ABSTRACT
Thirty-nine volatile compounds identified in hydrodistilled essential oil (HD) of Egyptian parsley, representing 97.87% of the total oil, while solid-phase microextraction (HS-SPME) revealed 16 components constituting 96.54% of the volatile material. Monoterpenes were the predominant in both extracts with considerable quantitative differences, while a dramatic decrease in myristicin from 26.21% in HD extract to 4.87% in HS-SPME extract occurred. Myristicin, β-phellandrene and myrecene were the major components among 38 identified components accounting for 92.87% of the total identified volatiles in Madinah hydrodistillate. Parsley essential oil of Madinah exhibited a higher scavenging ability for 2,2ʹ-diphenyl-1-picrylhydrazyl in comparison to Egyptian parsley oil.
Introduction
Antioxidants are important in the food industry, and thus manufacturers have strived to produce high-quality foods with superior texture, color, flavor, and nutritional values with extended shelf-life period. However, many foods are subject to various factors that lead to quality deterioration. One of the most undesirable factors is lipid autoxidation. The need for protecting food against oxidative degradation has prompted the wide usage of food additives.[Citation1] Although there are some synthetic antioxidant compounds available for this purpose, such as butylated hydroxytoluene and butylated hydroxyanisole, these compounds are associated with some adverse side effects. Alternatively, many herbs and spices that are commonly used to flavor dishes are excellent sources of phenolic compounds, which have been reported to show good antioxidant activity.[Citation2]
Parsley (Petroselinum crispum Mill., family Apiaceae) is a typical seasoning used in the Mediterranean and Middle East regions, and is consumed in large quantities as ingredients in various dishes and food preparations. Other reported traditional uses of parsley as a medicinal and food plant commonly used to flavor the cuisines of China, Mexico, South America, India, and Southeast Asia. As a well-known resource for aromatic leaves and roots, parsley also is a raw material for the production of resinoids, oleoresins, and lipids, such as palmitic, oleic, linoleic, and petroselinic acids.[Citation3] Fresh, dried, and dehydrated parsley leaves are used as condiments, garnishes, and flavoring ingredients. Essential oil can be extracted from the leaves and seeds of parsley, which is used as a flavoring agentor fragrance in perfumes, soaps, and creams. The commercial essential oils of parsley are largely derived from the seeds or the herbs harvested at seed formation prior to ripening.[Citation4] Aziz et al.[Citation5] and Sabry et al.[Citation6] studied the Egyptian hydrodistillate oils of parsley, and identified phenylpropanoids and terpenes as the major constituents; however, there are no reports of the antioxidant activities of these oils. Many medicinal plants, including parsley, are cultivated in the Arab peninsula, which is separated from Egypt by the Red Sea, but neither their chemical constituents nor bioactivities have been studied to date.
Different techniques are used for volatile extraction, such as hydrodistillation (HD), solvent extraction, static headspace (HS) extraction, and supercritical fluid extraction (SFE). However, there were negatives, e.g. solvent extraction which results in the recovery of non-volatile compounds,[Citation7] and SFE extraction which can provide reliable and efficient recovery but the expensive cost makes it limited for commercial applications. Moreover, suspicious results can be obtained by hydro- or steam-distillation,[Citation8] whereas monoterpenes may be susceptible to chemical changes during thermal treatments of HD or evaporation during solvent extraction.[Citation9] HS analysis offers a potentially rapid method to extract volatiles and requires very little plant material, but is associated with a risk of cross-contamination and has low sensitivity, as complete recovery is only possible for highly volatile materials.[Citation10] Solid-phase microextraction (SPME) is an alternative technique to the traditional HS extraction, which has been successfully applied qualitatively and quantitatively for the analysis of various food samples, and flavors and aromas of many medicinal plants, but has not been applied to parsley cultivated in Egypt or Arab peninsula. Therefore, the aim of the present study was to compare the volatile constituents of parsley cultivated in Madinah Monawara, Kingdom of Saudi Arabia (KSA), and Egypt, using the HD and HS-SPME extraction techniques, as well as to determine the antioxidant activity of the essential oils extracted by HD.
Material and methods
Plants and chemicals
The fresh green parts of parsley (P. crispum) were purchased from a central market for fruits and vegetables in Madinah, Saudi Arabia, while the Egyptian samples were purchased from a vegetable market in Gizain August 2014. The botanical identification was made by a taxonomist at the Department of Botany, Faculty of Science, Zagazig University. Diethyl ether and methanol were purchased from Fisher Chemicals. The mixture of n-alkanes C6–C26, authentic compounds, sodium bicarbonates, linoleic acid (≥99%), Tween 40, β-carotene (≥97%), Folin – Ciocalteu reagent for total phenolics, 2,2ʹ-diphenyl-1-picrylhydrazyl (DPPH), and gallic acid were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
Essential oil extraction by hydrodistillation (HD)
Fresh leaves of both types of parsley (100 g) in three replicates that were cut into small pieces (l × 1 cm) were subjected to HD for 3 h, using a Clevenger-type apparatus, according to the method of El-massry et al.[Citation11] The extracted essential oils were dried using anhydrous sodium sulfate and stored in airtight glass vials covered with aluminum foil at –20°C until analysis.
Headspace solid-phase microextraction (HS-SPME)
The dried leaves of parsley were cut into 1–2-cm-long pieces before being subjected to SPME. Two grams of leaves were introduced into a 20-mL SPME vial. The SPME device coated (fused-silica fiber) with a 100-µm layer of polydimethylsiloxane (Supelco, Bellefonte, PA, USA) was used for extraction of the plant volatiles, and the vial was sealed with a silicone septum. The samples were exposed to the SPME vial at 60°C for 30 min and immediately introduced in the gas chromatography injector. The above method was reported and optimized by many authors.[Citation12–Citation15]
Gas chromatography–mass spectrometry (GC–MS)
The components of the essential oil obtained by HD and the volatiles trapped by SPME fibre were analyzed using a GC–MS apparatus. Separation was performed on a Trace GC Ultra Chromatography system (Thermo Scientific, USA) equipped with an ISQ-mass spectrometer (Thermo Scientific, USA) with a 60 m × 0.25 mm × 0.25-µm-thick TG-5MS capillary column (Thermo Scientific, USA). The column separation was programmed from 50°C with a holding time of 3 min, and then the temperature was increased at a rate of 4°C per min to 140°C with a holding time of 5 min, and was then increased at a rate of 6°C per minute to 260°C for a 5-minisothermal hold. The injector temperature was 180°C, the ion source temperature was 200°C, and the transition line temperature was 250°C. The carrier gas was helium with constant flow rate 1.0 mL min−1. The mass spectrometer had a scan range from m/z 40 to m/z 450, and the ionization energy was set at 70 eV. The identification of compounds was based on matching with the MS computer library (NIST library version 2005) and comparison with those of authentic compounds and published data.[Citation16] The relative percentage of the oil constituents was calculated from the GC peak areas. Kovat’s index was calculated for each compound, using the retention times of a homologous series of C6–C26 n-alkanes.[Citation16]
Antioxidant activity measurements
DPPH radical scavenging assay
The potential antioxidant activity of P. crispum oils was assessed according to the methods reported by Hatano et al.[Citation17] in comparison to a synthetic antioxidant used in food industry, tert-butylhydroquinone (TBHQ). The absorbance was measured at 517 nm using a spectrophotometer (Evolution 300 Thermo UV-VIS); all tests were run in three replicates and the results were averaged.
β-carotene bleaching assay
The antioxidant activity of the aqueous solution was determined using a β-carotene/linoleic acid system, as described by Taga et al.[Citation18] in comparison to TBHQ. The absorbance was measured at 470 nm over a 60-min period.
Total phenolic content
The total phenolic content of the essential oils obtained from P. crispum was determined using the Folin–Ciocalteu reagent according to a modified method of Singleton et al.,[Citation19] using gallic acid as the standard. The reaction mixtures were incubated in a thermostat at 45°C for 45 min before the absorbance at 765 nm was measured.
Statistical analysis
Statistical analyses were performed using SPSS software version 16. The data were expressed as mean ± SD.
Results and discussion
HD of P. crispum provided a yellow liquid ranging from 0.28 ± 0.08% of the Egyptian essential oil to 0.21 ± 0.05% of the Madinah essential oil. Thirty-nine compounds were characterized in the Egyptian essential oil, representing 97.87% of the total oil (, ). Myristicin, which is a phenylpropanoid, was found to be the most abundant volatile oil component (26.41%), followed by monoterpene hydrocarbons, e.g., β-phellanderene (11.61%), α-phellandrene (10.54%), 1,3,8-p-menthatriene (9.41%), p-cymene (8.63%), myrcene (6.12%), α-pinene(1.79%), and p-cymene (1.09%). These results agreed with the findings of Aziz et al.,[Citation5] who found thatmyrsticin, β-phellanderene, and α-phellandrene were the major components of parsley essential oil, but not 1,3,8-p-menthatriene, which is likely due to differences between winter and summer parsley.[Citation20] In addition, Macleod et al.[Citation21] reported 45 volatile compounds from parsley leaves, and the most abundant components were identified as myristicin (20.6%), apiole (18.3%), α-phellandrene (12.4%), and p-1,3,8-menthatriene (9.2%).
Table 1. Volatile constituents identified from the essential oils of parsley cultivated in Egypt and Madinah using GC–MS with HD extraction and SPME.
Figure 1. Volatile extracts chromatograms for Egyptian parsley isolated by (a) hydrodistillation and (b) SPME.
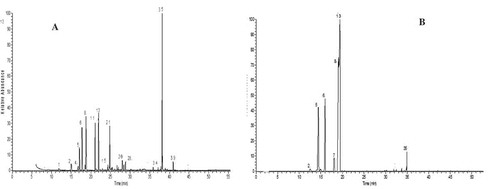
Sabry et al.[Citation6] studied different cultivars of parsley essential oils, including soft, Italian clause, rough clause, curly, and leaf parsley. Their results for essential oils of plain leaf parsley were most similar to our findings but with higher percentages of bisabolene (14.19%) and carotol (5.7%) owing to cultivation in winter. Again, myristicine was identified as the major component in parsley essential oils by Hassanpourghdam[Citation22] in the northwest of Iran and by Zhanget al.[Citation23] in Jiangsu Province, China. However, both studies showed that the major monoterpenes constituents were α-pinene, followed by β-pinene. Such discrepancies with the results of the present study are likely due to the environmental differences among the regions.[Citation24]
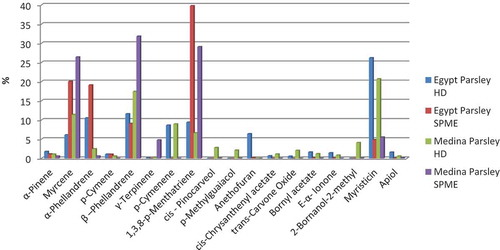
According to our literature search, there has been no report on the chemical constituents of parsley essential oils cultivated in the KSA. Saudi Arabia constitutes the major area of the Arab peninsula and is separated from Egypt by the Red Sea. Cultivation is one of the major activities in Madinah, including many famous crops such as parsley. As shown in and , the HD essential oil of Madinah parsley is characterized by the dominant presence of myristicine (20.7%), β-phellandrene (17.45%), myrcene (11.42%), p-cymene (8.95%), and 1,3,8-p-menthtriene (6.64%). In comparison to that in the essential oil of Egyptian parsley, there were considerable increases in the concentrations of myrcene, β-phellandrene, and trans-carvone oxide in the Madinah essential oil, whereas concentrations of α-phellandrene, 1,3,8-p-menthatriene, and myrsticine showed significant decreases, but were still dominant among the identified volatiles (). In addition, some compounds could be identified only in the Madinah parsley essential oil, such as cis-pinocarveol (2.8%), p-methyl guaiacol (2.14%), 2-bornanol, 2-methyl (4.09%), and linalool (0.76%). A recent study on the most potent odorants of parsley showed that 17 compounds contribute to the unique aroma of the cultivar.[Citation25] The authors reported that p-1,3,8-menthatriene, myrcene, 2-sec-butyl-3-methoxypyrazine, myristicin, linalool, 6-decenal, and 3-hexenal were the characteristic impact flavor compounds of parsley.
Figure 2. Volatile extracts chromatograms for Madinah, Saudi Arabia parsley isolated by (a) hydrodistillation and (b) SPME.
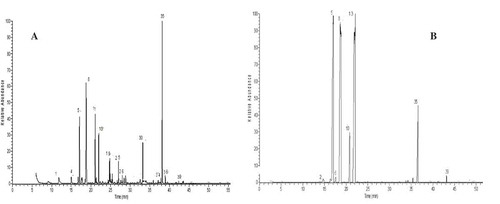
Figure 3. Effect of extraction technique on the area percentage of the main volatiles of Parsley cultivated in Egypt and Madinah.
Growing location, agronomical practices, as well as environmental conditions affect the composition of important sensory compounds and may be responsible for the disappearance of some components.[Citation26–Citation28] This suggests that the differences in environmental, climatic, and geographic conditions between Giza, Egypt and Madinah () likely contribute to the observed variations in the chemical constituents of essential oils from the parsley samples obtained from the two regions in this study.[Citation29]
Table 2. Climatic and geographical characterisitics of Giza, Egypt and Madinah Monawara, KSA.
Sixteen and 23 volatile constituents extracted by HS-SPME were identified in the Egyptian and Madinah parsley, respectively, by GC–MS (, , ). Dramatic quantitative changes could be noted between the SPME and hydrodistilled essential oils for both regions, where a higher content of monoterpenes could be identified among parsley volatiles of both Madinah and Egypt adsorbed by SPME (). 1,3,8-p-Menthtriene (39.76%), myrcene (20.07%), and α-phellandrene (19.11%) were the predominant volatile compounds of Egyptian parsley, whereas β-phellandrene (31.83%), 1,3,8-p-menthatriene (29.1%), and myrcene (26.42%) were identified as the major adsorbed volatiles from Madinah parsley. Sesquiterpenes, such as bornyl acetate and α-ionone could be identified in the SPME compounds, but were found in lower amounts in comparison to the hydrodistillates at 0.15% and 0.07%, respectively, in Egyptian parsley, and at 0.03% and not detected, respectively, in the Madinah parsley ( and ). The myrsticin concentrations dropped to 4.87% of the Egyptian parsley and 5.54% of the Madinah parsley adsorbed volatiles.
In comparison to the HD technique, HS-SPME analysis is a more simple, rapid, and economic procedure, which is currently widely used for studying the volatile chemical constituents of aromatic plants without essential oils. Such quantitative variation in the parsley volatile constituents extracted by HD and HS-SPME is likely due to the key differences in these extraction techniques. During HD, steam and oil vapors were condensed to form two main layers: hydrosol and essential oil. However, the use of this technique for the subsequent determination of the oil chemical composition is controversial, because of the possible transformation of aroma-active compounds by variations in heat, steam, and pH.[Citation30] Losses and degradation of some volatile compounds due to long extraction times and the degradation of unsaturated or ester compounds through thermal or hydrolytic effects are the principal disadvantages of this extraction method.[Citation31,Citation32] Zheljazkov et al.[Citation33] reported that HD extraction time negatively affected the low boiling point of the essential oil constituents, e.g., α-pinene, myrcene, and phellandrenes, whereas in HS-SPME, the affinity of compounds toward fiber is responsible for volatiles monitoring without any reported negative artifacts.[Citation34]
The antioxidant activity of the essential oils of parsley cultivated in Egypt and Madinah was tested by evaluating the DPPH radical scavenging activity and by a β-carotene–linoleate bleaching assay. The recorded activities are presented in , where lower half-maximal inhibitory activity (IC50) values indicate higher activity. Both essential oils tested exhibited clear antioxidant activity, in agreement with the results of Zhang et al.[Citation23] However, the Madinah essential oil exhibited higher scavenging ability for DPPH (IC50 = 1.48 mg mL−1) in comparison to the Egyptian oil (IC50 = 3.8 mg mL−1). In accordance with the above findings, the Madinah essential oil showed a higher inhibitory effect toward the oxidation of linoleic acid and the subsequent bleaching of β-carotene (47.3%) in comparison to the Egyptian oil (30%). According to Zhang et al.,[Citation23] antioxidant activity in parsley essential oil is due to apiol, which is described as the major contributor to the antioxidant activity of this oil, followed by myristicin, which exhibited moderate activity.
Table 3. Antioxidant activity of essential oils for parsley cultivated in Egypt and Madinah in comparison to the synthetic antioxidant TBHQ.
The presence of some exclusive phenolic compounds in Madinah parsley essential oil, e.g. carveol, methyl guaiacol, and monoterpenes as linalool, may be responsible for the higher antioxidant activity observed. Phenolic compounds are commonly used nowadays as natural antioxidants owing to their good bioactivity.[Citation35] Pripdeevech et al.[Citation36] reported the antioxidant activity of pure phenolic compounds, e.g. linalool, demonstrating their higher performance for DPPH scavenging in comparison to many extracted essential oils. However, there is no report of the bioactivity of parsley essential oil in either Egypt or Madinah. Al-Juhaimi and Ghafoor[Citation37] studied the antioxidant activity as well as the total phenolic content in leaf and stem extracts of parsley cultivated in Riyadh, KSA (≥1.02 mg GAE100 mL−1), and the phenolic content was lower than that of both oils in the present study (13.1 mg GAEmL−1 for Madinah oil and 3.2 mg GAE mL−1for Egyptian oil; ). Again, such higher phenolic content in the Madinah essential oil is likely responsible for the higher scavenging effect as well as the lower IC50 value with respect to those of the Egyptian oil.
Conclusions
In the present study, a comparative analysis of the volatile compounds of parsley (P. crispum) cultivated in Madinah Monawara and Egypt by HD and HS-SPME followed by GC–MS was conducted. Quantitative and qualitative differences were observed between both hydrodistillates due to geographic and environmental variations. In addition, the different extraction techniques applied resulted in respectable differences, with variations in the percentages and nature of the compounds adsorbed on the SPME fiber in comparison to the hydrodistilled essential oils. In addition to the presence of more volatiles with antioxidant activity, the Madinah essential oil showed a higher phenolic content in comparison to the Egyptian oil, which led to its higher scavenging ability as well as a greater inhibiting effect toward the oxidation of linoleic acid.
Additional information
Funding
References
- Pandian, D.; Nagarajan, N. Comparison of Chemical Composition and Antioxidant Potential of Hydrodistilled Oil and Supercritical Fluid CO2extract of Valerianawallichi DC. J. Nat. Prod. Res. 2015, 1, 25–30.
- Zheng, W.; Wang, S. Antioxidant Activity and Phenolic Composition in Selected Herbs. J. Agric. Food Chem. 2000, 149, 5165–5170.
- Snoussi, M.; Ameni, D.; Emira, N.; Guido, F.; Adele, P. Chemical Composition and Antibiofilm Activity of Petroselinum crispum and Ocimumbasilicum Essential Oils against Vibrio Spp. Strains. Microb. Pathog. 2016, 90, 13–21.
- Petropoulos, S.; Daferera, D.; Akoumianakis, C.; Passam, H.; Polissiou, M. The Effect of Sowing Date and Growth Stage on the Essential Oil Composition of Three Types of Parsley. Petroselinum crispum. J. Sci. Food Agric. 2004, 84, 1606–1610.
- Aziz, E.; Sabry, R.; Ahmed, S. Plant Growth and Essential Oil Production of Sage (Salvia Officinalis L.) And Curly-Leafed Parsley (Petroselinum crispum ssp. Crispum L.) Cultivated under Salt Stress Conditions. World Appl. Sci. J. 2013, 28, 785–796.
- Sabry, R.; Kandil, M.; Ahmed, S. Comparative Study of Some Parsley Cultivars Grown in Egypt for Some Potent Compound. J. Appl. Sci. Res. 2013, 9, 6419–6424.
- Burbott, A.; Loomis, W. D. Effects of Light and Temperature on the Monoterpenes of Peppermint. Plant Physiol. 1967, 42, 20–28.
- Takeoka, G.; Ebeler, S.; Jennings, W. Capillary Gas Chromatographic Analysis of Volatile Flavor Compounds and Their Application to Analysis of Volatiles in Foods. In: American Chemical Society Symposium Ser. 289, Amer. Chem. Soc.,Washington, D.C. Trends Anal. Chem. 1985, 19, 96–108.
- Chialva, F.; Gabri, G.; Liddle, P.; Ulian, F. Qualitative Evaluation of Aromatic Herbs by Direct Head Space (GC)2 Analysis. Applications of the Method and Comparison with the Traditional Analysis of Essential Oils. In Aromatic Plants–Basic and Applied Aspect; Margaris, N., Koedam, A., Vokou, D., Eds.; MartinusNijhoff Publishers: Netherlands, 1982; pp 183–195.
- Mazza, G.; Cottrell, T. Volatile Components of Roots, Stems, Leaves and Flowers Ofchinacea Species. J. Agric. Food Chem. 1999, 47, 3081–3085.
- El-Massry, K. F.; Farouk, A.; Abou-Zeid, M. Free Radical Scavenging Activity and Lipoxygenase Inhibition of Rosmarinus officinalis L.Volatile Oil. J. Essential Oil – Bearing Plants. 2008, 11, 536–543.
- Cătunescu, G. M.; Socaci, S. A.; Rotar, I.; Vidican, R.; Bunghez, F.; Tofană, M.; Pleșa, A.; Muntean, M. Volatile Profile of Minimally Processed Herbs during Cold Storage. Rom. Biotechnol. Lett. 2016, 21, 11923–11931.
- Yeon, B.; Sowndhararajan, K.; Jung, J.; Jhoo, J.; Kim, S. Comparison of Volatile Composition of Supercritical Carbon Dioxide Extract from Rhizomes of Korean Medicinal Plant ‘Chun-Kung’ (Cnidiumofficinale Makino) by Direct-And SPME-GC/MS. Int. J. Pharm. Pharm. Sci. 2014, 6, 355–358.
- Moradi, M.; Kaykhaii, M.; Ghiasvand, A. R.; Shadabi, S. Comparison of Solid Headspace Microextraction, Headspace Single Drop Microextraction and Hydrodistillation for Chemical Screening of Volatiles in Myrtus Communis L. Phytochem. Anal. 2012, 23, 379–386.
- Gazim, Z.; Rezende, C.; Fraga, S.; Filho, B.; Celso-Vataru, N.; Cortez, D. Analysis of the Essential Oils from Calendula Officinalisgrowing in Brazil Using Three Different Extraction Procedures. Braz. J. Pharmacol. Sci. 2008, 44, 391–395.
- Adams, R.;. Identification of Essential Oil Components Bygas Chromatography/Mass Spectroscopy; Carol Stream, IL: Allured Publishing Corp, 2007.
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root Their Relative Astringency and Radical Scavenging Effects. Chem. Pharm. Bull. 1988, 36, 2090–2097.
- Taga, M.; Miller, E.; Pratt, D. Chia Seeds as Source of Natural Lipid Antioxidants. J. Am. Oil Chemists’ Soc. 1984, 61, 928–931.
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178.
- Vokk, R.; Lougas, T.; Kravets, M. Dill (Anethumgraveolens L.) And Parsley (Petroselinum crispum (MilL.) Fuss) from Estonia: Seasonal Differences in Essential Oil Composition. Agron. Res. 2011, 9, 515–520.
- MacLeod, A. J.; Snyder, C. H.; Subramanian, G. Volatile Aroma Constituents of Parsley Leaves. Photochemistry. 1985, 24, 2623–2627.
- Hassanpouraghdam, M.;. GC/EI-MS Investigation of Cultivated Petroselinumhortense Hoffm. Fruit Volatile Oil from Northwest Iran. Chemija. 2010, 21, 123–126.
- Zhang, H.; Chen, F.; Wang, X.; Yao, H. Evaluation of Antioxidant Activity of Parsley (Petroselinum crispum) Essential Oil and Identification of Its Antioxidants Constituents. Food Res. Int. 2006, 39, 833–839.
- El-Massry, K.; El-Ghorab, A.; Farouk, A. Antioxidant Activity and Volatile Components of Egyptian Artemisia Judica L. Food Chem. 2000, 279, 331–336.
- Masanetz, C.; Grosch, W. Key Odorants of Parsley Leaves (Petroselinum crispum [Mill.] Nym.Ssp. Crispum) by Odour-Activity Values. Flavour Fragrance J. 1998, 13, 115–124.
- Vina, A.; Murillo, E. Essential Oil Composition from Twelve Varieties of Basil (Ocimumspp.) Grown in Columbia. J. Braz. Chem. Soc. 2003, 14, 744–749.
- Zheljazkov, V.; Cantrell, C.; Evans, W.; Ebelhar, M.; Coker, C. Yield and Composition of OcimumbasilicumL. And Ocimum Sanctum L. Grown at Four Locations. Hortic. Sci. 2008, 43, 737–741.
- Jamshidi, R.; Afzali, Z.; Afzali, D. Chemical Composition of Hydrodistillation Essential Oil of Rosemary in Different Origins in Iran and Comparison with Other Countries. American-Eurasian J. Agric. Environ. Sci. 2009, 5, 78–81.
- Peel, M.; Finlayson, B.; McMahon, T. Updated World Map of the Köppen–Geiger Climate Classification. Hydrol. Earth Syst. Sci. J. 2007, 11, 1633–1644.
- Jiménez-Carmona, M.; Ubera, J.; Luque, D. Comparison of Continuous Subcritical Water Extraction and Hydro Distillation of Marjoram Essential Oil. J. Chromatogr. 1999, 855, 625–632.
- Khajeh, M.; Yamini, Y.; Sefidkon, F.; Bahramifar, N. Comparison of Essential Oil Composition of Carumcopticumobtained by Supercritical Carbon Dioxide Extraction and Hydrodistillation Methods. Food Chem. 2004, 86, 587–591.
- Tuan, D.; Ilangantileke, S. Liquid CO2 Extraction of Essential Oil from Star Anise Fruits (Illiciumverum H.). J. Food Eng. 1997, 31, 47–57.
- Zheljazkov, V.; Astatkie, T.; Schlegel, V. Hydrodistillation Extraction Time Effect on Essential Oil Yield, Composition, and Bioactivity of Coriander Oil. J. Oleo Sci. 2014, 63, 857–865.
- Benyelles, B.; Allali, H.; Dib, M.; Djabou, N.; Tabti, B.; Costa, J. Essential Oil from Rhaponticumacaule L. Roots: Comparative Study Using HS-SPME/GC/GC-MS and Hydrodistillation Techniques. J. Saudi Chem. Soc. 2014, 18, 972–976.
- Moller, J.; Madsen, H.; Altonen, T.; Skibsted, L. Dittany (Origanumdictamnus) as a Source of Water-Extractable Antioxidants. Food Chem. 1999, 64, 215–219.
- Pripdeevech, P.; Chumpolsri, W.; Suttiarrorn, P.; Wongpornchai, S. The Chemical Composition and Antioxidant Activities of Basil from Thailand Using Retention Indices and Comprehensive Two-Dimensional Gas Chromatography. J. Serbian Chem. Soc. 2010, 75, 1503–1513.
- Al-Juhaimi, F.; Ghafoor, K. Total Phenols and Antioxidant Activities of Leaf and Stem Extracts from Coriander, Mint and Parsley Grown in Saudi Arabia. Pak. J. Bot. 2011, 43, 2235–2237.
