ABSTRACT
This work was aimed to investigate the chemical composition of pennyroyal (Mentha pulegium L.) aerial parts and castor (Ricinus communis L.) stems essential oils from Tunisia. Fumigant and repellent toxicities were assessed toward two major stored product beetles: Lasioderma serricorne and Tribolium castaneum. Pennyroyal essential oil was characterized by a clear predominance of the oxygenated monoterpenes fraction (97.10%) instead of phenol fraction (61.47%) in the castor essential oil. The major common compounds of Mentha pulegium were pulegone and isomenthone, whereas 2,4-bis (dimethylbenzyl)-6-t-butylphenol was the main volatile compound of castor essential oil. Pennyroyal essential oil showed a strong antiradical capacity (IC50 = 14 µg/mL) which is higher than synthetic standard. The effectiveness of pennyroyal essential oil against the coleopteran insects showed potential fumigant impact particularly against Lasioderma serricorne with LC50 = 8.46 µL/L air. Moreover, significant pest repellent activity was demonstrated with Ricinus communis and Mentha pulegium where the repellency effects reached 80 and 60% after 1 and 24h of exposure against Tribolium castaneum at doses of 0.31 µL/cm2 and 0.078 µL/cm2 respectively. Hence, these findings underlined the potential insecticidal effect of castor and pennyroyal essential oils and highlighted their use as valuable food and insecticide products instead of synthetic pesticides.
Introduction
Chemical pesticides used in agriculture represented a serious threat and a real environmental risk which affect essentially the groundwater and the atmosphere qualities. In fact, several studies have demonstrated the ecotoxicity of dozens of million cases of pesticide poisonings worldwide annually.[Citation1] In another hand, pesticides have harmful effects on human health where 15 to 20% of these chemicals are carcinogens and endocrine disruptors.[Citation2] Moreover, pesticides have significant chronic health effects, including respiratory and fetal diseases, diabetes, genetic, and neurological disorders.[Citation3] Current investigations have mentioned that insects were the first responsible for the excessive use of these chemical poisons.
Tribolium castaneum (the red flour beetle) and Lasioderma serricorne (the cigarette beetle) are cosmopolitan and polyphagous stored product pests in stored grains throughout the world. In Tunisia, tribolium and lasioderma represented the most important and destructive pests in mills.[Citation4,Citation5] These coleopteran insects constitute a real problem in damaging and contaminating food products. Of these, T. castaneum is the most serious pest where its severity relies on its short generation time (20 days) and its high rate of multiplication under favorable conditions.[Citation6] In addition, insect adults have glands that produce a nauseating secretion which strongly depreciates the foodstuffs.[Citation7]
Given the substantial impact and the potential link to pesticide exposure, it is critical to greatly restrict the use of these harmful chemicals instead of natural products. These later have been used successfully to improve crop protection.[Citation8] By this simple view, essential oils (EOs) constitute the bioactive fraction of aromatic plants and the most exploited products thanks to their harmlessness. Concerning pests’ control, many studies revealed that these secondary metabolites were well-known for their biological activities such as their insecticide effect.[Citation9] In addition, EOs exhibited acute toxicity, anti-feeding, and oviposition deterrents against a wide variety of insect-pests.[Citation10] Though, the EO effects vary regarding the insect species, its developmental stage, and the plant origin producing the EOs.[Citation9]
Among the sources of these natural products, Mentha (Mentha pulegium L.; Lamiaceae) and Ricin (Ricinus communis L., Euphorbiaceae) were very important wild medicinal and aromatic plants used in phytotherapy and traditional medicine.[Citation11,Citation12] Pennyroyal (Mentha pulegium) has been traditionally used for its antiflatulent, carminative, expectorant, diuretic, and antitussive effects and essentially for its antiseptic properties to treat cold, sinusitis, cholera, food poisoning, bronchitis, and tuberculosis.[Citation12] Castor (Ricinus communis) is also known for its anticancer, antidiabetic, antiprotozoal, insecticidal, larvicidal, and adult emergence inhibition activities.[Citation13] Since no reports were carried out, these two plants were selected in this current study for their insecticide effects against two selected beetles.
In this context, the present work aimed to investigate the chemical composition of Mentha pulegium and Ricinus communis EOs (i), to determine their antioxidant activities (ii) and to evaluate their repellent and fumigant potentials against T. castaneum and L. serricorne. (iii) The potential effect of the insecticidal activity was assessed by the determination of the respective repellent (RD50) and lethal doses (LD50). Such a study could be helpful to validate the traditional uses and valorize Mentha and Ricin as natural insecticides. This could be an encouragement trend to preserve the safety and the best quality of the industrial food and pharmaceutical products.
Materials and methods
Chemicals
All solvents used in the experiments were purchased from Merck (Darmstadt, Germany). Sodium phosphate (Na2HPO4), sodium monobasic phosphate anhydrous(NaH2PO4), potassium ferricyanide (K3Fe (CN)6), trichloroacetic acid (TCA), butylated hydroxytoluene (BHT), ascorbic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), homologous series of C8-C40 n-alkanes, and high-purity standards of EO were purchased from Sigma-Aldrich (Steinheim, Germany). Deionized water was used to prepare all solutions, unless otherwise indicated. These solutions were wrapped in aluminium foil and stored at 4°C. All other chemicals used were of analytical grade.
Plant material
Mentha pulegium (aerial parts) and Ricinus communis (stems) were harvested randomly by hands (three times) from plants growing wild in Tunis (Oued El Abid; latitude 36° 52ʹ 52.20ʹ’(N); longitude 14° 42ʹ 26.41ʹ’(E), altitude 1005 m) and Borj Cedria (North Western Tunisia; latitude 36° 41ʹ 17.22ʹ’(N); longitude 10° 22ʹ 40.31ʹ’(E), altitude 11904 m). The harvested plants were identified by Professor A. Smaoui (Biotechnology Center in BorjCedria Technopole, Tunisia) according to Tunisian flora[Citation14] and two voucher specimens were deposited at the herbarium of the Laboratory of Bioactive Substances, Biotechnology Center in Borj-Cedria Technopole. After that, the samples were freeze-dried and ground to fine powder by an electric mill and conserved in a dessicator at room temperature (~25°C) in darkness for further uses.
Essential oil extraction
Dried samples of the selected plants (100 g, three times) were subjected to hydrodistillation for Ricinus communis and Clevenger for Mentha pulegium for 90 min in accordance with European Pharmacopoeia method.[Citation15] This time was fixed after a kinetic survey during 30, 60, 90, and 120 min. In the case of Ricinus, the distillate (100 mL) was extracted with 100 mL of 2-methyl-butane for 30 min (three times) and dried over anhydrous sodium sulphate. The organic layer was then concentrated at 34.6°C using a vigreux column at atmospheric pressure and the resulting EOs were stored for a few hours at 4°C in dark and under a nitrogen atmosphere until analysis. EO yield percentage was calculated as volume (mL) of EO per 100 g of plant dry matter.
Gas chromatography analysis
The analysis of volatile compounds by gas chromatography (GC) was carried out on a Hewlett-Packard 6890 gas chromatograph (Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and an electronic pressure control (EPC) injector. A polar polyethylene glycol (PEG) HP Innowax and a 5% diphenyl, 95% dimethylpolysiloxane apolar HP-5 capillary columns (30 m x 0.25 mm, 0.25 mm film thickness; Hewlett-Packard, CA, USA) were used. The flow of the carrier gas (N2) was 1.6 mL/min. The split ratio was 60:1. The analysis was performed using the following temperature program: oven temperature kept isothermally at 40°C for 10 min, increased from 40 to 205°C at the rate of 3°C/min and kept isothermally at 205°C for 10 min. Injector and detector temperatures were held, at 250 and 300°C, respectively. The injected volume was 1 µL of diluted EO. The individual peaks were identified by retention times and indices (relative to C8-C40 n-alkanes), compared to known compounds. Percentage compositions of samples were calculated according to chromatographic peaks area using the total ion current.
Gas chromatography-mass spectrometry aanalysis
GC/MS analysis was performed on an Agilent GC system 7890A coupled with a mass spectrometer Agilent 5975C inert XL MSD with electron impact ionization (70 eV). An HP-5MS capillary column (30 m x 0.25 mm coated with 5% phenyl methyl silicone, 95% dimethylpolysiloxane, 0.25 µm film thickness) was used. Oven temperature was programmed at 40°C for 1 min, then heated to 100°C at a rate of 8°C/min, and kept constant at 100°C for 5 min, then heated to 200°C at a rate of 10°C/min, and kept constant at 200°C for 3 min. Finally it rose to 300°C at a rate of 12°C/min, transfer line temperature was 250°C. The carrier gas was He with a flow of 1 mL/min and a split ratio of 100/1. Scan time and mass range were 1 s and 50–550 m/z, respectively. The identification of volatile components was assigned by comparison of their retention indices (RI) relative to (C8-C40) n-alkanes with those of literature or with those of authentic compounds available in the authors’ laboratory. Further identification was made by matching their recorded mass spectra with those stored in the Wiley 09 NIST 2011 mass spectral library of the GC/MS data system.
Antioxidant activity
DPPH Assay: The electron donation ability of the EOs of the selected plants was measured by bleaching of the purple-colored solution of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) according to the method of Hanato et al.[Citation16] One half mL of 0.2 mM DPPH solution was added to EO solutions (2 mL, 1000–5000 µg/mL). After an incubation period of 30 min at room temperature, the absorbance was against a blank at 517 nm. The inhibition percentage of free radical DPPH (IP%) was calculated as follows:
where Ablank is the absorbance of the control reaction and Asample is the absorbance in the presence of EO. The EO concentration providing 50% inhibition (IC50) was calculated from the regression equation prepared from EO concentration and the inhibition percentage. BHT, a synthetic antioxidant used generally for food, cosmetics and pharmaceuticals was used as standard.
Reducing Power: The method of Oyaizu[Citation17] was used to assess the reducing power of R. communis and M. pulegium EOs. Of 1 mL different concentrations of EOs (1, 3, 5, and 7 mg/mL) in methanol were mixed with 2.5 mL of a 0.2 M sodium phosphate buffer (pH = 6.6) and 2.5 mL of 1% potassium ferricyanide (K3Fe (CN)6) and incubated in a water bath at 50°C for 20 min. Then, 2.5 mL of 10% trichloroacetic acid (TCA) were added to the mixture that was centrifuged at 650 g for 10 min. The supernatant (2.5 mL) was then mixed with 2.5 mL distilled water and 0.5 mL of 0.1% ferric chloride solution. The intensity of the blue-green colour was measured at 700 nm. The EO concentration at which the absorbance was 0.5 for the reducing power (EC50) was obtained from the linear regression equation prepared from the concentrations of the EO solutions and the absorbance values. High absorbance indicates high reducing power. Ascorbic acid was used as a positive control.
Insect rearing
For bioassays, 0–24 h old adult insects were collected from the rearing colony initiated in the laboratory of Plant Protection at the National Institute of Agricultural Research of Tunisia. The red floor beetle T. castaneum and the cigarette beetle L. serricorne were reared on wheat floor (Triticum aestivum) without exposure to insecticides. The rearing conditions were darkness in 25 ± 1°C, 65% ± 5%, relative humidity and 15h: 9h (light: dark) photoperiod. Adult insects were used for fumigant and repellency tests.
Fumigant toxicity
To determine the fumigant toxicity of Ricinus communis and Mentha pulegium EOs, 2 cm diameter filter papers (Whatman No. 1) were impregnated with the tested EO doses. They were then calculated to give equivalent fumigant concentrations. The impregnated filter paper was then attached to the screw caps of a 44 mL plexiglas bottle. Caps were screwed tightly on the vials; each one contained separately 10 adults (1–7 days old) of each species. When no leg or antennal movements were observed, insects were considered dead. The mortality was calculated using the Abbott correction formula.[Citation18] A second experiment was designed to assess 50% lethal doses. A series of dilutions was prepared to evaluate insect’s mortality after an initial dose-setting experiment. EO amounts tested on T. castaneum and L. serricorne were 0.5, 2, 5, and 25 µL corresponding to concentrations of 11.36, 45.46, 113.6, and 568.2 µL/L air, respectively. Control insects were kept under the same conditions without any EO and each dose was replicated three times. The number of dead and alive insects in each bottle was counted 48 h after initial exposure. The mortality was evaluated by direct observation of the insects every hour till total mortality. Probit analysis[Citation19] was used to estimate LC50 values (lethal concentration was designed to assess 50% of insect mortality).
Repellency bioassay
Filter Paper Tests: Repellency assays of Ricinus communis and Mentha pulegium EOs were carried according to the experimental method described by Jilani and Saxena.[Citation20] Watman filter papers (diameter 8 cm) were cut in half. Test solutions were preparing by dissolving 2.5, 5, 7.5, and 10 µL of EOs in 0.5 mL acetone. Doses were converted to give equivalent concentrations of respectively 0.078, 0.157, 0.235, and 0.314 µL/cm2. Each solution was applied to half a filter-paper disc as uniformly as possible with a micropipette. The other half of the filter paper was treated with acetone alone as a control. The treated and control half discs were air dried under a fan to evaporate the solvent completely. Treated and untreated halves were attached to their opposites using adhesive tape and placed in petri dishes. Twenty adult (7–10 days old) beetles of mixed sex (ratio 1:1) were released at the center of each filter paper disc. The dishes were then covered and sealed with parafilm. Observations on the number of insects present on both the treated and untreated halves were recorded after 1, 3, 5, and 24 h for each tested concentration. Four trials were made for each concentration.
Percentage Repellency (PR) and Median Repellent Doses (RD50 and RD95) of Essential Oil: Numbers of L. serricorne and T. castaneum presented on the treated and untreated portions of the experimental paper halves were recorded after various periods of exposure. Percentage repellency (PR) was calculated according to Nerio et al.[Citation21] as follows:
where Nc was the number of insects on the untreated area after the exposure interval and Nt was the number of insects on the treated area after the exposure interval. Four replications were used for each concentration. Probit analysis[Citation22] was used to calculate the median repellent doses (RD50 and RD95) (doses that repelled 50% and 95% of the exposed insects respectively) at 24 h of exposure. Results were presented as the mean of repellency percentage ± the standard error.
Data analysis
All analyses were performed in triplicate and the results were expressed as means values ± standard deviations (SD). One-way analysis of variance (ANOVA) using Statistica[Citation23] was performed on the data. A Duncan test was applied to the means to detect significant differences of repellence among concentrations and EOs at the 0.05% level. Data are presented in tables as means with standard errors. Probit analysis[Citation22] was conducted to estimate median repellent dose (RD50) with their 95% fiducial limits; RD was considered significantly different when their respective 95% fiducial limits did not overlap. To assess the impact of EO concentration and exposure time on repellent action, data underwent two-way ANOVA, converting percentage data into angular values; the averages were separated by LSD tests.
Results and discussions
Essential oil yields and chemical composition
Mentha pulegium: The extraction by steam distillation of pennyroyal (M. pulegium) aerial parts collected in the region of Oued el Abid (Tunisia) gave an EO (pale yellowish) with a yield reaching up to 1.5% (w/w on dry weight basis). This yield was relatively significant and pennyroyal could be considered as an appreciated source of EO. In fact, obtained yields were higher than other ones reported for Lamiaceae species such as Mentha aquatica (0.42%), [Citation24] Mentha longifolia (0.50–0.90%),[Citation25] Mentha spicata (0.72%), Mentha suaveolens (0.96%)[Citation26], and Mentha piperita (1.09%)[Citation27], but these yields were lower in case of Mentha pulegium from Morocco 2.33%).[Citation28] Hence, in addition to genetic control, variations in EO yield among mint species may be attributed to abiotic factors such as region of cultivation and cultural conditions (humidity, light, temperature and especially fertilizer use, and irrigation).[Citation29] Similarly, the effect of cultural practices and density of insect communities on the evolution of this parameter has also been described.[Citation30]
The chromatographic analysis of EO has identified 28 compounds that represented approximately 99.82% for Tunisian M. pulegium EO (). The EO was characterized by a clear predominance of monoterpenes and particularly the oxygenated fraction with a percentage of 97.10%. Pulegone (55.58%) and isomenthone (34.87%) were the responsible compounds of this rise. Pennyroyal EO can be considered as an important source of pulegone, a monoterpene ketone, which is presented in many other mint species and known for its numerous therapeutic effects such as anti-feedant, antibacterial, antifungal, and insecticide activities.[Citation31,Citation32] In addition, the study of De Sousa et al.[Citation33] has reported the analgesic, antinociceptive, and psychoactive effects of this volatile compound. Moreover, pulegone can be used in many other fields such as food, cosmetic, and pharmaceutical industries where it is used as a traditional remedy, a flavouring, and a spice.[Citation34,Citation35]
Table 1. Chemical composition (%, w/w) of the essential oils extracted from Mentha pulegium aerial parts and Ricinus communis stems growing wild in Tunisia.
These results were in accordance with other studies already carried out in Algeria, India, and Uruguay where M. pulegium EOs were characterized by their high rates of pulegone with percentages of 46.41%, (65.9–83.1%), and 73.4% respectively.[Citation36–Citation38] Nevertheless, this composition was different from Mentha pulegium EOs in Morocco and Iran and was characterized by two specific chemotypes: that of piperitone (35.56 and 38%) and piperitenone (21.12 and 33%) where the rate of pulegone did not exceed 2.3 and 6.42%, respectively.[Citation35]
Other compounds were poorly represented such as neoisomenthol (4.03%) and p-menth-3-ene (1.29%) (). The contribution of monoterpene hydrocarbons in pennyroyal EO did not exceed 2.08%. Of the same,the oxygenated sesquiterpenes and the phenols were detected in very small amounts which didn’t exceed 0.56% of total pennyroyal EO.
Ricinus communis: The results of Ricinus communis EO analysis were shown in . The components were identified and their percentages were listed according to their elution order on HP-5MS column. A total of 28 compounds were identified accounting for 99.19% of the EO with a yield of 0.26%. Ricinus EO was characterized by a clear dominance of phenolic compounds with 61.47% of the total EO. The main components were 2,4-bis (dimethylbenzyl)-6-t-butylphenol (28.03%) and phenol, 2,4-bis (1-methyl-1-phenylethyl) (20.99%). However, monoterpene aldehydes were detected in small amounts less than 5% and were represented essentially by benzene acetaldehyde (1.34%) ().
The major compound, 2,4-bis (dimethylbenzyl)-6-t-butylphenol was also detected as main volatile component in other species such as Parkinsonia aculeate aerial parts (Fabaceae) in Saudi Arabia.[Citation39] These authors showed the antimicrobial activity of this major compound. Nevertheless, a previous study focusing on Ricinus communis EO aerial parts in Tunisia showed that oxygenated monoterpenes had the highest percentage with 75.61% with α-thujone (31.71%) and 1,8-cineole (30.98%) as the main compounds of this EO.[Citation40] Thus, according to Lis et al.[Citation41], the chemical composition of the EO depends on several factors such as plant organ (leaves, flowers, stems, etc.). In fact, depending on this factor, several EOs of the same plant with different chemical compositions and biological activities can be formed. This property allowed us to offer a wide range of different natural species in many industrial outlets. Until now, it should be noted that no study has examined the composition of Ricinus communis EO stems.
Antioxidant activity of castor and pennyroyal essential oils
In order to limit the risk of free radicals and to evaluate the nutritional health food or plants, the appeal of natural antioxidants is crucial to ensure product stability.[Citation42] Indeed, an ideal and natural antioxidant is a preventive agent, capable to avoid free radicals by complexing catalysts, reacting with oxygen or by deflecting the food effects of light.
DPPH radical-scavenging activity
To evaluate the ability of M. pulegium and R. communis EOs to act as free radical scavengers, the DPPH method was used. By comparing the IC50 of both species, results showed that R. communis EO was less effective to inhibit the radical DPPH with value of 333.6 µg/mL instead of M. pulegium EO (14 µg/mL) (). This strong activity of pennyroyal EO (compared to BHT; 22 µg/mL) could be due to its high content of isomenthone, the monoterpene ketone, which is known for its powerful scavenging complexes.[Citation43] In addition, many other researchers showed that EOs rich in non phenolic compounds were characterized by their potential antioxidant activity.[Citation44]
Table 2. Antioxidant activity of pennyroyal and castor essential oils.
In another hand, it should be noted that the scavenging capacity of Tunisian M. pulegium was higher than that of mint species cited in the literature such as M. rotundifolia (29.52 µg/mL) from Tunisia,[Citation45] M. piperita (15.2 µg/mL) from Libya,[Citation46] and M. longifolia (10.7 mg/mL) from Turkey.[Citation47]
Iron reducing power
This test was used to determine if volatile compounds of the studied species were electron donors and can reduce the oxidized intermediates of lipid peroxidation processes.[Citation48] In this context, results from showed that the two EOs exhibited a similar reducing power (EC50 = 4.76 mg/mL and 4 mg/mL for Ricinus communis and Mentha pulegium EOs respectively). The higher reducing power of pennyroyal may be attributed to the presence of menthone and isomenthone, known for their potential antioxidant activity.[Citation49,Citation50]
Until now, there is negligible published report concerning the antioxidant activity of castor EO stems. However, the study of Kadri et al.[Citation40] has showed that the reducing power of the EO castor aerial parts in Sfax (Tunisia) was 39.32 µg/mL. This activity was better compared to that calculated in this work, which could be due to: the extraction solvent, the part of the plant having undergone extraction (leaves, fruit, and bark), and climatic or soil factors. By comparison to many other medicinal plants known for their antioxidant activity (Bidens pilosa,[Citation51] Bacopa moniera and Centella asiatica),[Citation52] M. pulegium and R. communis EOs showed an appreciable reducing power. This latter is generally associated with the presence of reductones which exert antioxidant action by breaking the free radical chain by donating an hydrogen atom.[Citation51] Hence, due to this property, these two EOs might protect or prevent food from oxidation.
Insecticidal activity of castor and pennyroyal essential oils
Fumigant toxicity
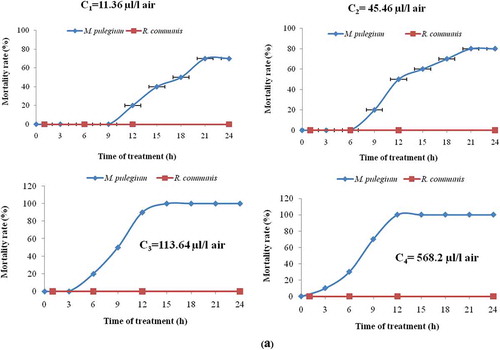
Against Tribolium castaneum results of the fumigant test were shown in . In fact, fumigant impact varied according to plant species, EO concentration and duration exposure. In another hand, in contrast to castor EO, fumigant impact of pennyroyal EO was clearly more significant to T. castaneum adults. The highest concentration of pennyroyal EO (586.2 μL/L air) caused 100% mortality of T. castaneum adults after only 11 h of exposure. Moreover, the lowest concentration of pennyroyal EO (11.36 μL/L air) caused 60% mortality of T. castaneum insects after 19 h of exposure. Meanwhile, at the highest concentration of castor EO (568.2 μL/L air), no mortality was caused showing unsusceptibilty of T. castaneum adults to castor EO.
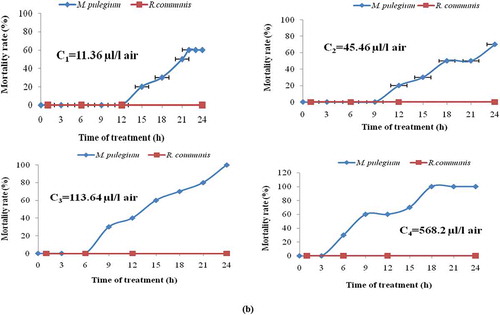
Figure 1. Percentage mortality of Tribolium castaneum (a) and Lasioderma serricorne (b) exposed for various periods of time to Mentha pulegium and Ricinus communis essential oils.
In conclusion, insect susceptibility varied according to plant species producing EO. With al, the analysis of the cause of plant resistance must include consideration of the behavioral and physiological characteristics of the insect and the plant host as was confirmed by Beck (Citation1965).[Citation53]
Against Lasioderma serricorne, as reported in , the same trend was reported as previously. In fact, the higher the concentration, the greater the percentage of mortality increased. In another hand, as compared to the other coleopteran pest (Tribolium castaneum), it appeared that the highest concentration of Mentha pulegium EO leading to 100% mortality on Lasioderma serricorne after 15 h of exposure was 568.2 µL/air. Hence, this potent fumigant effect might be attributed to the high content of monoterpenes ()[Citation54] and especially the main compounds; pulegone and isomenthone which have been screened for their insecticidal and genotoxic activities.[Citation55,Citation56] In this context, other reports highlighted the fumigant activity,[Citation57] the ovicidal and larvicidal effects of these volatile compounds against stored-product pests. [Citation34,Citation58,Citation59] In addition, pennyrotal EO was reported to be more toxic than many other EOs extracted from other plants such as Lippia citrodora Kunth (Verbenaceae), R. officinalis (Lamiaceae), and Juniperus Sabina (Pinaceae) against the beetle callosobruchus maculates.[Citation60] Thereby, M. pulegium EO has potential use against these two stored-product insects and could be considered as a natural fumigeant among the most promising botanical insect-control agents.
Lethal Concentrations: Probit analysis showed that the LC50 values of Mentha pulegium EO tested with Tribolium castaneum (LC50 = 11.57 µL/L air) was higher than those tested with cigarette beetle (LC50 = 8.46 µL/L air). Hence, these results demonstrated the higher sensitivity of Lasioderma serricorne to pennyroyal EO than Tribolium castameum ().
Table 3. LC50 values of Mentha pulegium essential oil against Lasioderma serricorne and Tribolium castaneum.
The insecticidal activity of Mentha pulegium EO against different insect species of stored food was reported by several researchers. In this context, Pavela[Citation26], Rim and Jee[Citation61] and Pavlidou et al.[Citation57] showed toxicity fumigation of Mentha pulegium EO against coleoptera species (Dermatophagoides pteronyssinus and Musca domestica). In addition, Lamiri et al.[Citation62] indicated that Mentha pulegium EO can cause 100% mortality of Mayetiola destructor adults (Hessian fly) at the dose of 8 µL/L. Among the other insects, Pavlidou et al.[Citation57] evaluated the sensitivity of Bactrocera oleae (olive fly) and Drosophila melanogaster larvae to M. pulegium EO and showed that the LD50 was between 0.22 and 2.09 µL/L.
Repellency effect
Ricinus communis: The repellency bioassay of Ricinus communis EO showed encouraging results against Tribolium and Lasioderma adults where the highest repellency potentials (80% and 50%) were detected at doses of 0.31 µL/cm2 and 0.235 µL/cm2 respectively after 1 h of exposure (). However, this activity decreased as a function of exposure time to reach respectively 60% and 30% after 24 h of exposure (for Tribolium) and from 50% after 5 h of exposure to 20% after 24 h of exposure (for Lasioderma). These results agreed with other reports where insect response varied according to exposure time. In fact, Mediouni-Ben Jemaa et al.[Citation4] found that the best repellent efficacy (92.5%) of Laurus nobilis EO against L. serricorne was observed at the dose of 0.12 µL/cm2 during short exposure period (1 h). In another experiment, Sahaf et al.[Citation63] found a strong insecticidal activity of Carum copticum EO against Tribolium castaneum (Tenebrionidae) which reached 100% mortality at a concentration higher than 185.2 µL/L air and after 12 h of exposure time.
Table 4. Percentage repellency (mean ± SE) of Ricinus communis essential oil against Lasioderma serricorne and Tribolium castaneum adults after various periods of exposure.
Mentha pulegium: In repellency bioassays, M. pulegium EO tested gave excellent results against Lasioderma serricorne and Tribolium castaneum. In fact, this EO showed strong repellent efficacy which was highly dependent upon the concentration and exposure time, as mentioned at . A significant repellent efficacy (60%) was observed even at low doses (0.078 µL/cm2) after 24 h of exposure period especially against Tribolium castaneum. Meanwile, at 0.31 µL/cm2, pennyroyal EO reached only 50% repellency after 5 h of exposure against Lasioderma sericorne (). Of the same, pennyroyal EO showed classes II and III repellency status at the majority of doses against the two beetles except for 5 h exposure with the concentration of 0.314 (T. castaneum) which showed class IV repellency status. Therefore, these findings showed that the tested coleopteran pests were more susceptible to M. pulegium than castor EO.
Table 5. Percentage repellency (mean ± SE) of Mentha pulegium essential oil against Lasioderma serricorne and Tribolium castaneum adults after various periods of exposure.
As proved by several authors, this strong repellency potential of pennyroyal could be due to its diaphoretic, antispasmodic, and anti-inflammatory properties.[Citation60] Of same, the presence of monoterpenoids and sesquiterpenes regularly appeared to be the responsible of this insecticidal activity by their neurotoxicity effect against theses insects.[Citation11,Citation64] Moreover, their lipophilicity facilitates penetration into the insects in order to interfere with their physiological functions.[Citation65]
Repellency Doses: The potent repellency of pennyroyal was evaluated by the lower corresponding doses RD50 and RD95 with values of 0.010 µL/cm2 and 0.526 µL/cm2 for L. serricorne and 0.015 and 0.370 µL/cm2 for T. castaneum respectively (). Thereby, we can note that Tribolium was more susceptible to pennyroyal EO than Lasioderma adults. In the meantime, the study of Olivero-verbel et al.[Citation66] has showed repellent doses of 21 µL/L and 84 µL/L for Cymbopogon citratus and Eucalyptus citriodora EOs respectively against Tribolium castaneum. On another hand, the sensitivity of Lasioderma serriorne to many other aromatic and medicinal plants was also reported by other researchers with higher RD50 such as Laurus nobilis (37.84 µL/cm2).[Citation4] To the best of our knowledge, this study was the first report focusing on the assessment of insecticidal activity of Mentha pulegium and Ricinus communis EOs against these two beetles.
Table 6. Repellency doses RD50 and RD95 values of Mentha pulegium essential oil against Lasioderma serricorne and Tribolium castaneum adults after 24 h of exposure.
Conclusion
Results of this work underlined the powerful scavenging activity, the potential reducing power of pennyroyal and castor EOs, respectively and their insecticidal capacities. That’s why, the use of these two medicinal plants could be considered as potential alternative to synthetic insect-control agents faced to their adverse impacts. However, further studiesshould be undertaken in order to formulate these two new natural products under controlled conditions and test their stability in many pharmaceutical and industrial fields.
Declaration of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgements
The authors were grateful to Pr. Abderrazek Smaoui for botanic identification.
Additional information
Funding
References
- Richter, E. D. Acute Human Pesticide Poisonings. In Encyclopedia of Pest. Management; Pimentel, D., ed.; Taylor & Francis: Boca Raton, FL, USA, 2002, 3–6.
- Meyer, A.; Chrisman, J.; Moreira, J. C.; Koifman, S. Cancer Mortality among Agricultural Workers from Serrana Region, State of Rio De Janeiro, Brazil. Environ. Res. 2003, 93, 264.
- Tago, D.: Andersson, H.; Treich, N. Pesticides and Health: A Review of Evidence on Health Effects, Valuation of Risks, and Benefit‐cost Analysis. Adv. Health Econ. Health Serv. Res. 2014, 24, 203–295.
- Mediouni-Ben Jemâa, J.; Tersim, N.; Khouja, M. L. Composition and Repellent Efficacy of Essential Oil from Laurus Nobilis against Adults of the Cigarette Beetle Lasioderma Serricorne (Coleoptera: Anobiidae). Tunisian J. Plant Prot. 2011, 6 (1), 29–41.
- Bachrouch, O.; Mediouni-Ben Jemâa, J.; Aidi Waness, W.; Talou, T.; Marzouk, B.; Abderraba, M. Composition and Insecticidal Activity of Essential Oil from Pistacia Lentiscus L. Against Ectomyelois Ceratoniae Zeller and Ephestia Kuehniella Zeller (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2010, 46, 242–247.
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal Properties of Mentha Species: A Review. Ind. Crops Prod. 2011, 34, 802–817.
- Appert, J. The Storage of Grains and Seeds. In Tropical Agriculture; Rene Coste; ed.; Macmillan Press London, 1992; pp 146.
- Forster, D.; Adamtey, N.; Messmer, M. M.; Pfiffner, L.; Baker, B.; Huber, B.; Niggli, U. Organic Agriculture-Driving Innovations in Crop Research. In Agricultural Sustainability: Progress and Prospects in Crop Research; Bhuller, G. S., Bhuller, N. K.; eds.; Elsevier Inc.: Oxford, UK, 2013. ISBN 978-0-12-404560-6.
- Lashgari, A.; Mashayekhi, S.; Javadzadeh, M.; Marzban, R. Effect of Mentha Piperita and Cuminum Cyminum Essential Oil on Tribolium Castaneum and Sitophilus Oryzae. Arch. Phytopathology Plant Prot. 2014, 47 (3), 324–329.
- Bosly, A. H. Evaluation of Insecticidal Activities of Mentha Piperita and Lavandula Angustifolia Essential Oils against House Fly, Musca Domestica L. (Diptera: Muscidae). J. Entomol. Nematol. 2013, 5 (4), 50–54.
- Bum, E. N.; Taiwe, G. S.; Moto, F. C. O.; Ngoupaye, G. T.; Vougat, R. R. N.; Sakoue, V. D.; Gwa, C.; Ayissi, E. R.; Dong, C.; Rakotonirina, A.; Rakotonirina, S. V. Antiepileptic Medicinal Plants Used in Traditional Medicine to Treat Epilepsy. In Clinical and Genetic Aspects of Epilepsy; Zaid Afawi, ed., In Tech, 2011, ISBN 978-953-307-700-0, 175-192.
- Boukhebti, H.; Chaker, A. N.; Belhadj, H.; Sahli, F.; Ramdhani, M.; Laouer, H.; Harzallah, D. Chemical Composition and Antibacterial Activity of Mentha Pulegium L. And Mentha Spicata L. Essential Oils. Der Pharmacia Lettre 2011, 3 (4), 267–275.
- Manpreet, R.; Dhamija, H.; Prashar, B.; Sharma, S. Ricinus Communis L. – A Review. Int. J. Pharm.Tech. Res. 2012, 4 (4), 1706–1711.
- Pottier-Alapetite, G. Tunisian Flora. Angiosperms-Dicotyledons Gamopetalaes; Official Printing of the Republic of Tunisia: Tunis, Tunisia, 1979; 1074.
- Council of Europe European Pharmacopoeia, 3rd ed.; Council of Europe: Strasbourg, France, 1997.
- Hanato, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effect. Chem. Pharmacol. 1988, 36, 1090–1097.
- Oyaizu, M. Studies on Products of Browning Reaction: Antioxidative Activity of Products of Browning Reaction. Jpn. J. Nutr. 1986, 44, 307–315.
- Abbott, W. S. A Method for Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267.
- Golob, P.; Webley, D. J. The Use of Plants and Minerals as Traditional Protectants of Stored Products. Trop. Prod. Inst. 1980, 138, 1–32.
- Jilani, G.; Saxena, R. C. Repellent and Feeding Deterrent Effects of Turmeric Oil, Sweetllag Oil. Neem Oil and a Neem-Based Insecticide against Lesser Grain Borer (Coleoptera: Bostrychidae). J. Econ. Entomol. 1990, 83, 629–634.
- Nerio, L. S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils from Seven Aromatics Plants Grown in Colombia against Sitophilus Zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 2009, 45, 212–214.
- Finney, D. J. Probit Analysis, 3rd ed.; Cambridge University: London, 1971; 261.
- Statsoft. STATISTICA for Windows (Computer Program Electronic Manual); Statsoft Inc.: Tulsa, OK, 1998.
- Ngoc Dai, D.; Dinh Thang, T.; Essien, E.; Oladimeji, O.; Isiaka, A.; Ogunwande, A. Study on Essential Oil of Mentha Aquatica L. from Vietnam. Am. J. Essential Oils Nat. Prod. 2015, 2 (4), 12–16.
- Sharopov, S. S.; Sulaimonova, V. A.; Setzer, W. N. Essential Oil Composition of Mentha Longifolia from Wild Populations Growing in Tajikistan. J. Med. Active Plants 2012, 1, 2.
- Pavela, R. Insecticidal Properties of Several Essential Oils on the House Fly (Musca Domestica L.). Phytotherapy Res. 2008a, 22, 274–278.
- Abbaszadeh, B.; Valadabadi, S. A.; Farahani, H. A.; Darvishi, H. H. Studying of Essential Oil Variations in Leaves of Mentha Species. Afr. J. Plant Sci. 2009, 3 (10), 217–221.
- Benayad, N.; Ebrahim, W.; Hakiki, A.; Mosaddak, M. Chemical Characterization and Insecticidal Evaluation of the Essential Oil of Mentha Suaveolens L. And Mentha Pulegium L. Growing in Morocco. Chem. Chem. Eng. Biotechnol. Food Ind. 2012, 13 (1), 27–32.
- Ravi, S.; D’Odorico, P.; Okin, G. S. Hydrologic and Aeolian Controls on Vegetation Patterns in Arid Landscapes. Geophys. Res. Lett. 2007, 34, L24S23.
- Msaada, K.; Salem, N.; Tammar, S.; Hammami, M.; Saharkhiz, M. J.; Debiche, N.; Limam, F.; Marzouk, B. Essential Oil Composition of Lavandula Dentata, L. Stoechas, and L. Multifida Cultivated in Tunisia. J. Essential Oil Bearing Plants 2012, 15 (6), 1030–1039.
- Skalicka-Wozniak, K.; Walasek, M. Preparative Separation of Menthol and Pulegone from Peppermint Oil (Mentha Piperita L.) By High-Performance Counter-Current Chromatography. Phytochem. Lett. 2014. doi:10.1016/j.phytol.06.007.
- Yanniv, Z.; Dubai, N. Medicinal and Aromatic Plants of Middle-East; Springer, Dordrecht Heidelberg New York London. 2014. ISBN 978-94-017-9276-9.
- De Sousa, D. P.; Nóbrega, F. F. F.; De Lima, M. R. V.; De Almeida, R. N. Pharmacological Activity of (R)-(+)-Pulegone, a Chemical Constituent of Essential Oils. Z. Naturforsch 2011, 66 (c), 353–359.
- Karousou, R.; Balta, M.; Hanlidou, E.; Kokkini, S. Mints Smells and Traditional Uses in Thessaloniki (Greece) and Other Mediterranean Countries. J. Ethnopharmacol. 2007, 109, 248–257.
- Zekri, N.; Smail Amalich, S.; Boughdad, A.; El Belghiti, M. A.; Zair, T. Phytochemical Study and Insecticidal Activity of Mentha Pulegium L. Oils from Morocco against Sitophilus Oryzae. Mediterr. J. Chem. 2013, 2 (4), 607–619.
- Ouakouak, H.; Chohra, M.; Denane, M. Chemical Composition Antioxidant Activities of the Essential Oil of Mentha Pulegium L, South East of Algeria. Int. Lett. Nat. Sci. 2015, 39, 49–55.
- Agnihotri, V.; Agarwal, S. G.; Dhar, P. L.; Qazi, G. N. Essential Oil Composition of Mentha Pulegium L. Growing Wild in the North-Western Himalayas India. Flavour Fragr. J. 2005, 20 (6), 607–610.
- Lorenzo, D.; Daniel Paz, D.; Eduardo Dellacassa, E.; Davies, P.; Vila, R.; Cañigueral, S. Essential Oils of Mentha Pulegium and Mentha Rotundifolia from Uruguay. Braz. Arch. Biol. Technol. 2002, 45 (4), 519–524.
- Al-Youssef, H. M.; Hassan, W. H. B. Antimicrobial and Antioxidant Activiti of Parkinsonia Aculeata and Chemical Composition of Their Essential Oils. Merit Res. J. Med. Med. Sci. 2015, 3 (4), 147–157.
- Kadri, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical Composition and in Vitro Antioxidant Properties of Essential Oil of Ricinus Communis L. J. Med. Plants Res. 2011, 5 (8), 1466–1470.
- Lis, A.; Liszkiewicz, R.; Krajewska, A. Comparison of Chemical Composition of the Essential Oils from Different Parts of Thuja Occidentalis L. ‘Brabant’ and T. Occidentalis L. ‘Smaragd’. Herba Polonica 2016, 62 (3), 20–27.
- Fr., M.; Davin, A.; Deglène-Benbrahim, L.; Ferrand, C. Méthodes D’évaluation Du Potentiel Antioxydant Dans Les Aliments. Erudit, M/S: Méd. Sci. 2004, 20 (4), 458–463.
- Mimica-Dukic, N. B.; Bozin, M.; Sokovic, B.; Mihajlovic, M. Matavulj Antimicrobial and Antioxidant Activities of Three Mentha Species Essential Oils. Planta Medica 2003, 69, 413–419.
- El-Massry, K. F.; El-Ghorab, A. H.; Farouk, A. Antioxidant Activity and Volatile Components of Egyptian Artemisia Judaica L. Food Chem. 2002, 79, 331–336.
- Riahi, L.; Elferchichi, M.; Ghazghazi, H.; Jebali, J.; Ziadi, S.; Aouadhi, C.; Chograni, H.; Zaouali, Y.; Zoghlami, N.; Mliki, A. Phytochemistry, Antioxidant and Antimicrobial Activities of the Essential Oils of Mentha Rotundifolia L. In Tunisia. Ind. Crops Prod. 2013, 49, 883–889.
- Singh, R.; Shushni, M. A. M.; Belkheir, A. Antibacterial and Antioxidant Activities of Mentha Piperita L. Arabian J. Chem. 2015, 8, 322–328.
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Dafarera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and Antioxidant Properties of the Essential Oils and Methanol Extract from Mentha Longifolia L. Ssp. Longifolia. Food Chem. 2007, 103 (4), 1449–1546.
- Chanda, S.; Dave, R. In Vitro Models for Antioxidant Activity Evaluation and Some Medicinal Plants Possessing Antioxidant Properties: An Overview. Afr. J. Microbiol. Res. 2009, 3 (13), 981–996.
- Braun, L.; Cohen, M. Herbs and Natural Supplements: An Evidence Based Guide, 4th edn., Vol. 2; Chatswood: Elsevier, 2015.
- Kim, S. I.; Park, C.; Ohh, M. H.; Cho, H. C.; Ahn, Y. J. Contact and Fumigant Activities of Aromatic Plant Extracts and Essential Oils against Lasioderma Serricorne (Coleoptera: Anobiidae). J. Stored Prod. Res. 2003, 39, 11–19.
- Goudom, A. B.; Abdou, A. T.; Ngamo, L. S.; Ngassoum, M. B.; Mbofungy, M. F. Antioxidant Activities of Essential Oil of Bidens Pilosa (Linn. Var. Radita) Used for the Preservation of Food Qualities in North Cameroon. Food Sci. Nutr. 2016, 4 (5), 671–678.
- Meena, H.; Pandey, H. K.; Pandey, P.; Arya, M. C.; Ahmed, Z. Evaluation of Antioxidant Activity of Two Important Memory Enhancing Medicinal Plants Baccopa Monnieri and Centella Asiatica. Indian J. Pharmacol. 2012, 44 (1), 114–117.
- Beck, S. D. Resistance of Plants to Insects. Annu. Rev. Entomol. 1965, 10, 207–232.
- Tong, F.; Coats, J. R. Effects of Monoterpenoid Insecticides on [3H]-TBOB Binding in House Fly GABA Receptor and 36 Cluptake in American Cockroach Ventral Nerve Cord. Pestic. Biochem. Physiol. 2010, 98 (3), 317–324.
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z. G.; Insecticidal, M.-T. P. Genotoxic Activities of Mint Essential Oils. J. Agric. Food Chem. 1997, 45 (7), 2690–2694.
- Pavlidou, V.; Karpouhtsis, I.; Franzios, G.; Zambetaki, A.; Scouras, Z.; Insecticidal, M.-T. P. Genotoxic Effects of Essential Oils of Greek Sage, Salvia Fruticosa, and Mint, Mentha Pulegium, on Drosophila Melanogaster and Bactrocera Oleae (Diptera: Tephritidae). J. Agric. Urban Entomol. 2004, 21 (1), 39–49.
- Kheirkhah, M.; Ghasemi, V.; Khoshnood Yazdi, A.; Rahban, S. Chemical Composition and Insecticidal Activity of Essential Oil from Ziziphora Clinopodioides Lam. Used against the Mediterranean Flour Moth, Ephestia Kuehniella Zeller. J. Plant Prot. Res. 2015, 55 (3), 260–265.
- Waliwitiya, R.; Kennedy, C. J.; Lowenberger, C. A. Larvicidal and Oviposition-Altering Activity of Monoterpenoids, Trans-Anethole and Rosemary Oil to the Yellow Fever Mosquitoes Aedes Aegypti (Diptera: Culicidae). Pest Manag. Sci. 2009, 65, 241–248.
- Laurent, D.; Vilaseca, A.; Chaintrane, J. M.; Ballivan, C.; Saavedra, G.; Ibanez, R. Insecticidal Activity of Essential Oils on Triatoma Infestans. Phytotherapy Res. 1997, 11 (4), 285–290.
- Mahmoudvand, M.; Abbasipour, H.; Rastegar, F.; Hosseinpour, M. H.; Basij, M. Efficacy of Some Plants as Post-Harvest Protectant against Some Major Stored Pests. Arch. Phytopathol. Plant Prot. 2011. First Online. doi:10.1080/03235408.2011.597151.
- Rim, I. S.; Jee, C. H. Acaricidal Effects of Herb Essential Oils against Dermatophagoides Farinae and D. Pteronyssinus (Acari: Pyroglyphidae) and Qualitative Analysis of an Herb Mentha Pulegium. Korean J. Parasitol. 2006, 44 (2), 133–138.
- Lamiri, A.; Lhaloui, S.; Benjilali, B.; Berrada, M. Insecticidal Effects of Essential Oils against Hessian Fly, Mayetiola Destructor (Say). Field Crops Res. 2001, 71, 9–15.
- Sahaf, B. Z.; Moharramipour, S.; Meshkatalsadat, M. H. Fumigant Toxicity of Essential Oil from Vitex Pseudo-Negundo against Tribolium Castaneum (Herbst) and Sitophilus Oryzae (L). J. Asia Pac. Entomol. 2008, 11, 175–179.
- Nerio, L. S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378.
- Brahmi, F.; Abdenour, A.; Brunoc, M.; Silviac, P.; Alessandrac, P.; Daniloc, F.; Drifa, Y.-G.; Fahmi, E. M.; Khodir, M.; Mohamed, C. Chemical Composition and in Vitro Antimicrobial, Insecticidal and Antioxidant Activities of the Essential Oils of Mentha Pulegium L. And Mentha Rotundifolia (L.) Huds Growing in Algeria. Ind. Crops Prod. 2016, 88, 96–105.
- Olivero-Verbel, J.; Nerio, L. S.; Stashenko, E. E. Bioactivity against Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon Citratus and Eucalyptus Citriodora Essential Oils Grown in Colombia. Pest Manag. Sci. 2010, 6 (6), 664–668.