ABSTRACT
The enzyme, β-glucosidase (β-Glu; EC 3.2.1.21), is a commercially important enzyme. First, β-Glu enzyme and specific activity were screened in total of 54 strains. In these strains, β-Glu-specific enzyme activity was assessed varying from 0.250 to 3.000 U/mg. Next, β-Glu enzyme belonging to the strains (Lactobacillus rhamnosus EA1 and Lactobacillus casei SC1) that exhibited high β-Glu-specific enzyme activity optimization were done. In these strains, optimum pH was 7.5, optimum temperature was 30°C, and optimum buffer was potassium phosphate. Then, the strains that displayed high β-Glu-specific enzyme activity were decided to hydrolyze the isoflavone glucosides using high-pressure liquid chromatography. Finally, following the partial purification of β-Glu enzyme, its molecular weight was detected to be approximately 78 K. Ultimately, in the present study, β-Glu enzyme acquired from L. casei SC1 strain became prominent because its activity was higher. It did not lose its activity under different environmental conditions and demonstrated high hydrolyzing the isoflavone.
Introduction
Lactobacilli are Gram-positive, catalase-negative bacilli belonging to the lactic-acid bacteria (LAB) group. The probiotic potential of lactobacilli has been extensively investigated. The term “probiotic” refers to “live microbial supplements that colonize the gut and possibly exert health benefit to the host.”[Citation1] Moreover, the lactobacilli, probiotic bacteria are Bifidobacterium[Citation2] and dairy propionibacteria.[Citation3]
Phytochemicals, a type of isoflavones, are an essential group of phytoestrogens. Isoflavones are diphenolic secondary metabolites of leguminous plants, particularly in soybeans.[Citation4,Citation5] They are structural homology to human estrogen hormone and have been informed in health benefits, including cancer, menopausal symptoms, cardiovascular diseases, and osteoporosis. [Citation6] There are two groups of isoflavones: aglycones and glycosides forms.[Citation7,Citation8] The biological effects of isoflavone are not because of the glycosides form (genistin, daidzin) but in place of this are predominantly from their aglycones, such as daidzein and genistein.[Citation9]
β-Glucosidase (β-Glu), β-glycoside bond breaking, produced by the lactobacilli, bifidobacteria, and dairy propionibacteria, is cell-associated. The enzyme can be used to convert glucosides isoflavones to bioactive aglycones.[Citation10,Citation11] The objective of this work was to identify β-Glu enzyme activities of Lactobacillus, Bifidobacterium, and Propionibacterium by using p-nitro0phenyl β-D-glucopyranoside (pNPG) as substrate. Optimization studies were applied to find out whether there is an impact of the pH, temperature, and buffer. Enzyme stability in gastric and intestinal juices was established. Whether the isoflavone can hydrolyze glycoside (daidzein, genistein) form to aglycone forms in strains with high enzyme activity by using high-performance liquid chromatography (HPLC) was found out.
Materials and methods
Assay of β-Glu activity
β-Glu activity was assigned at 24 h of incubation in de Man, Rogosa and Sharpe (MRS). The β-Glu activity was detected by measuring the rate of hydrolysis of p-nitrophenyl β-d-glucopyranoside (pNPG). The amount of p-nitrophenol released was measured using a spectrophotometer (Hithachi) at 420 nm. One unit of the enzyme activity was designated as the amount of β-Glu that released 1 nmol of p-nitrophenol from the substrate pNPG per milliliter per min under assay conditions. The specific activity (U/mg) is expressed as units of activity per milligram of protein. The protein concentration was designated with Bradford Reagent (Amresco). The enzymatic activity was decided in the supernatant of the cultures for extracellular β-Glu activity and in the cell-free extract for intracellular β-Glu activity.[Citation12]
β-Glu enzyme optimization
Lactobacillus rhamnosus EA1 and Lactobacillus casei SC1 strains were selected from the 54 strains according to their highest specific activity. Optimum pH was found through experimenting with various pH values (pH 4, 5, 6, and 7) to determine the maximum specific activity. To find out the optimum temperature, β-Glu activity assays were designated at pH 7.5 for 30 min at the temperatures of 37°C, 40°C, 50°C, and 60°C. To identify the influence of possible buffer, the activities of the β-Glu from EA1 and SC1 were assayed in the presence of 0.5 M potassium phosphate, 0.5 M sodium phosphate, and 0.5 M sodium citrate at pH 7.5.[Citation13]
Simulating gastric and intestinal digestion
In vitro conditions for simulating gastric and intestinal digestion were performed. The reaction mixture was incubated with 3 g/L pepsin (Sigma, 1:10000 ICN) (pH 2, 3, 4, and 7) for 30 min at 30°C defined as the gastric phase. The reaction mixture was incubated with 1 g/L pancreatin (Sigma, P-1500 USP), 0.30% bile salt (pH 5.5, 6.5, 7.5, and 8.0) for 30 min at 30°C, described as the intestinal phase. The specific activity was designated.[Citation13]
Hydrolysis of isoflavone glucosides
L. casei SC1 and L. rhamnosus EA1 strains that designated the highest β-Glu specific enzyme activity were inoculated at 2% (v/v) in MRS medium and incubated for 24 h at 37°C. Culture broth, 0.2 mL, was added to 1.8 mL 0.5 M potassium phosphate buffer, pH 7.5 containing 100 μg genistin or daidzin. The mixture was held at 45°C for 30 min and then boiled for 10 min. The composition of isoflavones was analyzed by HPLC.[Citation10]
Partial purification of β-Glu
The β-Glu enzyme owned SC1 strain that hydrolyzed a high rate at the isoflavone glycosides was used in the partial purification process. For partial purification purposes, all procedures were made at 4°C. The ammonium sulfate precipitation, dialyzed, and concentration procedures between the purification steps were done.[Citation13,Citation14] The enzyme was dialyzed using dialysis bag (Sigma, 0.4 in.) against 0.5 M potassium phosphate (pH 7.5) buffer.[Citation15] The dialysis buffer was exchanged and dialysis proceeded two nights at 4°C. The sample was concentrated with Amicon filtration system. All fractions were analyzed for β-Glu activity and protein content.
Electrophoresis
Protein separation was done by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresis was carried out in 4% stacking and 10% separating gels as defined by Laemmli.[Citation16] The gels were stained with 0.1% Coomassie Blue R-250 (Sigma-Aldrich, Germany) in a fixative (40% methanol, 10% acetic acid), and the excess colorant was removed using fixative without Coomassie Blue. The molecular weight marker for the SDS-PAGE was the Pierce Blue Prestained Protein Molecular Weight Marker Mix (Thermo Scientific).
Data analysis
The assessment tests were run in triplicate and averaged. The means, standard errors, and standard deviations were calculated from replicates within the experiments, and analyses were made using Microsoft Excel XP 2010. To find the difference in β-Glu-specific activity during different temperature, pH and buffer means were analyzed using one-way analysis of variance (ANOVA) and 95% confidence levels. ANOVA data with a p < 0.05 was classified as statistically significant.
Results and discussion
The isoflavones genistin and daidzin are the inactive forms of isoflavones. However, aglycones (genistein and daidzein), which are sugar-free component of a glycoside molecule, are the biologically active forms. Only a small amount of the total isoflavones yet exists in the aglycone forms in nature. β-Glu has been asserted to be the enzyme that is able to hydrolyze isoflavone glycosides to aglycones.[Citation17] Although there is one study about hydrolyze of isoflavones glycosides to aglycones by lactobacilli,[Citation10] our study is one of the first studies about the comparison, optimization, and partial purification of the β-Glu enzyme from different strains that have the ability of hydrolyze and the high enzyme activity.
β-Glu activity
In the present study, 54 Lactobacillus, Bifidobacterium, and Propionibacterium cultures were screened for β-Glu activity. These cultures exhibiting enzymatic activity had a yellow color during the reaction due to the release of p-nitrophenol from the substrate pNPG. The activity was defined in the cell-free extract and was not detected in culture supernatant. The cultures used in this study show different levels of β-Glu-specific activities, which ranged from 0.250 to 3.000 U/mg. L. casei SC1 and L. rhamnosus EA1 explained the highest specific activity. Generally, Propionibacterium strains had lower enzyme and specific activity than the other Lactobacillus and Bifidobacterium strains (data not shown). It is known that this enzymatic activity depends on the strain, growth medium, and culture conditions; moreover, the enzyme plays a key role in the bioconversion of isoflavones. These results are similar to those indicated previously for lactobacilli, including Marazza[Citation18] and Choi.[Citation10] Marazza[Citation18] informed that the ability to produce β-Glu was assessed in 63 strains of Lactobacillus. The maximum activity was acquired with L. rhamnosus CRL981 (22.93 UE/mg) at pH 6.4 and 42°C. Choi[Citation10] observed higher β-Glu activity of Lactobacillus delbrueckii KCTC 1047 (0.3 Unit) compared with Lactobacillus bulgaricus KCTC 3188, L. casei KCTC 3109, L. delbrueckii KCTC 1058, and L. lactis KCTC 2181. Generally, several studies explained that lactobacilli and bifidobacteria are able to produce the enzyme β-Glu,[Citation5,Citation19] but most of these were carried out in soymilk and not in MRS medium. Therefore, the behavior of the microorganisms as well as the production of the enzyme could depend, to some extent, on the culture medium employed. Tsangalis[Citation20] declared that Bifidobacterium longum-b presented a highest β-Glu activity when it was grown in MRS-glu (4.625 U/mg).
β-Glu enzyme optimization
The activity of enzymes is influenced by diverse environmental factors (temperature, pH, buffer) that can strongly affect the specific tridimensional structure of the protein.[Citation21] For β-Glu enzyme belonging to L. casei SC1 and L. rhamnosus EA1 strains that showed high β-Glu-specific activity, the effects of pH, temperature, and buffer were assessed. The influence of the pH on specific activities at SC1 and EA1 strains is display in . At pH 7.5, which were found to be the optimum pH level for SC1 and EA1 strains, the enzyme had specific activities of 2.750 and 2.100 U/mg, respectively. Marazza[Citation18] found that the optimum pH level for L. rhamnosus CRL981 strains was at 6.4. Our results indicate similar pH levels for β-Glu enzymes to those of other studies.
Table 1. The effects of pH, temperature, and buffer on the β-glycosidase enzyme and specific activity in L. casei SC1 strain and L. rhamnosus EA1 strain.
Temperature influence on specific enzyme activity was established between 30°C and 60°C (). The increase in β-Glu activity between 30°C and 60°C, between pH 4.0 and pH 7.5 and different buffer was significant (p < 0.05) for L. casei SC1 and L. rhamnosus EA1 strains. Marazza[Citation18] indicated that for β-Glu enzyme belonging to L. casei ATCC 393, optimum temperature was 35°C. In L. casei SC1 and L. rhamnosus EA1 strains, β-Glu enzyme functions are the highest at potassium phosphate buffer. It has been informed that for β-Glu enzyme optimum buffer was sodium phosphate buffer (supported our findings)[Citation22] and citrate buffer.[Citation23] The present study demonstrated significantly higher specific activity of β-Glu enzyme when the medium was acidic and temperature was 30°C. These data show that this enzyme may use industrial processes.
Simulating gastric and intestinal digestion
This is one of the first studies on β-Glu activity in artificial gastric and intestinal fluids from lactobacilli. The bacteria had the ability to survive in simulating stomach and intestine conditions by exhibiting a high β-Glu activity. It was intended to bring an alternative perspective to their probiotic properties in terms of their ability to tolerate the acid environment of the stomach and the bile in the intestine. β-Glu activity in artificial gastric and intestinal fluids was examined. The highest and the lowest β-Glu-specific activities occurred at pH levels of 4.0 (2.200 U/mg) and 2.0 (1.060 U/mg), respectively, in the simulated gastric phase () in L. casei SC1. In L. rhamnosus EA1, we designated that in the simulated gastric phase the highest and the lowest β-Glu-specific activities occurred at pH levels of 4.0 (1.250 U/mg) and 2.0 (0.880 U/mg), respectively (). On the other hand, in the simulated intestinal phase, the highest and the lowest β-Glu-specific activities were reported to take place at pH 8.0 (1.170 U/mg) and 5.5 (0.660 U/mg), respectively (), in L. casei SC1. In L. rhamnosus EA1, the highest and the lowest β-Glu-specific activities were designated to take place at pH 8.0 (0.730 U/mg) and 5.5 (0.560 U/mg), respectively ().
Table 2. L. casei SC1 and L. rhamnosus EA1 strains protein content, β-glycosidase enzyme and specific activity in the artificial gastric fluid and artificial intestinal fluid.
Hydrolysis of isoflavone glucosides
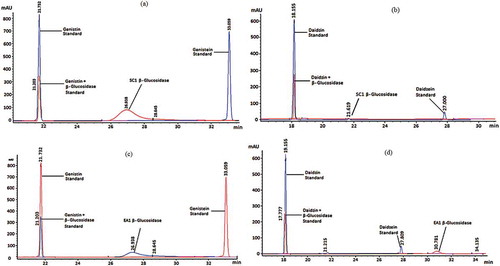
Several researches have shown that changes of isoflavones content in soymilk fermented with various LAB and bifidobacteria and their capacities to hydrolyze isoflavone glucosides to corresponding aglycones are analyzed.[Citation19,Citation22,Citation23] The strains that exhibited high β-Glu-specific enzyme activity with a capacity to hydrolyze isoflavone glucosides to corresponding aglycones were examined. In our study, both strains hydrolyzed the isoflavone glucosides (). Using HPLC, L. casei SC1 hydrolyzed 56% of genistin () and 74% of daidzin (), while L. rhamnosus EA1 hydrolyzed 56% of genistin () and 60% of daidzin (). In another study, Choi[Citation10] studied the ability of bacteria to hydrolyze the isoflavone glucosides after the isoflavone glycoside addition to the medium. Choi[Citation10] investigated the hydrolysis of genistin and daidzin at 50 μg/mL by lactic acid bacteria cultured in MRS medium for 24 h at 37°C. It has been indicated that L. delbrueckii subsp. delbrueckii KCTC 1047 hydrolyzed genistin and daidzin completely, while other strains hydrolyzed 70–80% of genistin into genistein and 25–40% of daidzin into daidzein.
Table 3. Hydrolysis of genistin and daidzin at 50 μg/mL by L. casei SC1 and L. rhamnosus EA1.
Partial purification of β-Glu
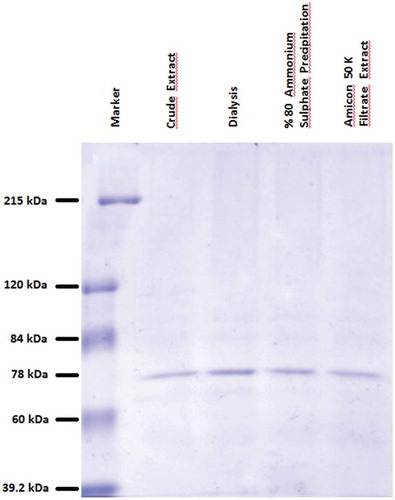
A 1.292-fold purification from the crude extract was achieved by the purification scheme developed for β-Glu enzyme obtained from L. casei SC1 strain that exhibited high hydrolyzed isoflavone (). β-Glu enzyme obtained from L. casei SC1 had a final specific activity of 10.667 U/mg. The SDS-PAGE gel revealed that a single protein species is produced by SDS-PAGE of partial purified. The stained band corresponded to approximately 78 K (). An 80 K band was indicated in L. brevis SK3 strain by Michlmayr[Citation24] and 55 K band in L. plantarum CECT 748T strain by Acebron.[Citation25]
Table 4. Partial purification of β-Glu enzyme obtained from L. casei SC1 strain.
Conclusion
This study investigated the ability of β-Glu belonging to lactobacilli strains that the source of the enzyme is abundant to biotransform isoflavone glycosides to aglycones. In order to achieve bioconversion of isoflavone glucosides, which is extracellular, enzyme β-Glu has to be released from the cell into soymilk. Thus, effective transformation of isoflavone glucosides to aglycones could be restricted by cell membrane permeability. The enzyme obtained in this study can be used directly in industrial applications. The strain that high enzyme activity and ability hydrolyzed the isoflavone glucosides, foods could be used as the starter and probiotic cultures.
Declaration of interest
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Abdulkadir, B.; Nelson, A.; Skeath, T.; Marrs, E. C.; Perry, J. D.; Cummings, S. P.; Embleton, N. D.; Berrington, J. E.; Stewart, C. J. Routine Use of Probiotics in Preterm Infants: Longitudinal Impact on the Microbiome and Metabolome. Neonatology. 2016, 109(4), 239–247. DOI: 10.1159/000442936.
- Moraes-Filho, J. P.; Quigley, E. M. M. The Intestinal Microbiota and the Role of Probiotics in Irritable Bowel Syndrome A Review. Arq. Gastroenterol. 2015, 52(4), 331–338. DOI: 10.1590/S0004-28032015000400015.
- Cousin, F. J.; Mater, D. D. G.; Foligne, B.; Jan, G. Dairy Propionibacteria as Human Probiotics: A Review of Recent Evidence. Dairy Sci. Technol. 2016, 91(1), 1–26. DOI: 10.1051/dst/2010032.
- Chun, J.; Kim, G. M.; Lee, W. K.; Choi, I. D.; Kwon, G. H.; Park, J. Y.; Jeong, S. J.; Kim, S. J.; Kim, J. H. Conversion of Isoflavone Glucosides to Aglycones in Soymilk by Fermentation with Lactic Acid Bacteria. J. Food Sci. 2007, 72(2), 39–44. DOI: 10.1111/j.1750-3841.2007.00276.x.
- Chun, J.; Jeong, W. J.; Kim, J. S.; Lim, J.; Park, C. S.; Kwon, D. Y.; Choi, I.; Kim, J. H. Hydrolysis of Isoflavone Glucosides in Soymilk Fermented with Single or Mixed Cultures of Lactobacillus Paraplantarum KM, Weissella Sp. 33, and Enterococcus Faecium 355 Isolated from Humans. J. Microbiol. Biotechnol. 2008, 18(3), 573–578.
- Smith, L. S.; Kalhan, R.; Wise, R. A.; Sugar, E. A.; Lima, J. J.; Irvin, C. G.; Dozor, A. J.; Holbrook, J. T. Effect of a Soy Isoflavone Supplement on Lung Function and Clinical Outcomes in Patients with Poorly Controlled Asthma. J. Am. Med. Assoc. 2015, 313(20), 2033–2043. DOI: 10.1001/jama.2015.5024.
- Setchell, K. D.; Adlercreutz, C. H. Mammalian Lignans and Phytoestrogens: Recent Studies on Their Formation, Metabolism and Biological Role in Health and Disease. In Role of the Gut Flora on Toxicity and Cancer; Rowland, I. R., ed.; Academic Press: San Diego, 1998; pp 315–345.
- Hughes, I.; Woods, H. F.; Bingham, S. A.; Brown, N. A.; Chipman, J. K.; Dibb, S.; Hindmarsh, P.; Joffe, M.; Kimber, I.; Rowland, I. R.; Salfield, J.; Sharpe, R. M. Phytoestrogens and Health, 1st ed.; Holborn: London, 2003. (Chapter, 11).
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of Regulative Functions between Dietary Soy Isoflavones Aglycone and Glucoside on Lipid Metabolism in Rats Fed Cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. DOI: 10.1016/j.jnutbio.2004.11.005.
- Choi, Y. B.; Kim, K. S.; Rhee, J. S. Hydrolysis of Soybean Isoflavone Glucosides by Lactic Acid Bacteria. Biotechnol. Lett. 2002, 24, 2113–2116. DOI: 10.1023/A:1021390120400.
- You, H. J.; Ahn, H. J.; Kim, J. Y.; Wu, Q. Q.; Ji, G. E. High Expression of β-glucosidase in Bifidobacterium Bifidum BGN4 and Application in Conversion of Isoflavone Glucosides during Fermentation of Soy Milk. J. Microbiol. Biotechnol. 2015, 25(4), 469–478. DOI: 10.4014/jmb.1408.08013.
- Matsuda, S.; Norimoto, F.; Matsumoto, Y.; Ohba, R.; Teramoto, Y.; Ohta, N.; Ueda, S. Solubilization of a Novel Isoflavone Glucoside-Hydrolyzing β-glucosidase from Lactobacillus Casei Subsp. Rhamnosus. J. Biosci. Bioeng. 1994, 77, 439–441. DOI: 10.1016/0922-338X(94)90021-3.
- Babaoğlu-Aydaş, S.; Şirin, S.; Aslim, B. Biochemical Analysis of Centaurea Depressa Phenylalanine Ammonia Lyase (PAL) for Biotechnological Applications in Phenylketonuria (PKU). Pharm. Biol. 2016, 54, 2838–2844. DOI: 10.1080/13880209.2016.1185634.
- Mesas, J. M.; Rodriguez, M. C.; Alegre, M. T. Basic Characterization and Partial Purification of β-glucosidase from Cell-Free Extracts of Oenococcus Oeni ST81. Lett. Appl. Microbiol. 2012, 55, 247–255. DOI: 10.1080/13880209.2016.1185634.
- Sarikaya, E.;. α-amilaz Üreten Bazı Bacillus Suşlarının Gelişme Parametreleri, Enzim Özellik Ve Üretim Koşullarının Optimizasyonu; Doktora Tezi, Ankara Üniversitesi Fen Bilimleri Enstitüsü: Ankara, 1995.
- Laemmli, U. K.;. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970, 277, 680–685. DOI: 10.1038/227680a0.
- Pham, T. T.; Shah, N. P. Hydrolysis of Isoflavone Glycosides in Soy Milk by β- Galactosidase and β-glucosidase. J. Food Biochem. 2009, 33, 38–60. DOI: 10.1111/jfbc.2009.33.issue-1.
- Marazza, J. A.; Garro, M. S.; Giori, G. S. Aglycone Production by Lactobacillus Rhamnosus CRL981 during Soymilk Fermentation. Food Microbiol. 2009, 26, 333–339. DOI: 10.1016/j.fm.2008.11.004.
- Donkor, O. N.; Shah, N. P. Production of β-glucosidase and Hydrolysis of Isoflavone Phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis and Lactobacillus casei in Soymilk. J. Food Sci. 2008, 73(1), M15–M20. DOI: 10.1111/j.1750-3841.2007.00547.x.
- Tsangalis, D.; Ashton, J. F.; Mcgill, A. E. J.; Shah, N. P. Enzymic Transformation of Isoflavone Phytoestrogens in Soymilk by β-glucosidase Producing Bifidobacteria. Food Microbiol. Saf. 2002, 67, 8.
- Jurado, E.; Camacho, F.; Luzon, G.; Vicaria, J. M. Kinetic Models of Activity for β-galactosidases: Influence of pH, Ionic Concentration and Temperature. Enzyme Microb. Technol. 2004, 34, 33–40. DOI: 10.1016/j.enzmictec.2003.07.004.
- Rekha, C. R.; Vijayalakshmi, G. Bioconversion of Isoflavone Glycosides to Aglycones, Mineral Bioavailability and Vitamin B Complex in Fermented Soymilk by Probiotic Bacteria and Yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. DOI: 10.1111/j.1365-2672.2010.04745.x.
- Hu, Y.; Luana, H.; Haoa, D.; Xiao, H.; Yang, S.; Yang, L. Purification and Characterization of a Novel Ginsenoside-Hydrolyzing β-glucosidase from the China White Jade Snail (Achatina fulica). Enzyme Microb. Technol. 2007, 40, 1358–1366. DOI: 10.1016/j.enzmictec.2006.10.011.
- Michlmayr, H.; Kneifel, W. β-Glucosidase Activities of Lactic Acid Bacteria: Mechanisms, Impact on Fermented Food and Human Health. FEMS Microbiol. Lett. 2014, 352(1), 1–10. DOI: 10.1111/1574-6968.12348.
- Acebron, I.; Curiel, J. A.; Rivas, B.; Munoz, R.; Mancheno, J. M. Cloning, Production, Purification and Preliminary Crystallographic Analysis of a Glycosidase from the Food Lactic Acid Bacterium Lactobacillus plantarum CECT 748T. Protein Expr. Purif. 2009, 68, 177–182. DOI: 10.1016/j.pep.2009.07.006.