of low-density lipoprotein (LDL) cholesterol significantly; on the other hand, trans fatty acids decrease the amount of high-density lipoprotein (HDL) cholesterol in metabolism.[Citation7,Citation8] The labelling of trans-fats-containing foods becomes mandatory. In many countries (products containing less than 2% trans-fat shall legally have an expression on the package saying that it does not contain trans fat)[Citation9], labelling and banning trans fat in foods became compulsory because of adverse effects on health.[Citation10] Recently, numerous studies focused on reducing the amount of SFA and trans fats in foods[Citation4,Citation11] by transforming liquid oils into solid-like form using non-conventional methods. Oleogelation or organogelation is a novel technique that represents the forming of liquid oils into a gel-like structure by entrapping the liquid phase into a thermo-reversible and three-dimensional gel network.[Citation12] Oleogelation recently has been of great interest in many areas like pharmaceutics[Citation13], food, cosmetic, petrochemistry, etc.[Citation4,Citation14] In the food industry, oleogels (OGs) prepared by different oils and waxes with various concentrations were used instead of adding shortenings into many food products such as cookies[Citation15–Citation18], spreads[Citation19,Citation20], chocolate pastes[Citation20], cakes[Citation20,Citation21], and compound chocolate[Citation22,Citation23] Spreads and margarine-like products were studied by several researchers[Citation19,Citation20], who used virgin olive oil and rapeseed oil as an oil phase and carnauba wax (CW), beeswax, shellac, rice bran, and sunflower wax as an oleogelator. The result indicated that the characteristics of OGs were remarkably affected by the oil and wax types and their concentrations. Therefore, further studies are required to produce OGs to fill the lacunae by achieving a good quality of shortenings or margarines by optimization of formulation. In this case, it is necessary to observe the usage possibilities of different oil/oleogelator combinations in the production of OG production.
Previous works have shown that waxes are the most efficient oleogelators because of their ability to start crystallization at lower concentrations (<10%), easy to find, and being economic.[Citation24] Regarding the good crystallization behaviour of waxes, it can be mentioned that some specific characteristics of waxes, including low polarity, high melting point, and long chain length, are affected by the crystallization of oils.[Citation25]
SO has been widely used for cooking and frying during food processing and is a great source for the biodiesel industry.[Citation26] SO has a high amount of polyunsaturated fatty acids (the majority of fatty acids is linoleic acid).[Citation27] Based on its fatty acid composition, SO is classified into four different groups: high oleic sunflower oil (HOSO), mid-oleic sunflower oil (MOSO), high stearic high oleic sunflower oil (HSHOSO), and high palmitic high oleic sunflower oil (HPHOSO).[Citation28] OGs were prepared from SO[Citation21,Citation16] and HOSO[Citation29] to use in the preparation of cookies, cakes, and ice creams. In this study, it was aimed to produce wax OGs using different percentages of SO and HOSO and to observe quality similarities with conventional margarines in terms of some physicochemical properties.
The first objective of this study was to investigate the potential usage of SO wax-OGs in margarine formulation, which had low SFA and trans fat, as an alternative to spreadable products like margarines. Second, it also aimed to increase the unsaturated fatty acid content by using HOSO instead of regular SO in OG formulation.
Material and methods
Materials
Industrial blend oil (BO) produced by In-Es (inter-esterification) technology containing 50% palm oil, 20% palm stearin, 20% cotton oil, and 10% palm kernel SO and HOSO were purchased from a local market in Istanbul. Food-grade CW (2442, Kahlwax; Kahl GmbH & Co. KG) was supplied by Ejder Kimya Inc. The ingredients of margarine formulation were procured from a company in Istanbul, Turkey.
Methods
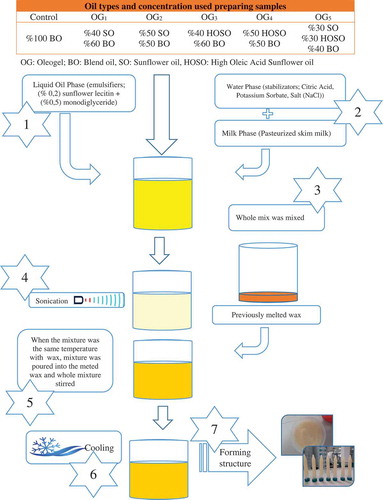
The formulation and the production procedure of OGs and margarine are showed in . The water content in watery formulations was 16%, which is equal to the water content of the commercial margarines sold in Turkey. Approximately 5% CW was used in all the OG formulations. The preparation of all OGs and margarine was similar to the production of commercial margarine.
Production of margarine
For margarine production, BO was melted and emulsifiers ((‰ 2‰) sunflower lecithin + (5‰) monodiglyceride), (2‰) β-carotene, (0,15‰) Vitamin A, and (0,015‰) vitamin AD3 were solved in a small amount of melted oil. For the water phase of margarine, 1‰ citric acid, 1‰ potassium sorbate, and 2.5% sodium chloride were solved in tap water and 40‰ skim milk was added into the mixture. Afterwards, water and oil phases were emulsified and sonicated for 10 min at 100% amplitude by an ultrasonic homogenizator (Hielscher-UP200 Ht, Germany). The second homogenization was applied using ultra-turrax for 10 min and the final product was gradually cooled down to room temperature and kept in a refrigerator for upcoming analysis.
Production of OGs
Before the preparation of OGs, previously weighed waxes and oils were placed in a water bath at 90°C. While emulsifiers ((‰ 2‰) sunflower lecitin + (‰ 5‰) monodiglyceride), (‰ 2‰) β-carotene, (‰ 0,15‰) Vitamin A, and (‰ 0,015‰) vitamin AD3 were solved in oil phase, stabilizators (citric acid, potassium sorbate, and NaCl) were solved in water phase. The OGs were produced as follows.
First, water phase (W) and oil phase (O) were mixed. For better emulsion, a two-phase mix was sonicated for 10 min at 100% amplitude by an ultrasonic homogenizator (Hielscher-UP200Ht, Germany). Emulsion (W/O) and wax (X) were heated until 90°C for the second time after sonication. When the W/O reached a similar temperature where wax was melted, W/O was poured into the melted wax. To proceed with the same emulsion quality, the wax/emulsion (X/O/W) mixture was homogenized with ultra-turrax for 10 min and cooled down in room temperature and kept in the refrigerator.
Determination of the fatty acid composition of OGs
GC-FID (Agilent 6890 GC, USA) equipped with an HP-88 column (100 m x 0.25 mm ID x 0.2 µm) was used to determine the fatty acid composition of the samples. The methyl esters of the fatty acids were prepared according to the method in AOCS[Citation30] with some modifications. Around 10 ml of n-hexane was poured into a centrifuge tube and 0.1 g sample was added. The whole mixture was mixed for 30 s in a vortex mixer (VELP-ZX3, Italy). A total of 100 µl of 2 N potassium hydroxide (KOH) solution prepared in methyl alcohol was added and mixed for 30 s by a vortex mixer and centrifuged at 40 rpm for 5 min. After centrifugation, 1–2 ml supernatant was transferred to GC vials and placed in an auto sampler. Temperature and oven program of the method were as follows: inlet temperature: 250°C; injection volume: 1 µl; split ratio: 1/50; carrier gas: helium; pressure flow: 2 ml/min; oven temperature: 120°C for 1 min, increasing to 175°C at 10 °C/min, to 210°C at 5°C/min, to 230°C at 5°C/min and waiting at that temperature for 5 min; detector temperature: 280°C; detector gases: hydrogen: 40 ml/min, air: 450 ml/min, helium: 30 ml/min.
Determination of solid fat content of OGs
Solid fat content (SFC) of the samples was determined using NMR (Bruker Minispec 7.5 MHz, USA) at three different temperatures (10, 20, and 30°C). Around 3.5 ml of water-free samples were placed in NMR glass tubes and kept at 60°C for 5 min and then at 0 °C for 1 h. Afterwards, they were maintained in a water bath at 20°C for 30 min. The hardened sample was selected and then put into NMR.[Citation31]
Rheological properties of OGs
Stress/strain controlled rheometer (Anton Paar, MCR 302, Australia) equipped with a Peltier heating/cooling system was used for determining the viscoelastic characteristics of the samples. Parallel plate geometry (diameter = 50 mm) was used for rheological analysis. The gap was adjusted to 1 mm and analysis was carried out at 5°C. Before the velocity test, linear viscoelastic region (LVR) was determined by an amplitude test with the strain range between 0.1% and 100%. According to the results, the velocity sweep test was performed at 0.5%. Storage modulus (G’), loss modulus (G’’), complex modulus (G*), complex viscosity (η*) and tan (δ) for viscoelastic characteristics of the samples were measured as a function of velocity.
Instrumental texture analysis
Firmness values of the samples were determined by a texture analyser (Stable Micro Systems, TA.XT2 Plus, UK) equipped with 5 kg load cell. A cylindrical probe (P/2) was used to determine the textural properties of OGs at 25°C. Penetration test was performed and the force required for penetration of 1 cm was determined using the displacement–force curve obtained.
Statistical analysis
One factor analysis of variance (ANOVA) was performed to determine whether the differences between the corresponding quality parameters of the samples were statistically significant or not (p < 0.05).
Results
Fatty acid composition of OGs
The fatty acid compositions of reference (control margarine) and OGs including 5% CW prepared with different percentages of commercial In-Es blend fat, SO, and HOSO are given in . Regarding control margarine, as mostly fractions of palm oil are generally used in In-Es blend fat, palmitic acid (16:0), one of the SFA, was found to have the highest concentration (40.69%), whereas the lowest amount was observed in OG5, which contained the lowest (20.52%) blend fat amount among all the samples produced.Oleic acid content of control margarine was found to be 28.74%. While OG4 had the highest oleic acid content (%51.21), OG2 had the lowest (26.96) among all oleogel samples.
Table 1. Fatty acid composition of the samples (n = 3).
The total unsaturated fatty acids (TUFA) content of the samples changed between 43.52% (control margarine) and 69.08% (OG5). The mono-unsaturated fatty acids (MUFA) concentrations were found to be between 27.47% (OG2) and 51.59% (OG4) and 14.22% (OG3) and 29.43% (OG2). Regarding saturated fatty acids (SFA) contents, it changed between 30.26% (OG5) and 56.16% (control margarine). The findings of the present study indicated that OGs had higher TUFA and lower SFA contents when compared with the control margarine.
Solid Fat Content
SFC of the samples at three temperature levels (10, 20, and 30°C) was determined and the results are shown in . The reference sample had the highest SFC, whereas OG5 had the lowest SFC at all temperature levels analysed. Among the prepared OG samples, the highest SFC was observed in the OG1 sample prepared from SO and BO at concentrations of 40 and 60%, respectively. At 10°C, after control margarine, OG1 had the highest SFC value and OG5 had the lowest compared with the others. Both the highest and lowest SFC values of all the samples after reference displayed the same pattern at 20 and 30°C.
Table 2. SFC values of the samples (10°C, 20°C, and 30°C).
Rheological properties
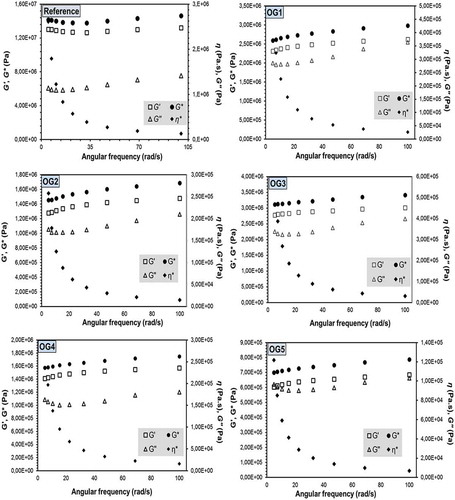
Different rheological properties of the control margarine and OGs are shown in and , where changes in the viscoelastic parameters, namely, G’, G’’, G*, and η* values, as a function of angular velocity are presented. As can be seen from , the G’ values were found to be significantly higher than the G’’ values in all samples at all angular velocity values performed and no crossover point was observed, indicating that the solid-like character of the samples was predominant within the velocity range studied. The viscoelastic results demonstrated that G′ slightly increased with angular velocity like G″. The obtained velocity versus the G’ and G’’ values were modelled using the power law functions as follows:
Table 3. Power-law model parameters calculated for describing the relation between angular velocity and the G’ and G’’ values.
Model coefficient calculations are presented in . The R2 values obtained from fitting of the G’ values changed between 0.9647 and 0.9966, except for the reference, and those for G’’ were found to be between 0.2667 and 0.7416. The findings showed that the relationship between angular velocity and G’’ values cannot be explained by the power law model, especially for the OG1 and OG4 samples. The K’ values of the samples were found to be higher than the K’’ values, implying that the control and OG samples had solid-like behaviour rather than viscous character. In addition, using Equation 3, where G* is expressed as a function of angular velocity, the gel strength of the samples was calculated.
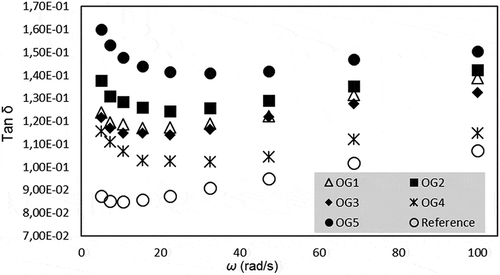
where AF and z are the gel strength and interaction factor, respectively.[Citation32] The obtained data were fitted to the model and the corresponding parameters are tabulated in . As can be seen, the AF values changed between 650,571 and 13,752,174, implying that OG formulation significantly affected the gel strength of the samples. The findings indicated that there is a positive correlation between the AF values and the SFA content (r = 0.9067). The AF value of the control sample was very large compared with that of the OGs. Increasing the blend fat concentration or changing the fatty acid composition of the OGs may affect the AF value. Variation of tan δ (G’’/G’) with respect to angular velocity is shown in . The tan δ values of the all samples ranged between 0.08 and 0.17 within all the velocity ranges studied, also indicating the solid character of the samples analysed.
Textural properties
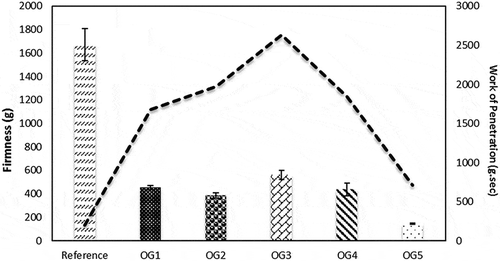
Another quality parameter determined in this study was texture analysis, which was conducted to determine the hardness and spreadability of the samples. Firmness and work of penetration values are presented in . As expected, the hardness value of margarine was the highest, followed by OG3, OG1, OG4, OG2, and OG5, respectively. The results indicated that fatty acid composition of the samples remarkably affected textural properties like the rheological characteristics. Considering the fact that the textural properties were determined at room temperature, the results are consistent with the SFC profile of the OGs (). Positive correlation between hardness and SFA content or hardness and AF value was also observed, with correlation coefficients of 0.9312 and 0.9067, respectively. The lowest penetration was observed in the control sample, penetration of OG3 was found to be the higher than those of oleogel samples.
Table 4. Magnitudes of AF and z values calculated by the fitting of Equation 3.
Discussion
Fatty acid composition samples
As expected, the sample including HOSO (OG4) had the highest oleic acid concentration. Increasing the oleic acid content of fat-based materials is important for health since it may decrease coronary heart disease risk by reducing the oxidation susceptibility of LDL cholesterol to oxidation.[Citation33]
In a related study, Si et al.[Citation34] compared the fatty acid composition of soybean oil and soybean OGs produced with different types of oleogelators such as glycerol monostearate (MAG), sorbitan tristearate (STS), and lecithin (Lec) and different compositions of oleogelators, which are 3, 6% MAG and 6, 8, 10% Lec-STS, confectionery-fitting fat (CFF) and coating fat. Soybean oil had the lowest SFA (16,63) and the highest polyunsaturated fatty acid (58,11) contents compared with the others. On the other hand, coating fat contained the highest SFA content, which was 99,68, and after coating fat, CFF had the second highest SFA (43,58%) among all the samples. As a result of fatty acid composition of the samples, after soybean oils, OGs were a better solid-like material compared with fitting and coating fat. The highest SFA content (56,2%) was found to be in control margarine in our work, whereas the CFF was the highest in the study mentioned above. On the other hand, OG5, which had 40% BF, was found to be the sample that included the lowest SFA, similar to that of soybean oil. The results indicated that lowering blend fat affected the SFA content.
In another study, Jang et al.[Citation17] evaluated the fatty acid composition of cakes prepared with shortening, 3% and 6% canola oil/candelilla wax OGs. They found that while shortening included the highest amount of palmitic acid (16:0) (41.9%), the other two samples were rich in unsaturated fatty acids, namely, oleic (18:1, 62%), linoleic (18:2, 20%), and linolenic (18:3, 7%) acids. Comparing the palmitic acid amounts of shortening and control margarine in our work, both were found to be higher than those of canola oil and wax OGs, as expected. Moreover, the highest oleic, linoleic, and linolenic acid contents were 51.21% in OG4, 26.94% in OG1, and 0.48% in OG2.
Solid Fat Content
The melting behaviour is one of the most important quality factors of margarine. According to the quality of margarine, its rapid meltability in the mouth and firmness at room temperature are substantial quality indicators.[Citation35] Using HOSO in the OG formulation resulted in a decrease in SFC of OGs, which seems to be a negative aspect. However, the SFC of such OGs can be adjusted by increasing the SFA present in the OG formulation. Results of the present study indicated that a higher SFA content resulted in an increase in SFC. Miskandar et al.[Citation6] found that the SFC values of margarines at 20°C, namely table refrigerated, pastry, palm oil, and table non-refrigerated, were 14, 32, 23, and 26%, respectively.
For margarine, SFC must be lower than 6% at 37°C.[Citation35] In the SFC values of this study, the OG1 and OG4 samples had approximately acceptable SFC values compared with that of margarine. SFC is associated with the plasticity and consistency of OGs or fats[Citation37]; it is also one of the important properties of an oil or fat, defined as the ratio of the solid content to the total matrix at a particular temperature.[Citation36] The SFC and SMP (slip melting point) values are usually used for the estimation of products’ suitability for different uses and climatic conditions.[Citation38]
Rheological properties
Rukke et al.[Citation39] found that the G’ value of vegetable oil-based margarines was higher than 107. Regarding the findings in this study, the control sample gave similar results; however, the value of OGs was found to be lower than that reported by Rukke et al.[Citation39], which might have resulted from the reduction of SFA present in the formulation. The slight increases in G’ and G’’ were determined with respect to angular velocity, which represents the characteristics of solid-like gels.[Citation40]
When the G’ and G’’ values are slightly increased with angular velocity, this situation is called the “plateau region”, which is typically found in highly entangled polymeric systems.[Citation41] On the other hand, this behaviour displays properties similar to those of a weak gel.[Citation40] When the tan δ values of the samples were lower than 1, these systems show gel-like consistency.[Citation41] The tan δ value can be used to characterize the relative elasticity of materials.[Citation42]
Textural properties
In , the oil types used in the OG formulation significantly affected the hardness value of the samples, consistent with the previous study.[Citation43] The types and concentrations of oil and wax were the major parameters influencing the physical properties of the OGs.
Conclusion
Selected important properties (fatty acid composition, SFC, textural, and rheological properties) of the water-containing wax-OGs produced by SFO and HOSFO compared with commercial margarine were determined. The wax-based oleogelation method for structuring liquid oil into gel-like materials was successfully demonstrated. The water-containing OGs showed a gel-like characteristic as well as the water-free OGs. Results showed that the oil type used in the formulation was one of the major parameters that significantly affected the thermal, rheological, and textural properties of the wax-OGs. OG with high oleic acid content can be produced by optimization of formulation. OG5 had the highest TUFA and the lowest SFA, whereas the control margarine was the exact opposite. In the SFC values, control margarine had the highest values at all temperatures analysed. This study showed that wax OGs have some similar properties (textural, rheological, and SFC) to those of commercial margarines and that spreadable products can be produced using oleogelation technology. As a result, these findings showed that the development of SO-based soft matter systems including oleic acid at high concentrations could be used in food formulas. This is important to the industry and people regarding reduction of SFA in their diet.
References
- Jones, S. A. Issues in Fat Replacement. In Handbook of Fat Replacers, Eds; Roller, S., and Jones, S. A; CRC Press: USA, 1996; pp. 3–26.
- Basoglu, F. Edible Oil Technology. Nobel Yayın Dağıtım: Turkey, 2014; Vol. 3, p. 342.
- Yu, D.; Qi, X.; Ren, Y.; Wang, W.; Sun, L.; Xu, D.; Zhang, H.; Hu, L.; Jiang, L.; Elfalleh, W. Thermal and Crystal Characteristics of Enzymatically Interesterified Fats of Fatty Acid-Balanced Oil and Fully Hydrogenated Soybean Oil in Supercritical CO2 System. Int. J. Food Properties. 2017, 20(11) 2675–2685.
- Marangoni, A. G.; Garti, N. Edible Oleogels: Structure and Health Implications; AOCS Press: Urbana, IL, 2011.
- Sopelana, P.; Arizabaleta, I.; Ibargoitia, M. L.; Guillén, M. D. Characterisation of the Lipidic Components of Margarines by 1 H Nuclear Magnetic Resonance. Food Chem. 2013, 141(4), 3357–3364. DOI: 10.1016/j.foodchem.2013.06.026.
- Hamm, W. Trends in Edible Oil Fractionation. Trends Food Sci. Technol. 1995, 6, 121–126. DOI: 10.1016/S0924-2244(00)88995-5.
- Dhaka, V.; Gulia, N.; Ahlawat, K. S.; Khatkar, B. S. Trans Fats—Sources, Health Risks and Alternative approach-A Review. J. Food Sci. Technol. 2011, 48(5), 534–541. DOI: 10.1007/s13197-010-0225-8.
- O’Brien, R. D. Fats and Oils: Formulating and Processing for Applications, 2nd ed.; CRC press: Boca Raton, 2004.
- Anonymous. Turkish Food Codex — Labeling and Consumer Information Regulation; Ankara, Turkey, 2017, No: 29960/35.
- Hwang, H. S.; Kim, S.; Evans, K. O.; Koga, C.; Lee, Y. Morphology and Networks of Sunflower Wax Crystals in Soybean Oil Organogel. Food Struct. 2015, 5, 10–20. DOI: 10.1016/j.foostr.2015.04.002.
- List, G. R.; Pelloso, T. Zero/Low Trans Margarine, Spreads, and Shortening. In Trans Fats in Foods; List, G. R., Kritchevsky, D., Ratnayake, N., Eds.,; AOCS Press: Urbana, IL. 2007; pp. 155–176.
- Marangoni, A. G. Organogels: An Alternative Edible Oil-Structuring Method. J. Am. Oil Chemists’ Soc. 2012, 89(5), 749–780. DOI: 10.1007/s11746-012-2049-3.
- Hughes, N. E.; Marangoni, A. G.; Wright, A. J.; Rogers, M. A.; Rush, J. W. E. Potential Food Applications of Edible Oil Organogels. Trends Food Sci. Technol. 2009, 20, 470–480. DOI: 10.1016/j.tifs.2009.06.002.
- Dassanayake, L. S. K.; Kodal, D. R.; Ueno, S. Formation of Oleogels Based on Edible Lipid Materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 432–439. DOI: 10.1016/j.cocis.2011.05.005.
- Mert, B.; Demirkesen, I. Reducing Saturated Fat with Oleogel/Shortening Blends in Baked Product. Food Chem. 2016a, 199, 809–816. DOI: 10.1016/j.foodchem.2015.12.087.
- Mert, B.; Demirkesen, I. Evaluation of Highly Unsaturated Oleogels as Shortening Replacer in Short Dough Product. LWT – Food Sci. Technol. 2016b, 68, 477–484. DOI: 10.1016/j.lwt.2015.12.063.
- Jang, A.; Bae, W.; Hwang, H. S.; Lee, H. G.; Lee, S. Evaluation of Canola Oil Oleogels with Candelilla Wax as an Alternative to Shortening in Baked Goods. Food Chem. 2015, 187, 525–529. DOI: 10.1016/j.foodchem.2015.04.110.
- Yılmaz, E.; Ogutcu, M. The Texture, Sensory Properties and Stability of Cookies Prepared with Wax Oleogels. Food Funct. 2015, 6(4), 1194–1204. DOI: 10.1039/C5FO00019J.
- ÖǧüTcü, M.; Yılmaz, E. Oleogels of Virgin Olive Oil with Carnauba Wax and Monoglyceride as Spreadable Products. Grasas Y Aceites. 2014, 65(3), e040. DOI: 10.3989/gya.2014.v65.i3.
- Patel, A. R.; Cludts, N.; Sintang, M. D. B.; Lesaffer, A.; Dewettinck, K. Edible Oleogels Based on Water-Soluble Food Polymers: Preparation, Characterization and Potential Application. Food Funct. 2014a, 5(11), 2833–2841. DOI: 10.1039/C4FO00624K.
- Patel, A. R.; Rajarethinem, P. S.; Gredowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W. H.; Dewettinck, K. Edible Applications of Shellac Oleogels: Spreads, Chocolate Paste and Cakes. Food Funct. 2014b, 54, 645–652. DOI: 10.1039/C4FO00034J.
- Stortz, T. A.; Marangoni, A. G. Ethylcellulose Solvent Substitution Method of Preparing Heat Resistant Chocolate. Food Res. Int. 2013, 51(2), 797–803. DOI: 10.1016/j.foodres.2013.01.059.
- Stortz, T. A.; De Moura, D. C.; Laredo, T.; Marangoni, A. G. Molecular Interactions of Ethylcellulose with Sucrose Particles. RSC Adv. 2014, 4(98), 55048–55061. DOI: 10.1039/C4RA12010H.
- Hwang, H. S.; Kim, S.; Singh, M.; Winkler-Moser, J. K.; Liu, S. X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chemists’ Soc. 2012, 89(4), 639–647. DOI: 10.1007/s11746-011-1953-2.
- Mukkamala, R.; Weiss, R. G. Physical Gelation of Organic Fluids by Anthraquinone− Steroid-Based Molecules. Structural Features Influencing the Properties of Gels. Langmuir. 1996, 12(6), 1474–1482. DOI: 10.1021/la950666k.
- Rashid, U.; Anwar, F.; Moser, B. R.; Ashraf, S. Production of Sunflower Oil Methyl Esters by Optimized Alkali-Catalyzed Methanolysis. Biomass Bioenergy. 2008, 32(12), 1202–1205. DOI: 10.1016/j.biombioe.2008.03.001.
- Salgın, U.; Döker, O.; Çalımlı, A. Extraction of Sunflower Oil with Supercritical CO 2: Experiments and Modeling. J. Supercrit. Fluids. 2006, 38(3), 326–331. DOI: 10.1016/j.supflu.2005.11.015.
- Gotor, A. A.; Rhazi, L. Effects of Refining Process on Sunflower Oil Minor Components: A Review. Oil Seeds and Fats, Crops Lipids. 2016, 23(2), D207, 1–10.
- Zulim Botega, D. C.; Marangoni, A. G.; Smith, A. K.; Goff, H. D. The Potential Application of Rice Bran Wax Oleogel to Replace Solid Fat and Enhance Unsaturated Fat Content in Ice Cream. J. Food Sci. 2013, 78(9), 1334–1339. DOI: 10.1111/1750-3841.12175.
- AOCS Official Method Ce 2-66. Preparation of Methyl Esters of Fatty Acids; 2009, Champaign, IL: AOCS Press.
- AOCS Official Method Cd 16b-93. Solid Fat Content (SFC) by low-resolution nuclear magnetic resonance - the direct method. Official Methods and Recommended Practices of the AOCS. 6th Ed. 2009, Champaign, IL:AOCS Press.
- Gabriele, D.; De Cindio, B.; D’Antona, P. A. Weak Gel Model for Foods. Rheological Acta. 2001, 40, 120–127. DOI: 10.1007/s003970000139.
- Ashton, E. L.; Best, J. D.; Ball, M. J. Effects of Monounsaturated Enriched Sunflower Oil on CHD Risk Factors Including LDL Size and Copper-Induced LDL Oxidation. J. Am. Coll. Nutr. 2001, 20(4), 320–326. DOI: 10.1080/07315724.2001.10719052.
- Si, H.; Cheong, L. Z.; Huang, J.; Wang, X.; Zhang, H. Physical Properties of Soybean Oleogels and Oil Migration Evaluation in Model Praline System. J. Am. Oil Chemists’ Soc. 2016, 93(8), 1075–1084.
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical Properties and Storage Stability of Margarine Containing Opuntia Ficus-Indica Peel Extract as Antioxidant. Food Chem. 2015, 173, 382–390. DOI: 10.1016/j.foodchem.2014.10.025.
- Miskandar, M. S.; Man, Y. C.; Yusoff, M. S. A.; Rahman, R. A. Quality of Margarine: Fats Selection and Processing Parameters. Asia Pac. J. Clin. Nutr. 2005, 14(4), 387.
- Jacob, J.; Leelavathi, K. Effect of Fat-Type on Cookie Dough and Cookie Quality. J. Food Eng. 2007, 79(1), 299–305. DOI: 10.1016/j.jfoodeng.2006.01.058.
- Chateris, W.; Keogh, K. Fats and Oil in Table Spread. Lipid Technol. 1991, 3, 16–22.
- Rukke, E. O.; Kottage, D.; Abrahamsen, R. K.; Schüller, R. B. Rheological Studies of Butter and Margarine Exposed to Repeated Breaks in the Cooling Chain. Annu. Trans. Nordic Rheology Soc. 2010, 18, 11–17.
- Sánchez, R.; Franco, J. M.; Delgado, M. A.; Valencia, C.; Gallegos, C. Rheological and Mechanical Properties of Oleogels Based on Castor Oil and Cellulosic Derivatives Potentially Applicable as Bio-Lubricating Greases: Influence of Cellulosic Derivatives Concentration Ratio. J. Ind. Eng. Chem. 2011, 17(4), 705–711. DOI: 10.1016/j.jiec.2011.05.019.
- Gallego, R.; González, M.; Arteaga, J. F.; Valencia, C.; Franco, J. M. Influence of Functionalization Degree on the Rheological Properties of Isocyanate-Functionalized Chitin-And Chitosan-Based Chemical Oleogels for Lubricant Applications. Polymers. 2014, 6(7), 1929–1947. DOI: 10.3390/polym6071929.
- Peyronel, F.; Campos, R. Methods Used in the Study of the Physical Properties of Fats. Structure—function analysis of edible fats, Ed.; Marangoni, A.G., AOCS Press: Urbana, IL, 2012; pp. 231–294.
- Zetzl, A. K.; Marangoni, A. G.; Barbut, S. Mechanical Properties of Ethylcellulose Oleogels and Their Potential for Saturated Fat Reduction in Frankfurters. Food Funct. 2012, 3(3), 327–337. DOI: 10.1039/c2fo10202a.