ABSTRACT
The present work was designed to compare the vitamin C (ascorbic acid, AsA) content of pulp, peel, and juice of 64 apple cultivars. These cultivars were carefully identified as ‘true to type’ by molecular genetic tools, grown in the same site under identical conditions and processed by a standardized protocol. Twenty-one of them, accounting for more than 95% of the apple production of South Tyrol, were chosen to represent the current market, 16 were old or local cultivars formerly grown in the area, and 27 were new cultivars, including 15 with scab resistance and 12 with red flesh fruit. For the determination of the AsA content, a new High-Performance Liquid Chromatography with Diode-Array Detection method was developed and validated. While old cultivars stood out for their high AsA content in pulp and peel, the red-fleshed cultivars are the ones maintaining most of their AsA content during processing. Our data thus suggest a potential for old and red-fleshed cultivars for healthy juices or further processed food components.
Introduction
The World Health Organization recommends the consumption of five servings of fruits and vegetable per day. The intake of fruits and vegetables was linked to positive health effects, reduced risk for cardiovascular disease, and some forms of cancer.[Citation1–Citation3] Plants produce antioxidant phytochemicals with cytoprotective properties, the health benefits of which are well documented; these compounds include polyphenols, carotenoids, and vitamins C and E.
Apples are among the most consumed fruits in the Western world, either as fresh fruit, juice, or further processed. Therefore, apples represent a major source of antioxidant nutrients in Western countries. The content of antioxidants, and AsA in particular, has been widely studied[Citation4,Citation5] but shown to depend on the genotype, the geographic location, environmental conditions during the growing season, agricultural practices, storage conditions, and processing.[Citation6–Citation15] The increased attention to healthy food has led to a growing interest for cultivars with high contents of AsA and other functional nutrients; however, studies focussing on the genotype while holding the multiple other factors that influence the AsA content constant are rare.[Citation10,Citation11,Citation16–Citation18]
While more than 7,500 apple cultivars are known today, orchard economics, demand for long storage times, and consumer preferences have led to a progressive varietal impoverishment towards few commercially available apple cultivars.[Citation19,Citation20] In South Tyrol, with 18,500 hectares, Europe’s largest contiguous apple-growing area, three cultivars (‘Golden Delicious’, ‘Gala’, and ‘Red Delicious’) account for more than 65% of the total commercial production of the region.[Citation21] Nevertheless, the increased demand for healthy food opens new market opportunities for old and innovative cultivars, like red-fleshed ones. This holds especially for processed products like cloudy juices. Therefore, data on the antioxidants of these cultivars are highly desired but frequently lacking. In addition, the decrease in the number of commercially available apple cultivars is accompanied by a decrease of knowledge in the identification of the cultivars, old ones in particular. Traditionally, apple cultivars are identified based on morphological and agronomic traits, which depend on environmental factors and are susceptible to misidentification. Thus, an accurate identification of apple cultivars by molecular genetic tools[Citation20] is an important prerequisite for studies on constituents, including antioxidants, in order to avoid an incorrect assignment of the results.
This study reports on the AsA content of pulp, peel, and monovarietal cloudy juice of 64 accurately identified apple cultivars from the Laimburg varietal collection, grown in the same site, under identical conditions and processed by a standardized protocol in order to limit the variation to the cultivar. Twenty-one of them, accounting for more than 95% of South Tyrol’s apple production,[Citation21,Citation22] were chosen to represent the current market, 16 were old or local cultivars formerly grown in the area, and 27 were new cultivars, including 15 with scab resistance and 12 with red flesh fruit. AsA contents among commercial and non-commercial cultivars were compared to evaluate the potential of yet non-commercial apple cultivars for functional food products, rich in AsA.
Materials and methods
Plant material
Harvest and storage: Apple (Malus x domestica Borkh.) trees were grown on M9 rootstocks in the experimental orchard of Laimburg Research Centre in Auer (Ora) at 220 m a.s.l. (South Tyrol, Italy) according to the regional guidelines for integrated fruit production in South Tyrol.[Citation21] All cultivars used in this study were from the Laimburg core collection and certified to be true to type involving two or more reference accessions. Fruits were harvested at the optimal harvest time for each cultivar between August and October 2013 from four different trees per cultivar. Apples were collected randomly from the central canopy, avoiding the tops and bottoms of the tree.
Fruit weight, firmness, soluble solid content, and total titratable acidity were measured for each cultivar (Table S3) using the semiautomatic instrument ‘Pimprenelle’ (Setop Giraud Technology, France) on 10 individual apples, except for total titratable acidity, for which the bulk juice of nine fruits was analysed. Starch index was determined using the iodine-starch test according to Streif et al.[Citation23]
After harvest, apples were stored at 2°C and 90% humidity under ambient air for 60 ± 10 days. The only exceptions were the early-ripening cultivars ‘Gravensteiner’, ‘Lureprec’, and ‘Weirouge’, which were stored for 14 days only. Five apples per cultivar were peeled and cut into three equal equatorial discs. The central disc was cored and immediately frozen in liquid nitrogen together with the peel and stored at −80°C. Both peel and pulp were freeze-dried separately, ground to a fine powder, and stored at −30°C in dark glass vials until further analysis. The water loss of each sample during freeze-drying was recorded.
Juice production
After storage, 50 apples were washed with cold water (~16.8°C), transferred to a centrifugal mill RM1.5 (Voran Maschinen GmbH, Austria) with an 8 mm insert, crushed to mash, and transferred immediately to the press. The mash was pressed with a hydraulically driven piston-cylinder system (Bucher Unipektin press HPL 14) using a pressure up to 5 × 105 Pa generated by a pneumatic press. Drainage elements inside the press were used for prefiltration and to support draining. Three pressing cycles of 90 s each were carried out using three pressure steps (2 x 105, 3 x 105, 5 × 105 Pa) for 30 s each in the first cycle and a single pressure step of 5 × 105 during the last two cycles. Before pasteurization at 78–83°C in a PA90 Voran (Voran Maschinen GmbH, Austria) pasteurization unit, the juice was stood overnight at 4°C for decantation. Finally, the hot juices were filled in green bottles (1 L) and cooled immediately to 30°C. An aliquot of 60 mL was stored at −80°C in an amber vial until analysis.
Ascorbic acid analysis
Reagents
Ascorbic acid (AsA) 99% was purchased from Sigma Aldrich (St. Louis, MO, USA), acetic acid (96%) was obtained from Merck KGaA (Darmstadt, Germany), methanol (HPLC-grade) was purchased from VWR Chemicals (Milan, Italy), and meta-phosphoric acid (≥99%) and monopotassium phosphate (≥ 99%) was from Thermo Fisher Scientific Inc. (Waltham, MA, USA). The ultrapure water was prepared with a Millli-Q-water purification system (EMD Millipore Corporation, Billerica, MA, USA).
Extraction from peel and pulp
An aliquot of ca. 50 mg of freeze-dried apple pulp or peel was extracted using 1 mL of extraction solution (700 µL deionized H2O containing 8% (v/v) acetic acid and 3% (w/v) metaphosphoric acid[Citation24] added with 300µL of methanol) mixed using a Vortex-Genie 2 at 3200 rpm for about 20 s at room temperature and filtered through a 0.20 µm PTFE filter.
Extraction from juices
Juice was centrifuged at 6300rcf and the supernatant was collected. An aliquot (0.5 mL) and an aqueous solution of 10% (w/v) metaphosphoric acid (0.5 mL) were mixed using a Vortex-Genie 2 at 3200 rpm for 20 s at room temperature and filtered through a 0.20 µm PTFE filter.[Citation25] Each extraction was carried out in triplicate for each monovarietal juice.
Chromatographic conditions
Agilent 1260 Infinity LC system (Santa Clara, CA, USA) with a diode array (1260 DAD VL) detector was used for the analysis of AsA. Chromatographic separation was carried out at 25°C using a Kinetex 5μ C18 100 Å column (150 mm × 4.6 mm, 5 μm particle size; Phenomenex, Torrance, CA, USA) and a pre-column (4.6 mm, Security Card, Phenomenex, Torrance, CA, USA). Detection wavelength: 260 nm. Mobile phase: solvent A: aqueous KH2PO4 (5 mM, pH 4.8); solvent B: methanol; flow rate: 1.0 mL min−1. Gradient: 0 min, 100% A; 2.5 min, 100% A; 6 min, 80% A; 8 min, 100% A and 13 min, 100% A. The temperature of the autosampler was 4°C and injection volume was 5 μL. Agilent ChemStation™ (ver. C.01.03) (Agilent, Palo Alto, CA) was used for system control and data processing.
Method validation
The High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) method was validated for precision (inter-day and intraday), linearity, sensitivity (limit of detection, LOD, and limit of quantification, LOQ), and recovery. Precision was determined by six measurements of an AsA standard (10 mg/L), on the same day (intraday) and on six consecutive days. Every calibration curve was acquired in triplicate for each concentration (0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, 100, and 200 mg/L). LOD and LOQ were calculated by linear regression of HPLC peak areas.[Citation26] Recovery was determined in two representative apple cultivars: ‘Weirouge’ (red-fleshed) and ‘Golden Delicious’ (white-fleshed). Three levels of AsA (1, 20, and 40 mg/L) were added to each matrix under investigation (peel, pulp, and juice) and the recovery was determined in duplicate for each matrix and cultivar.
Results and discussion
Quantification of ascorbic acid in apple pulp, peel, and juices
AsA was analysed with a rapid HPLC-DAD method. AsA was eluted after 1.9 min using an initial isocratic gradient and then a gradient of methanol and KH2PO4 buffer (5 mM, pH 4.8) with a total analysis time of 13 min, including reconditioning of the column. The linearity of the calibration curve was excellent (r2 > 0.999) in the range from 0.05 to 200 mg/L (). In addition, both instrumental detection limit (ILOD) and instrumental quantification limit (ILOQ), calculated on the standard deviation of the response and the slope,[Citation26] were 0.016 mg/L and 0.049 mg/L, respectively. Instrument precision from six consecutive injections during the same day and on six consecutive days was satisfactory, with intraday and inter-day RSDs of 1.1% and 4.0%, respectively. Extractions were performed in the presence of metaphosphoric acid, a stabilizer for AsA[Citation27] known to prevent oxidation, inhibit ascorbate oxidase and metal catalysis, and finally to precipitate proteins. Indeed, the extraction conditions enabled a very good recovery of at least 89% in all matrices (Table S2) of both red-fleshed (‘Weirouge’) and white-fleshed (‘Golden Delicious’) apples. Moreover, AsA was sufficiently stable in the extraction solution, with 98 ± 1% of the analyte still being present after standing for 24 h at room temperature. The performance of our method is in line with that of previous reports, involving HPLC-DAD[Citation25,Citation28–Citation30] or High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS).[Citation31,Citation32]
Table 1. HPLC-DAD method instrumental validation parameters.
Ascorbic acid content in peel and pulp
The AsA content of 64 accurately identified apple cultivars grown in the same site under identical conditions was analysed. The sample set included 16 old, 12 red-fleshed, 15 scab-resistant, and 21 commercial cultivars, including the 10 most cultivated in South Tyrol in 2013[Citation22]: ‘Golden Delicious’, ‘Gala’, ‘Red Delicious’, ‘Fuji’, ‘Granny Smith’, ‘Braeburn’, ‘Cripps Pink’, ‘Nicoter’, ‘Pinova’, and ‘Jonagold’ (Table S1 and ). In the peel, the AsA content ranged from 2.7 mg per 100 g fresh weight (FW) for ‘Gala’ to 56.0 mg/100g FW for ‘MC 38’. In the pulp, the AsA content was between 0.1 mg/100g FW (‘Harmensz’) and 13.9 mg/100g FW (‘Weißer Winterkalvill’). The edible part of the apples, based on the applied sample preparation, consisted of 16% peel and 84% pulp (considering the core as the non-edible part of apples). All 64 cultivars showed a higher concentration of AsA in the peel than in the pulp. The ratio of AsA content in peel versus pulp, however, was highly variable between cultivars. ‘Goldparmäne’, for instance (Figure S1), contained most AsA in its peel (98.8%), whereas ‘Bay 3675ʹ showed a more balanced distribution (70.3% in the peel). Some cultivars were even found to present a higher content of AsA in their pulp than others in their peel. ‘Osnabrücker Renette’, for instance, while ranking eighth with respect to the AsA content in the pulp, was exceeded by 33 cultivars with respect to the peel content. These results are in good agreement with previous data from Planchon et al., which reported AsA contents between 2.9 and 25.6 mg/100g FW for whole fruits of 30 old Belgian apple cultivars.[Citation16] However, Łata et al. reported significantly higher values for the peel of freshly harvested commercial cultivars.[Citation17] Kevers and co-workers noted that AsA was degraded by 80% during three months of cold storage, offering thus a possible explanation of the lower values in our study.[Citation7] Although AsA degradation during storage is evident, low standard deviations and standardized treatments of our samples suggest that the values are well comparable between cultivars. Importantly, after 60 days of cold storage in normal air, apples are representative for real-market samples.
Figure 1. Ascorbic acid (AsA) content of peel and pulp of 64 apple cultivars. Data are ordered according to AsA pulp content. Error bars indicate standard deviations (n=5; biological replicates).
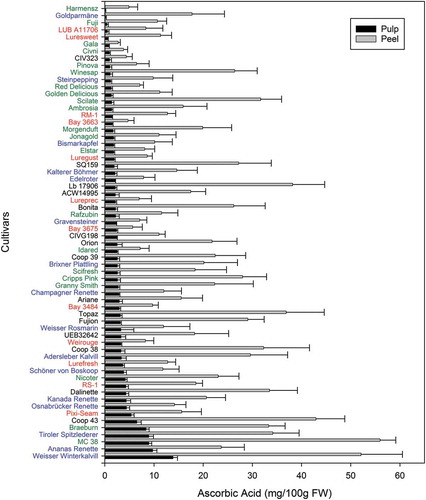
None of the cultivar groups in our study stood out with a uniformly high or low AsA content, but when focusing on the pulp we noticed that old cultivars tended to present high pulp AsA contents (). Indeed, among the 10 cultivars with the highest pulp AsA content, five were old cultivars (‘Weißer Winterkalvill’, ‘Ananas Renette’, ‘Tiroler Spitzlederer’, ‘Osnabrücker Renette’, and ‘Kanada Renette’), and only two were commercial ones (‘Braeburn’ and ‘MC 38ʹ). ‘Braeburn’ is already well known for its high AsA content.[Citation33] The three most cultivated varieties not only in South Tyrol but also in the world (excluding China, with its Fuji plantations) – ‘Golden Delicious’, ‘Gala’, and ‘Red Delicious’ – had AsA contents of less than 1.5 mg/100g FW in the pulp, 10 times lower than the old variety ‘Weißer Winterkalvill’ for instance. In general, we noticed that the commercial cultivars showed lower AsA contents in the pulp (<2.0 mg/100g FW, ), except for ‘Granny Smith’, ‘Cripps Pink’, and ‘Nicoter’ (2.6, 2.7, and 4.2 mg/100g FW, respectively). New cultivars with scab resistance or red flesh did not show a clear pattern: among the red-fleshed cultivars, ‘LUB A11706ʹ contained the least AsA (0.5 mg/100g FW) and ‘Pixi-Seam’ contained the most (5.4 mg/100g FW) in the pulp, whereas the scab-resistant cultivars ranged from 1.0 mg/100g FW (‘CIV323ʹ) to 6.5 mg/100g FW (‘Coop 43ʹ).
Ascorbic acid content in monovarietal cloudy juices
As a water-soluble vitamin, AsA is readily extracted into juices from the mash, but rapidly degraded after pressing.[Citation18] Consequently, the concentration in juices was lower compared with the intact fruit or its parts (Table S1 and ). In fact, among the 64 monovarietal juices in our study, we only found 14 with AsA levels higher than 3.0 mg/L. Concentrations ranged from 0.74 (‘Scilate’) to 7.50 mg/L (‘LUB A11706ʹ), and the scab-resistant ‘UEB32642ʹ was below the LOQ. Varming et al. reported 3.1–10 mg/L in freshly pressed juices of old Danish apple cultivars, but only 5 min standing after pressing was enough to reduce AsA to 0.8–3.6 mg/L. ‘LUB A11706ʹ was the cultivar with the highest AsA content in juice (7.50 mg/L), followed by ‘Bay 3663ʹ (6.68 mg/L) and ‘Bay 3675ʹ (6.50 mg/L). Interestingly, among the 10 varieties showing the highest concentration of AsA, six were red-fleshed (‘LUB A11706ʹ, ‘Bay 3663ʹ, ‘Bay 3675ʹ, ‘Weirouge’, ‘RS-1ʹ, and ‘Bay 3484ʹ). In fact, the red-fleshed cultivars were found over the whole concentration range, but mostly in the upper quartile. Old cultivars, instead, were found around the median to upper quartile, whereas the commercial varieties, especially the most cultivated ones in South Tyrol, were found in the lower quartile of the concentration range. Among these commercial cultivars, the juice of ‘Braeburn’ presented the highest AsA content (2.09 mg/L). ‘Gala’, ‘Red Delicious’, and ‘Golden Delicious’ juices showed concentrations around 1.20 mg/L and ‘Fuji’ was below 1 mg/L (0.80 mg/L). Comparing data from the literature[Citation34–Citation36] and from our own unpublished results, we noted common characteristics in AsA-rich monovarietal cloudy juices: a pH below 3.4 was observed in most juices of red-fleshed (e.g. ‘Weirouge’ had a pH of 3.05, ‘Bay 3675ʹ of 3.18, and ‘LUB A11706ʹ of 3.20) and some old varieties (e.g. ‘Bismarckapfel’ had a pH of 3.14, ‘Osnabrücker Renette’ of 3.17, and ‘Champagner Renette’ of 3.20). A pH of 3.4 is known to preserve AsA, whose rate of degradation is maximal at pH 4 and minimal at pH 2.[Citation28] Most juices of the commercial cultivars, including ‘Gala’ and ‘Red Delicious’, had a pH near 4. More acidic juices, instead, contained higher concentrations of organic acids, in particular malic and citric acid (data not shown), which are supposed to protect AsA from degradation.[Citation37] In addition, the high concentration of anthocyanins in red juices, in particular cyanidin-3-O-galactoside, can protect AsA from oxidation through its antioxidant capacity.[Citation38]
Figure 2. Ascorbic acid (AsA) concentration of 64 monovarietal cloudy apples juices. Error bars indicate standard deviations (n=3; technical replicates).
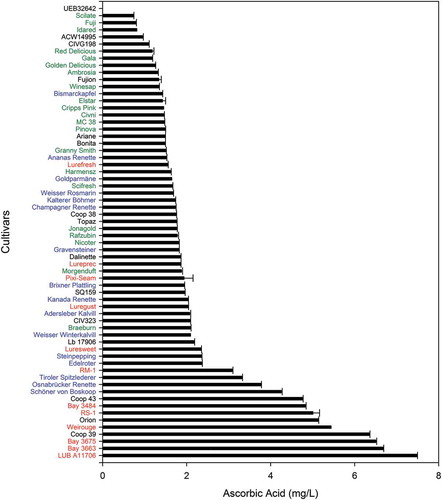
Surprisingly, AsA content in the pulp and peel did not correlate well with the concentration found in the monovarietal juices; rather, we observed that varieties presenting a low content of AsA in the fruit showed high concentrations in the juice and vice versa. For example, the old cultivar ‘Weißer Winterkalvill’ had 13.9 mg/100g FW in the pulp and 52.1 mg/100 g FW in the peel, but a low concentration of AsA in the juice (2.09 mg/L). The old cultivar ‘Weißer Winterkalvill’ and the commercial ‘MC 38ʹ were the two cultivars with the highest AsA content in the fruit, but they showed a low concentration in juice (13.9 mg/L and 2.09 mg/L, respectively). Explanations for the missing correlation between fruit and juice AsA contents might be found in the complex interplay of AsA stability, the content of other polyphenols, pH, pressing yield (water content), or juice production conditions. Red-fleshed fruits, for instance, were found to be relatively poor in AsA in the fruit, but apparently preserved that content during juice production due to their low pH and high polyphenol and anthocyanins contents.[Citation34] ‘LUB A11706ʹ, for instance, had 8.3 and 0.5 mg/100g FW in peel and pulp, respectively, but showed 7.50 mg/L in juice. Scab-resistant cultivars were generally high in AsA content but showed variable concentration in their respective juices.
Conclusion
The present study explored the amount of water-soluble antioxidant AsA in 64 accurately identified apple cultivars, including commercial, old, scab-resistant, and red-fleshed genotypes. We developed an HPLC-DAD method with excellent extraction recovery, linearity, precision, and sensitivity. With a low LOQ, our method enabled the quantification of AsA in all our cultivars. Consistently, the AsA content was higher in peel than in pulp, but did not show a clear pattern according to the four categories. As a general trend, however, the peel and the pulp of old cultivars were found to be rich in AsA, while those of commercial ones were poor. During juice production, the AsA content was reduced dramatically for most cultivars, and in one case (‘UEB32642ʹ) it disappeared completely. With high contents of acids, malic and citric in particular, and the presence of the antioxidant cyanidin-3-O-galactoside, the juice of red-fleshed genotypes, instead, maintained high concentrations of AsA. Our results suggest that old and red-fleshed cultivars offer a considerable potential for the production of healthy juices or further processed food components, provided that AsA can be stabilized for old cultivars. This study complements nutritional databases and previous studies on the AsA content of different apple cultivars with new (red-fleshed and scab resistant) hybrids and old cultivars with local relevance to South Tyrol. Importantly, all cultivars were grown in the same site and accurately identified by genetic fingerprints. To fully appreciate the chemodiversity in apple cultivars, further work is required, providing comprehensive data on antioxidants in different apple cultivars and their chemical transitions during storage, processing, and in the human microbiome. Eventually, it will improve our understanding of the beneficial health effects of fruit in our diet.
LJFP_A_1381705_Supplementary_materials.zip
Download Zip (443.9 KB)Acknowledgement
Laimburg Research Centre is funded by the Autonomous Province of Bolzano – South Tyrol. Financial support from the European Regional Development Fund Program 2007–2013 (Project POMOSANO, Nr. 5-1a-238, CUP: H21J12000060001 and Project APFELFIT, Nr. 1-1a-56, CUP: H21J08000370006) is gratefully acknowledged.
Supplemental data
Supplemental data for this article can be access on the publisher’s website.
References
- Joshipura, K. J.; Hu, F. B.; Manson, J. E.; Stampfer, M. J.; Rimm, E. B.; Speizer, F. E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D.; Willett, W. C. The Effect of Fruit and Vegetable Intake on Risk for Coronary Heart Disease. Ann. Intern. Med. 2001, 134(12), 1106–1114.
- Riboli, E.; Norat, T. Epidemiologic Evidence of the Protective Effect of Fruit and Vegetables on Cancer Risk. Am. J. Clin. Nutr. 2003, 78(3), 559S–569S.
- Genkinger, J. M.; Platz, E. A.; Hoffman, S. C.; Comstock, G. W.; Helzlsouer, K. J. Fruit, Vegetable, and Antioxidant Intake and All-Cause, Cancer, and Cardiovascular Disease Mortality in a Community-Dwelling Population in Washington County, Maryland. Am. J. Epidemiol. 2004, 160(12), 1223–1233.
- Haytowitz, D. B.; Lemar, L. E.; Pehrsson, P. R. USDA’s Nutrient Databank System–A Tool for Handling Data from Diverse Sources. J. Food Composition Anal. 2009, 22(5), 433–441.
- Bodner-Montville, J.; Ahuja, J. K.; Ingwersen, L. A.; Haggerty, E. S.; Enns, C. W.; Perloff, B. P. USDA Food and Nutrient Database for Dietary Studies: Released on the Web. J. Food Composition Anal. 2006, 19, S100–S107.
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56(15), 6520–6530.
- Kevers, C.; Pincemail, J.; Tabart, J.; Defraigne, J. O.; Dommes, J. Influence of Cultivar, Harvest Time, Storage Conditions, and Peeling on the Antioxidant Capacity and Phenolic and Ascorbic Acid Contents of Apples and Pears. J. Agric. Food Chem. 2011, 59(11), 6165–6171.
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J. O.; Dommes, J.; Pincemail, J. Evolution of Antioxidant Capacity during Storage of Selected Fruits and Vegetables. J. Agric. Food Chem. 2007, 55(21), 8596–8603.
- Macheix, J. J.; Fleuriet, A. Fruit Phenolics; CRC Press: Boca Raton, FL, 1990.
- Jiang, H.; Ji, B.; Liang, J.; Zhou, F.; Yang, Z.; Zhang, G. Changes of Contents and Antioxidant Activities of Polyphenols during Fruit Development of Four Apple Cultivars. Eur. Food Res. Technol. 2006, 223(6), 743–748.
- Van Der Sluis, A. A.; Dekker, M.; De Jager, A.; Jongen, W. M. Activity and Concentration of Polyphenolic Antioxidants in Apple: Effect of Cultivar, Harvest Year, and Storage Conditions. J. Agric. Food Chem. 2001, 49(8), 3606–3613.
- McGhie, T. K.; Hunt, M.; Barnett, L. E. Cultivar and Growing Region Determine the Antioxidant Polyphenolic Concentration and Composition of Apples Grown in New Zealand. J. Agric. Food Chem. 2005, 53(8), 3065–3070.
- RöSsle, C.; Wijngaard, H. H.; Gormley, R. T.; Butler, F.; Brunton, N. Effect of Storage on the Content of Polyphenols of Minimally Processed Skin-On Apple Wedges from Ten Cultivars and Two Growing Seasons. J. Agric. Food Chem. 2009, 58(3), 1609–1614.
- Drogoudi, P. D.; Pantelidis, G. Effects of Position on Canopy and Harvest Time on Fruit Physico-Chemical and Antioxidant Properties in Different Apple Cultivars. Sci. Hortic. 2011, 129(4), 752–760.
- Veberic, R.; Schmitzer, V.; Petkovsek, M. M.; Stampar, F. Impact of Shelf Life on Content of Primary and Secondary Metabolites in Apple (Malus Domestica Borkh.). J. Food Sci. 2010, 75(9), S461–S468.
- Planchon, V.; Lateur, M.; Dupont, P.; Lognay, G. Ascorbic Acid Level of Belgian Apple Genetic Resources. Sci. Hortic. 2004, 100(1), 51–61.
- Łata, B.; Przeradzka, M.; Binkowska, M. Great Differences in Antioxidant Properties Exist between 56 Apple Cultivars and Vegetation Seasons. J. Agric. Food Chem. 2005, 53(23), 8970–8978.
- Varming, C.; Petersen, M. A.; Toldam-Andersen, T. B. Ascorbic Acid Contents in Danish Apple Cultivars and Commercial Apple Juices. LWT-Food Sci. Technol. 2013, 54(2), 597–599.
- Donno, D.; Beccaro, G. L.; Mellano, M. G.; Torello Marinoni, D.; Cerutti, A. K.; Canterino, S.; Bounous, G. Application of Sensory, Nutraceutical and Genetic Techniques to Create a Quality Profile of Ancient Apple Cultivars. J Food Qual. 2012, 35(3), 169–181.
- Storti, A.; Dalla Via, J.; Baric, S. Comparative Molecular Genetic Analysis of Apple Genotypes Maintained in Germplasm Collections. Erwerbs-Obstbau. 2012, 54(3), 137–141.
- Dalla Via, J.; Mantinger, H. Agricultural Research in the Field of Fruit Growing in South Tyrol. Erwerbs-Obstbau. 2012, 54, 83–115.
- Relazione agraria e forestale, 2014. Retrieved September 14, 2015 from: http://www.provincia.bz.it/agricoltura/download/ita_relazione_2014.pdf
- Streif, J.;. Optimum Harvest Date for Different Apple Cultivar in the “Bodensee” Area. In Determination and Prediction of Optimum Harvest Date of Apples and Pears. COST 94. The Postharvest Treatment of Fruit and Vegetables; De Jager, A., Johnson, D., Hohn, E., Eds; European Commission: Luxembourg, 1996; pp 15–20.
- Association of Official Analytical Chemists. Vitamin C in Juices and Vitamin Preparations. Official Method 967.21. In AOAC Official Methods of Analysis, 18th; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005; pp 45.1.14.
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC Methods for Determination of Vitamin C. Food Chem. 2015, 175, 100–105.
- International Conference on Harmonization (ICH). Q2b: Validation of Analytical Procedures: Methodology. 1996, Vol. 62, 27463. US FDA Federal Register.
- Fatariah, Z.; Tengku Zulkhairuazha, T. Y.; Wan Rosli, W. I. Ascorbic Acid Quantification in Benincasa Hispida Fruit Extracted Using Different Solvents. Int. Food Res. J. 2015, 22(1), 208–212.
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative Evaluation of UV-HPLC Methods and Reducing Agents to Determine Vitamin C in Fruits. Food Chem. 2007, 105(3), 1151–1158.
- Valente, A.; Albuquerque, T. G.; Sanches-Silva, A.; Costa, H. S. Ascorbic Acid Content in Exotic Fruits: A Contribution to Produce Quality Data for Food Composition Databases. Food Res. Int. 2011, 44(7), 2237–2242.
- Spínola, V.; Mendes, B.; Câmara, J. S.; Castilho, P. C. An Improved and Fast UHPLC–PDA Methodology for Determination of L-Ascorbic and Dehydroascorbic Acids in Fruits and Vegetables. Evaluation of Degradation Rate during Storage. Anal Bioanal Chem. 2012, 403(4), 1049–1058.
- Fenoll, J.; Martínez, A. Simultaneous Determination of Ascorbic and Dehydroascorbic Acids in Vegetables and Fruits by Liquid Chromatography with Tandem-Mass Spectrometry. Food Chem. 2011, 127(1), 340–344.
- Frenich, A. G.; Torres, M. E. Determination of Ascorbic Acid and Carotenoids in Food Commodities by Liquid Chromatography with Mass Spectrometry Detection. J. Agric. Food Chem. 2005, 53(19), 7371–7376.
- Davey, M. W.; Auwerkerken, A.; Keulemans, J. Relationship of Apple Vitamin C and Antioxidant Contents to Harvest Date and Postharvest Pathogen Infection. J. Sci. Food Agric. 2007, 87(5), 802–813.
- Malec, M.; Le Quéré, J. M.; Sotin, H.; Kolodziejczyk, K.; Bauduin, R.; Guyot, S. Polyphenol Profiling of a Red-Fleshed Apple Cultivar and Evaluation of the Color Extractability and Stability in the Juice. J. Agric. Food Chem. 2014, 62(29), 6944–6954.
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic Compounds and Antioxidant Activity in Red-Fleshed Apples. J Funct Foods. 2015, 18, 1086–1094.
- Sadilova, E.; Stintzing, F. C.; Carle, R. Chemical Quality Parameters and Anthocyanin Pattern of Red-Fleshed Weirouge Apples. J. Appl. Bot. Food Qual. 2012, 80(1), 82–87.
- CoSeteng, M. Y.; McLellan, M. R.; Downing, D. L. Influence of Titratable Acidity and pH on Intensity of Sourness of Citric, Malic, Tartaric, Lactic and Acetic Acids Solutions and on the Overall Acceptability of Imitation Apple Juice. Can. Inst. Food Sci. Technol. J. 1989, 22(1), 46–51.
- Rupasinghe, H. P.; Huber, G. M.; Embree, C.; Forsline, P. L. Red-Fleshed Apple as a Source for Functional Beverages. Can. J. Plant Sci. 2010, 90(1), 95–100.
