ABSTRACT
In this study, investigations of hydro-distilled essential oils of Tamarix dioica flowers and leaves performed by gas chromatography-mass spectrometry (GC-MS) revealed 31 volatile constituents. Nearly 24 (comprising 77.15% of the total volatiles) and 14 compounds (encompassing 63.15% of the total volatiles) were identified in flowers and leaves, respectively. Major constituents in flower were 1-hexadecene (6.93%), hexahydrofarnesyl acetone (5.64%), octadecane (5.60%), dodecanoic acid (5.22%), E-15-heptadecenal (4.98%), docosane (4.76%), 2-methoxy-4-vinylphenol (4.44%), 1-tetradecene (4.29%), tetracosane (4.00%), 1-docosene (3.89%), hexadecane (3.76%), nonanal (3.69%), nonanoic acid (1.33%), dihydroactindiolide (3.33%), and cyclotetracosane (3.04%), whereas the major constituents in leaves were 2-methoxy-4-vinylphenol (17.70%), dihydroactindiolide (10.27%), megastigmatrienone (5.53%), 1-hexadecene (5.10%), β-lonone (4.28%), safranal (3.35%), vitispirane (3.35%), trans-geranylacetone (2.72%), and hexahydrofarnesyl acetone (2.03%). These essential oils were screened in vitro against gram negative (G−ve) bacteria Escherichia coli (E. coli) and gram positive (G+ve) bacteria Staphylococcus aureus (S. aureus) by using disc diffusion method to determine minimum inhibitory concentration (MIC). However, it is noteworthy that essential oils from Tamarix dioica flower and leaves showed a higher activity against E. coli (MIC at 10 μg/mL) compared to S. aureus (MIC at 100 μg/mL).
Introduction
Essential oils are aromatic and volatile liquid that are expressed by a strong and pleasant odor and flavor. These are complex mixtures low molecular weight compounds that mostly comprise hydrocarbons, monoterpenes, and sesquiterpenes. Additionally, oxygenated compounds include aldehydes, ketones, ethers, alcohols, phenols, and esters derived from these hydrocarbons. Essential oils are significant products of the agricultural industry and have been utilized as flavoring agents in food products, perfumeries, pharmaceuticals, drinks, and cosmetics industries.[Citation1–Citation6] Plants essential oils have a broad range of biological activities, particularly antimicrobial effects and are usually used to treat different diseases in folk medicine.[Citation7,Citation8]
Recently, essential oils from plant sources have increased their consideration in the invention of perfumes, fragrance lotions, aromatic soaps, cosmetics, and pharmaceuticals.[Citation5] The beneficial pharmacological properties of essential oils and their main components, terpenes, have been well-known as powerful potent sedatives, antipyretics, expectorants, anxiolytics, fungal, and bacterial inhibitors.[Citation9] Among all natural compounds, essential oils exhibit biological activities exposed and gain specific attention because of their radical scavenging properties.[Citation10] Essential oils have medicinal uses in human medicines because of their antiphlogistic, anticancer, antiviral, antinociceptive, antioxidant, and antibacterial properties.[Citation11,Citation12] The essential oils, as well as its volatile components, can be used by inhalation or massage therapy. Essential oils can be beneficial in treating people suffering from dementia. Its use can develop food safety and protect our body against bacteria that contain food poisoning.[Citation4] Essential oils have the capacity to prevent the respiration process plus ion circulation and hence the destruction of the bacterial cell.[Citation13] Essential oils have shown fumigant properties.[Citation14] In addition, essential oils have a short existence in the plant, they degrade easily in the environment and many of them have insufficient negative disadvantages.[Citation15]
The genus Tamarix composed of about 60 species of flowering plants in the family Tamaricaceae. T. dioica species is one of them having binomial name Tamarix dioica. Roxb. ex Roth is an evergreen shrub or small tree growing from 1 to 18 m high. It has vaginate leaves reddish bark and purple flowers. The genus Tamarix feeds on more than 250 species of invertebrates and camels and cattle.[Citation16] Although Tamarix contains the size of a small tree, which has a deep taproot that can extend 30 m or more below the ground. The tree is originally from Pakistan, Afghanistan, Iran, India, Bangladesh, Bhutan, Kashmir, Nepal, and Myanmar. In Pakistan, it is found in abundance in the Khyber Pakhtun Khwa (KPK) and Sindh provinces.[Citation17]
T. dioica leaves are used as a diuretic, carminative and cure liver infection and splenic or inflammation. In addition, this plant is used as an astringent for indications such as vaginal discharge.[Citation16] Tamarix dioica has shown antifungal activity against three microbes, Trichophyton rubrum, Candida glabrata, and Aspergillus fumigatus, and antibacterial activity against Pseudomonas aeruginosa and Klebsiella pneumoniae.[Citation2,Citation18] The phytochemical preliminary screening of T. dioica revealed that almost all parts of the plant contain steroids and phlobatannins. Leaves, flowers, and roots contain tannins, phenols, and flavonoids; while the stem, flowers, and leaves contain saponins and terpenoids.[Citation2]
A bibliographical research shows that negligible reports are found on T. dioica flowers and leaves essential oils and its antibacterial activity. For this reason, the purpose of this study was to explore the chemical composition of the essential oils of Tamarix dioica and the determination of its antibacterial activity.
Materials and methods
Collection and identification of plant materials
T. dioica flowers and leaves (800 g each) were collected from District Jamshoro (longitude: N 25.430400 and latitude: E 68.280900), Sindh, Pakistan in August 2016 and identified by a Taxonomist, Institute of Plant Sciences, University of Sindh, Jamshoro, Pakistan. A voucher specimen of the species was deposited in the herbarium of the same Institute under the acquisition numbers 2671317.
Essential oils isolation
Dried and powdered T. dioica flowers and leaves (three samples of 100 g each) were subjected to hydro-distillation using a Clevenger-type apparatus for 3 h in triplicate. The obtained oils were separated using n-Hexane, dried over anhydrous magnesium sulfate, and immediately analyzed by GC-MS.
Analyses of the essential oils
Composition of T. dioica flowers and leaves essential oils was investigated by gas chromatography-mass spectrometry (GC-MS). The GC-MS analysis was carried out using Agilent 6890 N GC instrument joined with inert XL mass selective detector MS-5975. The 7683-B auto sampler injector was used. The HP-5MS capillary column of 30 m × 0.25 mm i.d., 0.25 µm film thickness was used. The oven temperature was kept at 90°C for 1 min, raised to 200°C at 5°C/min (6 min hold) and then to 290°C at 20°C (10 min hold). 1 µL HPLC grade n-hexane sample was injected by using splitless mode. And for the MS detection the electron ionization (EI) mode at 70 eV was used. At a flow rate of 1.5 mL/min the helium gas was used as a carrier gas. The injector and MS transfer line temperatures were held at 220 and 290°C, respectively. To calculate retention indices solution of standard alkanes (C8–C32) was analyzed using same conditions.
Identification of components
The individual chemical constituents of T. dioica flowers and leaves were identified by comparing their RI, HP-5MS (retention indices) with those reported in the literature (with the help of Kovats method, retention indices of all the components were determined by using standard (C8–C32) n-alkanes) and NIST (National Institute of Standards and Technology) mass spectral library with a resemblance percentage above 90%. The percentage composition of the oils was computed from peak areas of GC (gas chromatography).
Evaluation of in vitro antibacterial activity
The antibacterial activity of T. dioica flower and leaves essential oils was tested against two bacterial strains: S. aureus and E. coli using the disc diffusion method on Muller Hinton Medium (MHA). The modified disc diffusion method was preferred to check the antibacterial activity of both parts.[Citation3] With the help of the American Type Culture Collection (ATCC), antibacterial activity was studied against two different microorganisms. MHA was used for the growth of microorganism species.[Citation19]
Four successive concentrations of 1000, 500, 100, and 10 μg/mL were prepared in 100% DMSO (dimethylsulfoxide) to check the antibacterial activity of flowers and leaves essential oils. Whereas, DMSO was used as the negative control.[Citation13] The bacterial suspensions were adjusted to 106 CFU/mL and spread on the solid Petri dishes with a sterile cotton swab moistened with the bacterial suspension.
Subsequently, a soaked Whatman No. 1 (5 mm in diameter) filter paper with 15 μL of essential oils at different concentrations (Diluted in 100% DMSO) was placed on the surface of the microbial Petri dishes (size 90 mm). They were placed in an incubator at 37°C for 24 hours. At the end of the incubation period, the antibacterial activity of essential oils of flowers and leaves was recorded against each microbial species by measuring the radius of inhibition diameter in mm (millimeter) around the discs[Citation20] and calculated MIC values. All assays were performed in triplicate and results are expressed as mean ± standard deviation (SD).
Results and discussion
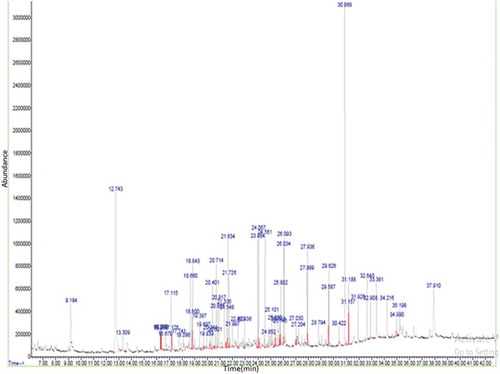
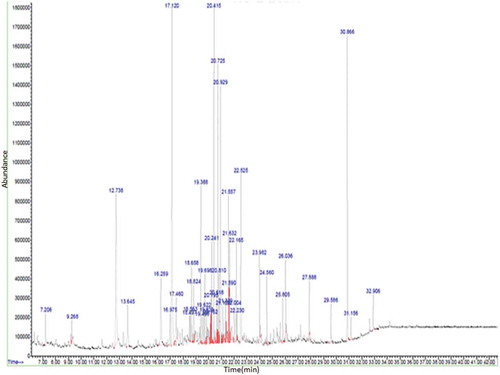
In this study, hydro-distillation method was employed for the isolation of essential oils from the T. dioica flowers and leaves. This method is one of the famous, cost-efficient and a regularly adopted method used world over to isolate volatile compounds.[Citation21] Hydro-distillation of flower and leaves yielded 0.1% and 0.07% (dry weight) of essential oils, respectively. In total, nearly 24 (comprising 77.15% of the total volatiles) were identified in flower, whereas, 14 compounds (encompassing 63.15% of the total volatiles) were identified in the leaves by GC-MS. Results are reported in , and according to their elution order on the HP-5MS column.
Table 1. Chemical composition of essential oil from T. dioica flowers and leaves.
The major constituents of the flowers oil are 1-hexadecene (6.93%), hexahydrofarnesyl acetone (5.64%), octadecane (5.60%), dodecanoic acid (5.22%), and E-15-heptadecenal (4.98%). While the major components of the leaves oil are 2-methoxy-4-vinylphenol (17.70%), dihydroactindiolide (10.27%), megastigmatrienone (5.53%), and 1-hexadecene (5.10%).
T. dioica flowers and leaves chemical constituents are composed of a complex mixture of various substances and are presented in . Several classes of components were identified including terpenoids (monoterpenes, oxygenated mono-terpenes, and compounds related to terpenes), acids, esters, phenolic compounds, hydrocarbons, ketones, and others. It is observed that the main chemical constituents are hydrocarbons in flowers (51.54%) but less concentration in leaves (8.36%) has been found. Whereas, the main chemical constituents are phenolic compounds in leaves (17.70%) however, low concentration in flowers (5.62%) has been found. The monoterpenes, oxygenated monoterpenes, and terpene-related compounds were absent in flowers but present in leaves, representing 3.35%, 5.19%, and 6.46% with the number of compounds 1, 2, and 2 identified, respectively. Esters and acids were absent in leaves and present in flowers, representing 1.36% and 6.55% with the number of 1 and 2 compounds identified, respectively. Ketones were found in the two parts of T. dioica; in leaves 16.55% and flowers 12.08% with the same number of constituents identified.
shows that there are some similarities between the chemical composition of flower constituents and leaves of T. dioica. The results demonstrate that 2-methoxy-4-vinylphenol, trans-geranylacetone, dihydroactiindolide, 1-hexadecene, hexahydrofarnesyl acetone, 1-docosene and cyclotetracosane are similar chemical constituents of flowers and leaves essential oils. The data shown in expressed that leaves and flower oil had variable antimicrobial activity against the strains tested. The flowers essential oils exhibited a maximum zone of inhibition 16, 11, 8, and 3 mm in diameter, respectively at the concentrations 1000, 500, 100, and 10 μg/mL, whereas against S. aureus flower essential oils exhibit the 12, 7, and 3 mm in diameter inhibition zone at concentrations 1000, 500, and 100 μg/mL, respectively; but show no zone of inhibition at 10 μg/mL concentration. Whereas, antibacterial activity against E. coli leaves essential oils had a maximum inhibition zone of 14, 11, 6, and 2 mm in diameter, respectively at concentrations of 1000, 500, 100, and 10 μg/mL, whereas against S. aureus leaves essential oils have shown inhibition zone 10, 5, and 2 mm in diameter at concentrations 1000, 500, and 100 μg/mL, respectively; but no zone of inhibition at 10 μg/mL concentration.
Table 2. Inhibition zones (mm in diameter) for antibacterial activities of T. dioica flowers and leaves essential oils.
Essential oils of flowers and leaves are good antibacterial agents against bacteria including E. coli and S. aureus as shown in . However, it is noteworthy that the essential oils of flowers and leaves show higher activity against E. coli (MIC at 10 μg/mL) compared to S. aureus (MIC at 100 μg/mL), while control DMSO showed no antibacterial activity against E. coli (G-ve) and S. aureus (G+ve). The antibacterial activity of T. dioica flowers and leaves essential oils was carried out by inhibiting growth against two strains of bacteria and activity was presented according to the four criteria. In this criterion, the inhibition diameter zone was considered as 12–16 mm in diameter (very high activity), 7–12 mm in diameter (high activity), 2–7 mm in diameter (relatively high activity), and less than 2 mm in diameter (no activity), respectively.
At 500 μg/mL of flower essential oils show high activity against E. coli and S. aureus; while leaves essential oils show relatively high against S. aureus and E. coli. However, flower essential oils and leaves essential oils show relatively high activity against E. coli and show no activity against S. aureus at the concentration of 10 μg/mL. The results show that T. dioica flowers and leaves essential oils are more efficient to reduce colonies of bacteria of E. coli compared to bacteria of S. aureus. On the other hand, flower and leaves essential oils are more effective against E. coli (MIC at 10 μg/mL) compared to S. aureus (MIC at 100 μg/mL). The control DMSO does not exhibit antibacterial activity against both bacteria E. coli (Gram negative) and S. aureus (Gram positive).
MIC was the lowest oil concentration which completely inhibited bacterial growth, causing the visualization of an inhibition zone.[Citation22,Citation23] In vitro different concentrations of essential oils from flowers and leaves such as 1000, 500, 100, and 10 μg/mL were prepared against two strains of bacteria. It is also shown in that flower essential oils were observed to be highly effective in action against E. coli with an MIC of 10 μg/mL compared to S. aureus with an MIC of 100 μg/mL. Correspondingly, essential oils from leaves were also observed to be highly effective against E. coli with an MIC of 10 μg/mL compared to S. aureus with a MIC of 100 μg/mL. Thus, it was found that T. dioica flowers and leaves essential oils were more effective against E. coli with a MIC value 10 μg/mL compared to S. aureus with an MIC value of 100 μg/mL.
Previous studies on of Tamarix boveana volatile oil revealed that, total 62 components were identified. Hexadecanoic acid (18.14%), docosane (13.34%), germacrene D (7.68%), fenchyl acetate (7.34%), benzyl benzoate (4.11%) were found to be the major components in the whole aerial parts.[Citation24] Comparing the obtained data from T. dioica () with those previously reported on T. boveana shows that nonanoic acid, dodecanoic acid, heptadecane, nonadecane, farnesyl acetone, heneicosane, 1-docosene, docosane, tetracosane, and pentacosane are common essential oils constituents.
Conclusion
Essential oils profiles of T. dioica flower and leaves are reported in this study. Overall, results demonstrated total compounds in flower to be higher than the leaves. Further, flowers and leaves showed that it was predominantly composed of hydrocarbon (51.54%) and phenolic compounds (17.70%), respectively. Besides this, present study also represents a contribution to the use of T. dioica as a source of antibacterial agent which might be presence of essential oils. Further investigation of compound isolation as well as their in vivo studies should be examined.
Acknowledgment
We are highly thankful to Dr. M. A. Kazi Institute of Chemistry, University of Sindh, Jamshoro and National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro for financial support as well as required facilities.
References
- Mehdizadeh, L.; Ghasemi Pirbalouti, A.; Moghaddam, M. Storage Stability of Essential Oil of Cumin (Cuminum cyminum L.) As a Function of Temperature. Int. J. Food Prop. 2017. DOI: 10.1080/10942912.2017.1354018.
- Samejo, M. Q.; Memon, S.; Bhanger, M. I.; Khan, K. M. Essential Oil Constituents in Fruit and Stem of Calligonum Polygonoides. Indust. Crops Prod. 2013, 45, 293–295. DOI: 10.1016/j.indcrop.2013.01.001.
- Rahman, M. M.; Sultana, T.; Ali, M. Y.; Rahman, M. M.; Al-Reza, S. M.; Rahman, A. Chemical Composition and Antibacterial Activity of the Essential Oil and Various Extracts from Cassia sophera L. Against Bacillus Sp. From Soil. Arabian J. Chem. 2013. DOI: 10.1016/j.arabjc.2013.07.045.
- Rahman, A.; Shanta, Z. S.; Rashid, M. A.; Parvin, T.; Afrin, S.; Khatun, M. K.; Sattar, M. A. In Vitro Antibacterial Properties of Essential Oil and Organic Extracts of Premna integrifolia Linn. Arabian J. Chemistry. 2011. DOI: 10.10.1016/j.arabjc.2011.06.003.
- Adeosun, C. B.; Olaseinde, S.; Opeifa, A. O.; Atolani, O. Essential Oil from the Stem Bark of Cordia sebestena Scavenges Free Radicals. J. Acute Med. 2013, 3, 138–141. DOI: 10.1016/j.jacme.2013.07.002.
- Gao, X.; Lv, S.; Wu, Y.; Li, J.; Zhang, W.; Meng, W.; Wang, C.; Meng, Q. Volatile Components of Essential Oils Extracted from Pu-Erh Ripe Tea by Different Extraction Methods. Int. J. Food Prop. 2017, 20, S240–S253. DOI: 10.1080/10942912.2017.1295256.
- Kazemi, M.;. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int. J. Food Properties. 2015, 18, 1784–1792. DOI: 10.1080/10942912.2014.939660.
- Edris, A. E.;. Pharmaceutical and Therapeutic Potentials of Essentials Oils and Their Individual Volatile Constituents: A Review. Phytother Res. 2007, 21, 308–323. DOI: 10.1002/ptr.2072.
- Kacem, N.; Roumy, V.; Duhal, N.; Merouane, F.; Neut, C.; Christen, P.; Hostettmann, K.; Rhouati, S. Chemical Composition of the Essential Oil from Algerian Genista quadriflora Munby and Determination of Its Antibacterial and Antifungal Activities. Indust. Crops Prod. 2016, 90, 87–93. DOI: 10.1016/j.indcrop.2016.06.016.
- Yildiz, H.;. Chemical Composition, Antimicrobial, and Antioxidant Activities of Essential Oil and Ethanol Extract of Coriandrum sativum L. Leaves from Turkey. International. J. Food Prop. 2016, 19, 1593–1603. DOI: 10.1080/10942912.2015.1092161.
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N. R.; Nogueira, J. M.; Saraiva, J. A.; Nunes, M. L. Chemical Composition and Antibacterial and Antioxidant Properties of Commercial Essential Oils. Indust. Crops and Prod. 2013, 43, 587–595. DOI: 10.1016/j.indcrop.2012.07.069.
- Ghani, A.; Akramian, M.; Hassanzadeh-Khayyat, M.; Joharchi, M. R. The Essential Oil Analysis of Pseudohandelia umbellifera (Boiss.) Tzvel. Growing in Iran. J. Essen. Oil Bear. Plants. 2010, 13, 568–574. DOI: 10.1080/0972060X.2010.10643864.
- Bouchekrit, M.; Laouer, H.; Hajji, M.; Nasri, M.; Haroutounian, S. A.; Akkal, S. Essential Oils from Elaeoselinum asclepium: Chemical Composition, Antimicrobial and Antioxidant Properties. Asian Pacific J. Trop. Biomed. 2016, 6, 851–857. DOI: 10.1016/j.apjtb.2016.07.014.
- Brahmi, F.; Abdenour, A.; Bruno, M.; Silvia, P.; Alessandra, P.; Danilo, F.; Drifa, Y. G.; Fahmi, E. M.; Khodir, M.; Mohamed, C. Chemical Composition and in Vitro Antimicrobial, Insecticidal and Antioxidant Activities of the Essential Oils of Mentha pulegium L. And Mentha rotundifolia (L.) Huds Growing in Algeria. Indust. Crops Prod. 2016, 88, 96–105. DOI: 10.1016/j.indcrop.2016.03.002.
- Karimi, K.; Ahari, A. B.; Weisany, W.; Pertot, I.; Vrhovsek, U.; Arzanlou, M. Funneliformis Mosseae Root Colonization Affects Anethum Graveolens Essential Oil Composition and Its Efficacy against Colletotrichum nymphaeae. Indust. Crops Prod. 2016, 90, 126–134. DOI: 10.1016/j.indcrop.2016.06.024.
- Baum, B. R.;. Introduced and Naturalized Tamarisks in the United States and Canada. Baileya. 1967, 15, 19–25.
- Khan, S.; Khan, G. M.; Mehsud, S.; Rahman, A.; Khan, F. Antifungal Activity of Tamarix dioica-An in Vitro Study. Gomal J. Med. Sci. 2004, 2, 40–42.
- Khan, S.; Ullah, F.; Mahmood, T. In Vitro Antimicrobial and Cytotoxic Activity of Tamarix dioica Roxb. Leaves. Turkish J. Bio. 2013, 37, 329–335.
- Rawat, S.; Saini, R.; Sharma, A. Phytochemical Study and Antimicrobial Activities of Cordia dichotoma. Int. Res. J. Phar. 2013, 4(12), 53–56. DOI: 10.7897/2230-8407.041212.
- Pavithra, P. S.; Sreevidya, N.; Verma, R. S. Antibacterial Activity and Chemical Composition of Essential Oil of Pamburus missionis. J. Ethnopharma. 2009, 124, 151–153. DOI: 10.1016/j.jep.2009.04.016.
- Rajan, N. S.; Bhat, R. Volatile Constituents of Unripe and Ripe Kundang Fruits (Bouea macrophylla Griffith). Int. J. Food Prop. 2017, 20, 1751–1760. DOI: 10.1080/10942912.2016.1218892.
- Costa, J. G.; Brito, S. A.; Nascimento, E. M.; Botelho, M. A.; Rodrigues, F. F.; Coutinho, H. D. Antibacterial Properties of Pequi Pulp Oil (Caryocar coriaceum–WITTM.). Int. J. Food Prop. 2011, 14, 411–416. DOI: 10.1080/10942910903207744.
- Coutinho, H. D.; Costa, J. G.; Lima, E. O.; Siqueira-Júnior, J. P. Anti-Staphylococcal Activity of Eugenia Jambolana L. Against Methicillin–Resistant Staphylococcus aureus. International. J. Food Prop. 2010, 13, 1405–1410. DOI: 10.1080/10942910903108488.
- Saidana, D.; Mahjoub, M. A.; Boussaada, O.; Chriaa, J.; Chéraif, I.; Daami, M.; Mighri, Z.; Helal, A. N. Chemical Composition and Antimicrobial Activity of Volatile Compounds of Tamarix boveana (Tamaricaceae). Microbio. Res. 2008, 163, 445–455. DOI: 10.1016/j.micres.2006.07.009.