ABSTRACT
A long ripening period is essential for the production of dry-cured ham. This ripening process results in the generation of a large number of small peptides that are responsible for the ham’s flavour. In this study, peptides extracted from Jinhua ham were separated and purified using a tangential flow filter and Sephadex G-25 gel filtration chromatography and identified using liquid chromatography-tandem mass spectrometry. Sixty-three peptides were identified within the range between 227.1 and 1540.8 Da, including 10 dipeptides, 14 tripeptides, 12 tetrapeptides, eight pentapeptides, eight hexapeptides, four heptapeptides, one octopeptide, three nonapeptides, one decapeptide, one dodecapeptide, and one tridecapeptide. The most abundant peptides (and their relative peak areas) were Leu-Pro-Lys (10.49%), Ser-Gly-Leu (8.72%), Ala-Ala-Pro (7.36%), Val-Glu (5.62%), Pro-Leu (3.83%), Ser-Gly-Val (3.77%), Ala-His (3.40%), Ala-Arg (3.37%), and Leu-His-Ala (3.03%). The peptide sequence results provide novel information that contributes to a better understanding of proteolysis and fundamental peptide characterizations.
Abbreviations: L, Leu; P, Pro; K, Lys; S, Ser; G, Gly; L, Lys; A, Ala; V, Val; E, Glu; H, His; R, Arg; T, Thr; C, Cys; I, Ile; N, Asn; D, Asp; Q, Gln; Y, Tyr; M, Met; W, Trp; F, Phe; DPP, dipeptidyl peptidase; TPP, tripeptidyl peptidase; LAP, Leu aminopeptidase; AAP, Ala aminopeptidase; RAP, Arg aminopeptidase; TFF, tangential flow filter
Introduction
Jinhua ham is the most famous traditional Chinese dry-cured meat product. It has a characteristic flavour, colour, taste, and appearance. During its processing, many biochemical reactions occur, including lipid oxidation, Strecker degradation, and Maillard reactions.[Citation1,Citation2] One of the main biochemical changes was the degradation of protein fractions.[Citation3] The cathepsin and calpain endopeptidases played crucial roles in muscle proteolysis during dry-cured ham processing.[Citation4] High molecular weight proteins could be degraded into low molecular weight proteins, polypeptides, and free amino acids. Polypeptides might be further degraded by peptidyl peptidases, aminopeptidases, and carboxypeptidases, leading to the generation of smaller peptides and free amino acids.[Citation5,Citation6]
Specific combinations of peptides and free amino acids might make a major contribution to the characteristic taste of Jinhua ham and provide substrates for the generation of typical volatile compounds.[Citation7] Italian Parma, San Daniele, and Toscano dry-cured hams, which were produced using different processing methods, exhibited significant differences in their sensory characteristics.[Citation8] Studies of the functional peptides generated during dry-cured ham processing worldwide have isolated and identified peptides that showed high angiotensin-converting enzyme inhibitory activity and antioxidant activity from Spanish Teruel ham, Xuanwei ham, and Jinhua dry-cured ham.[Citation9,Citation10] These studies have shown that the peptide profile of each type of dry-cured ham plays a key role in determining both its nutritional and sensory qualities.
Studies of the peptides generated in European dry-cured hams have used proteomic analysis to investigate the proteolysis that occurs during processing and to identify the peptides produced. The activities of the peptidyl peptidases, aminopeptidases, and carboxypeptidases responsible for proteolysis have also been well studied.[Citation11–Citation14] However, proteomics is not able to identify the processing sites of precursor proteins. Efforts were made during the last decade, and some peptide sequences have been described.[Citation15–Citation19] Peptide sequences derived from myofibrillar proteins and sarcoplasmic proteins have been identified in dry-cured hams.[Citation12,Citation14,Citation20] Peptidomic characterization of proteins was recently suggested for the study of peptides such as titin and ubiquitin, and the proteins in dry-cured ham processing have been evaluated.[Citation21,Citation22] Size-exclusion chromatography has been frequently used as an initial step for the analysis of dry-cured ham peptides,[Citation16] which were fractionated according to their molecular size. Most peptides that accumulated in dry-cured ham were small peptides below 1200 Da, mainly dipeptides. The effects of peptides on the nutritional and sensory qualities of dry-cured ham were very complex because of their different chain lengths as well as the composition and sequences of their amino acids.[Citation23,Citation24] One study reported that fractions eluted from 200 mL in size-exclusion chromatography corresponded to peptides smaller than 2500 Da.[Citation25] The peptides in the water-soluble extract proved to have a wide range of flavours, from bitterness to more desirable savoury flavours.
Knowledge of the peptide profile in dry-cured ham could be very useful for understanding the processing effects and thus developing strategies for better control right from the stage of pig genetics. The objective of this study was to separate, purify, and identify low molecular weight peptides generated in Jinhua ham and then determine the relationship between peptides and peptidases during the processing of the ham, to increase understanding of the influence of specific sequences on flavour development. Knowledge of the specific peptide sequences will help clarify their functional effects in dry-cured ham. The different specific peptide sequences that result from proteolysis in the different dry-cured ham products could then be used as an authentication tool to enable purchasers to detect possible fraud in the production and sale of dry-cured hams.
Materials and methods
Jinhua ham processing and sampling
Jinhua hams were processed according to traditional methods by Zhejiang Provincial Food Company, PR China. The process began in winter and finished in the following autumn (eight months in total). The processing consisted of six stages: green ham preparation, salting, washing, sun-drying and shaping, ripening, and post-ripening. At the end of post-ripening, five hams were chosen at random as samples. The entire biceps femoris was taken from each ham for analysis. Samples were labelled, packed, and stored at −40°C.
Extraction
The peptide crude extracts were prepared according to the method described by Escudero et al. [Citation26] with slight modifications. Samples were thawed at 4°C, trimmed off visible fat and connective tissue, and minced. A 20 g portion of minced sample was homogenized in 100 mL of 0.2 M phosphate buffer (PBS, pH 7.2) using an IKA T18 basic disperser (Staufen, Germany) (three processes of 10 s each at 22,000 rpm with cooling on ice). The homogenate was held at 4°C for 12 h and then centrifuged at 12,500 g for 20 min at 4°C. The supernatant was filtered through glass wool, and then three volumes of ethanol were added to the filtrate. The mixture was kept for 12 h at 4°C and then centrifuged at 12,500 g for 15 min at 4°C for de-proteinizing. The final supernatant was then dried in a vacuum freeze dryer (LYOVAC GT2, SRK, Germany) for 48 h and stored at −20°C.
Separation
A 2 g sample of extract powder was dissolved in 50 mL of deionized water and filtered through a 0.45 μm membrane (Millipore, Bedford, MA, USA). The filtrate was separated using a TFF system equipped with an ultrafiltration membrane with a molecular weight cut-off (MWCO) of 5,000 Da. The flow rate and pressure were 3.5 mL/min/cm2 and 2.5 × 105 Pa, respectively. Fractions passed through the 5,000 MWCO membrane were recovered and dried in a vacuum freeze dryer for 48 h and stored at −20°C.
A 1 g sample of the powder passed through the 5,000 MWCO membrane was dissolved in 10 mL of deionized water, and a 5 mL aliquot of the fraction was filtered through a 0.45 μm membrane (Millipore, Bedford, MA, USA). The sample was subjected to size-exclusion chromatography with a column packed with Sephadex G-25 (Sigma Aldrich, St. Louis, MO, USA) to fractionate the peptides according to their different molecular masses. Separation was performed at a constant flow rate of 30 mL/h eluted with deionized water at room temperature. Fractions were monitored at 214 nm using an ultraviolet detector (HD-1, HUXI, Shanghai, China), and fractions were collected every 2 min using an automatic fraction collector (HD-1, HUXI, Shanghai, China). Fractions in the same peak were pooled and dried in a vacuum freeze dryer for further separation.
Identification
A 10 μL aliquot of the redissolved fraction was injected into a high-performance liquid chromatography tandem mass spectrometry system. Peptides were separated using high-performance liquid chromatography (ACQUITY UPLC, Waters, Milford, MA, USA) with a BEH C18 column (100 × 2.1 mm; 1.7 µm particle size, Waters) and a photodiode array detector (ACQUITY PDA, Waters, Milford, MA, USA) with a range of 200–700 nm at 45°C. The eluents were solvent A, containing 0.9% trifluoroacetic acid in water, and solvent B, containing 0.1% trifluoroacetic acid in water/acetonitrile (10:90). Both eluents were filtered using a 0.45 μm filter and degassed before analysis. Peptides were eluted at a flow rate of 0.3 mL/min, using a 5 min isocratic gradient with solvent A, followed by a linear gradient from 0 to 60% of solvent B within 40 min. The elution peaks were monitored using the photodiode array detector at a wavelength of 214 nm.
Tandem mass spectrometry (MALDI SYNAPT Q-TOF MS, Waters, Milford, MA, USA) was used with multiple reactions measuring (MRM). Matrix-assisted laser desorption ionization positive ion pattern (MALDI) was used for ionization. The MS scanning range was from 200 to 4000 Da. Nitrogen was used as the collision gas. The capillary voltage was set at 3.5 kV, and the cone voltage was 20 V. The source block temperature was set at 100°C, and the desolvation temperature was 250°C. The collision energy and detector voltage were set at 15 V and 1600 V, respectively. The desolvation gas flow and cone gas flow were 500 L/h and 50 L/h, respectively.
Data analysis
Information on the polypeptides was inputted to the Mass Lynx 4.1 (Waters, USA) data processing system to obtain accurate molecular masses and amino acid sequences. The molecular masses of the peptides purified by HPLC were determined by mass spectrometry, and sequences were determined according to the secondary ion mass spectra of the singly charged ions ([M + H]+) performed with the same system. Because many different amino acid combinations can correspond to a given mass, the matched peptides required further manual verification. Peptides with theoretical molecular masses that agreed well with the detected molecular masses were identified as the objective peptides.
Results and discussion
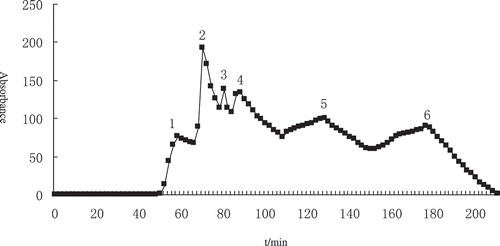
Peptide extracts were fractionated by size-exclusion chromatography (). Fractions corresponding to the eluted peak (a total of six fractions) were obtained based on their variations in molecular weight, and labelled as 1 to 6. The retention time of the first four peaks was narrow, from 50 to 100 min, indicating that the molecular masses of the peptides in these fractions were similar, so they were pooled before drying in a vacuum freeze dryer and were labelled “a”. Fractions 5 and 6 were also dried and were labelled “b” and “c,” respectively.
Figure 1. Sephadex G-25 gel chromatographic profile of Jinhua ham. a, mixture peaks of 1–4; b, peak 5; c, peak 6 of gel chromatographic profiles.
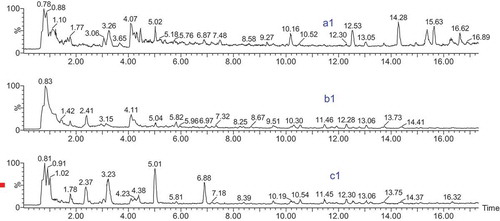
The fractions went through further purification using HPLC, and the peaks that exhibited absorbance at 214 nm were detected. Peptide profiles of fractions a, b, and c, named “a1”, “b1”, and “c1” after HPLC, respectively, are shown in . There were about 11 major peaks of fraction a, 12 of fraction b, and 13 of fraction c. The retention time and hydrophobic character revealed by the chromatography analysis indicated that they were probably small peptides. A large number of peptide peaks were observed in a1, b1, and c1, indicating that a large number of peptides was present in these three fractions.
Figure 2. HPLC elution profiles of Sephadex G-25 gel chromatography. a1, b1, and c1 are in accordance with the HPLC elution profiles a, b, and c, respectively.
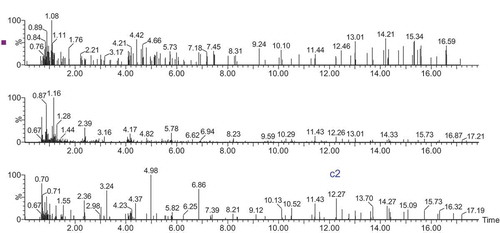
Total ion chromatograms for fractions a1, b1, and c1 are shown in . Fractions named a2, b2, and c2 corresponded to fractions a1, b1, and c1, respectively (). Combined with the fragment information of profiles a2, b2, and c2, precise masses of the peptides were determined. The amino acid compositions of the separated peptides were identified using secondary ion mass spectrometry.
Figure 3. Total ion chromatograms of MS/MS. a2, b2, and c2 are in accordance with the MS profiles a1, b1, and c1, respectively.
lists the identified peptides along with their expected mass, repeatability, and relative peak area expressed as a percentage of total area. Sixty-three peptides were identified between 227.1 Da and 1540.8 Da, including 10 dipeptides, 14 tripeptides, 12 tetrapeptides, eight pentapeptides, eight hexapeptides, four heptapeptides, one octopeptide, three nonapeptides, one decapeptide, one dodecapeptide, and one tridecapeptide. This result is in accordance with the previous results reported for European dry-cured hams.[Citation27] Research on European dry-cured hams has shown that most peptides that accumulate in ham products were small. Five size ranges of peptides extracted from traditional Spanish dry-cured ham at different processing times were identified using HPLC, and a clear increase in peptides below 2700 Da was observed.[Citation24] The proteolysis of troponin T in dry-cured ham generated 27 peptides, with masses of 1706.80 Da–3162.76 Da identified using MALDI-TOF/TOF.[Citation12] Small peptides are released from glycolytic enzymes in muscle during dry-cured ham processing, and a total of 45 specific fragments of these enzymes within the range of 510.21–940.26 Da were observed.[Citation14]
Table 1. The important peptides identified by MS/MS.
Different pig genotypes are associated with the generation of different peptides in dry-cured hams. Peptides generated in Spanish Teruel, Italian Parma, and Belgian dry-cured hams have been identified and quantified using a label-free method to assess the main differences in proteolysis between the three types of ham.[Citation27] The main peptides responsible for the differences detected were three types of myosin light chain from Sus scrofa proteins and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), myosin heavy chain-7, and myosin 4 from S. scrofa. Different processing parameters (such as salt content, processing temperature, and curing time) also lead to different peptide characteristics. Spanish Teruel dry-cured ham exhibited more angiotensin-converting enzyme inhibitory activity than Parma and Belgian hams for the same range of eluted volume of peptide. This fact could be due to the longer curing period of Spanish Teruel hams (14 months) in comparison with Italian Parma (12 months) and Belgian (9 months) hams. The effect of proteolytic enzymes on the generation of small peptides and free amino acid is widely known.[Citation21,Citation28] The different types and activities of endogenous muscular enzymes are the main reasons for the different hydrolysis and peptide profiles in dry-cured hams.
In the 10-month processing period of Jinhua ham, muscle proteins were hydrolysed continuously, and small molecular products such as small peptides of molecular weight < 1 kDa and free amino acids were generated.[Citation2] The differences between Jinhua ham and European hams might be attributed to different raw materials, the relatively short processing duration, and the high temperature in the final phase of processing Jinhua ham compared with most European dry-cured hams, which result in relatively strong activities of proteolytic enzymes that hydrolyse soluble proteins, as described next.[Citation1]
Among the 63 identified peptides, the total relative peak areas of dipeptides, tripeptides, tetrapeptides, pentapeptides, hexapeptides, and heptapeptides were 23.59%, 48.28%, 21.08%, 3.72%, 1.96%, and 0.86%, respectively. Thirty peptides had relative peak areas of more than 1.00%, especially LPK, SGL, AAP, VE, PL, SGV, AH, AR, and LHA.
Dipeptidyl peptidases (DPPs) can hydrolyse polypeptides to generate dipeptides. Different kinds of DPP act on different sites of polypeptides and generate different dipeptides. AR and GR were first generated by the effect of DPP I, and GP was produced by DPP II and DPP IV, whereas DPP III generated RR and AR.[Citation29–Citation32] However, other peptides can also be produced by these peptidases at a slower rate. All of these DPPs had a better stabilization and activity even after the fifteenth month of post-ripening of Jinhua dry-cured hams. DPP І remained strongly active throughout the processing of Jinhua ham, whereas the activity of DPP IV was always weak despite its strong activity shown in vitro.[Citation33] This suggests that DPP I might be a key enzyme responsible for the generation of dipeptides during Jinhua ham processing. Dipeptide LK might come from the cleavage of the pentapeptide EEELK or the nonapeptide THAFASELK, and SE might come from the cleavage of the hexapeptide VGTGSE or the nonapeptide FLDVSEAAP. The decomposition of the tetrapeptide LLAH and the heptapeptide PPGSFEL might result in the generation of AH and FE, respectively. In this study, AR, which had relatively higher contents, might be attributed to DPP I and DPP III. Considering this, our result was in accordance with the regular pattern, and these DPPs could be responsible for the observed high content of dipeptides.
Two kinds of tripeptidyl peptidase (TPP I and TPP II) were found that could generate different tripeptides in dry-cured ham by hydrolysing proteins and polypeptides. TPP I can produce GPX, where X could be any amino acid, but the optimal choice would be a hydrophobic amino acid. TPP II can produce many types of tripeptides except for those with P in the terminal position. AAF is generated by TPP II. Considering this, the generation of the tripeptide EEE observed in our study might be the result of TPP hydrolysing peptides EEELK and EEELKVL, and peptide SGV might be attributable to the TPP hydrolysing peptides EPSGV and ESGV. AAP might be derived from LAAP and FLDVSEAAP; LPK and LLP might come from LLLLPKDKKLYPV; and GSF and LHA might come from PPGSFEL and AEPLHAV, respectively.
Aminopeptidases are enzymes that are responsible for the loss of N-terminal amino acids in peptides. Studies have revealed the activity of all aminopeptidases during the processing of dry-cured hams, even at more than 12 months, suggesting that these enzymes might be involved in protein degradation in the later stages of processing.[Citation34,Citation35] Zhao et al.[Citation4,Citation6] reported that LAP, AAP, and RAP were the most important aminopeptidases in Jinhua ham. LAP exhibited greater activity than AAP or RAP during ageing and post-ageing, especially at the later stage of ripening and throughout post-ripening, suggesting that it is the main contributor to the generation of free amino acids during the processing of Jinhua ham. The N-terminal sites of peptides LAAP and VPL are L and V, respectively. This could explain the generation of peptides AAP and PL by the continuous activity of aminopeptidases. It is likely that peptides SGV, LK, and VE were produced by the loss of E from the N-terminal sites of peptides ESGV, ELKS, and EVEE, which suggests that aminopeptidases play an important role in the generation of peptides throughout the curing process. These results are in accordance with those previously reported by Escudero et al.[Citation26] for traditional Spanish dry-cured ham. They reported that aminopeptidase activity was detected even after 8 months of processing, suggesting that these enzymes are still involved in the last period of curing.
Carboxypeptidases are responsible for the hydrolysis of amino acids from the C-terminal site of peptides. This group of enzymes has not been as widely studied in dry-cured ham as have the aminopeptidases.[Citation36] In our study, EEEL, LLAHL, and PPGSFEL had an additional L at the C-terminal site, compared with EEE, LLAH, and FE, and LKE and EVEE had an additional E compared with LK and VE. Moreover, EEELK had an additional K compared with EEEL, and ELKS had an additional S compared with LK. These findings confirmed the activity of carboxypeptidases on the C-terminal of peptides, in accordance with previously reported results.[Citation25] The difference between DSRGNPTVEVDLHTAKG and DSRGNPTVEVDLHTAKGR can be attributed to the action of carboxypeptidases, which are responsible for the loss of C-termini amino acids.
LAP, AAP, RAP, and carboxypeptidases might be the most important contributors to the generation of free amino acids, whereas the generation of dipeptides and tripeptides is mainly attributed to DPP and TPP, respectively. Aminopeptidases, carboxypeptidases, and peptidyl peptidases may play an important role in the generation of peptides in dry-cured ham.
Conclusion
The peptide profile of Jinhua ham has been described. The differences obtained in the relative quantification of different peptide types and in the sequences of peptides are mainly due to the action of endogenous enzymes in dry-cured hams. The peptides identified in our study proved that in the post-ageing stage of dry-curing, many small peptides of MW less than 1600 kDa were generated, including dipeptides, tripeptides, tetrapeptides, pentapeptides, and hexapeptides. Their relative peak areas indicate that dipeptides, tripeptides, and tetrapeptides are the three main peptide types present in the post-ageing period. Further research is under way in our laboratory to determine the quantity and biochemical characteristics of the different peptides to provide a theoretical basis for modernizing the processing of Jinhua ham.
Acknowledgements
The authors gratefully acknowledge the staff of the National Key Laboratory of Food Science and Technology, Jiangnan University, PR China, for their valuable contribution to the UPLC-MALDI-TOF/TOF MS analysis.
Additional information
Funding
References
- Zhao, G. M.; Zhou, G. H. The Properties and Traditional Processing Technology of “Jinhua Ham”. Food Sci. Technol(China) (Zl). 2003. 322–326.
- Zhao, G. M.; Tian, W.; Liu, Y. X.; Zhou, G. H.; Xu, X. L.; Li, M. Y. Proteolysis in Biceps Femoris during Jinhua Ham Processing. Meat Sci. 2008, 79, 39–44. DOI: 10.1016/j.meatsci.2007.07.030.
- Zhou, G. H.; Zhao, G. M. Biochemical Changes during Processing of Traditional Jinhua Ham. Meat Sci. 2007, 77, 114–120. DOI: 10.1016/j.meatsci.2007.03.028.
- Zhao, G. M.; Zhou, G. H.; Wang, Y. L.; Huan, Y. J.; Wu, J. Q. Time-Related Changes in Cathepsin B and L Activities during Processing of Jinhua Ham as a Function of pH, Salt and Temperature. Meat Sci. 2005, 70(2), 381–388. DOI: 10.1016/j.meatsci.2005.02.004.
- Zhao, G. M.; Liu, Y. X.; Guo, S. L.; Li, H.; Zhang, Q. H. Changes of Free Amino Acids in Jinhua Ham during Processing. Food Chem. 2009, 30(21), 152–154.
- Zhao, G. M.; Zhou, G. H.; Liu, Y. X.; Xu, X. L.; Hou, Y. X. Muscle Changes of Non-Protein Nitrogen and Free Amino Acids during Jinhua Ham Processing. Food Chem. 2006, 27(2), 33–37.
- Huan, Y. J.; Zhou, G. H.; Zhao, G. M.; Xu, X. L.; Peng, Z. Q. Changes in Flavor Compounds of Dry-Cured Chinese Jinhua Ham during Processing. Meat Sci. 2005, 71, 291–299. DOI: 10.1016/j.meatsci.2005.03.025.
- Mora, L.; Escudero, E.; Toldrá, F. Characterization of the Peptide Profile in Spanish Teruel, Italian Parma and Belgian Dry-Cured Hams and Its Potential Bioactivity. Food Res. Int. 2016, 89, 638–646. DOI: 10.1016/j.foodres.2016.09.016.
- Zhu, C. Z.; Zhang, W. G.; Zhou, G. H.; Xu, X. L. Identification of Antioxidant Peptides of Jinhua Ham Generated in the Products and through the Simulated Gastrointestinal Digestion System. J. Sci. Food Agric. 2016, 96, 99–108. DOI: 10.1002/jsfa.7065.
- Xing, L.; Hu, Y. Y.; Hu, H. Y.; Ge, Q. F.; Zhou, G. H.; Zhang, W. G. Purification and Identification of Antioxidative Peptides from Dry-Cured Xuanwei Ham. Food Chem. 2016, 194, 951–958. DOI: 10.1016/j.foodchem.2015.08.101.
- Théron, L.; Sayd, T.; Pinguet, J.; Chambon, C.; Robert, N.; Santé-Lhoutellier, V. Proteomic Analysis of Semimembranosus and Biceps Femoris Muscles from Bayonne Dry-Cured Ham. Meat Sci. 2011, 88, 82–90. DOI: 10.1016/j.meatsci.2010.12.006.
- Mora, L.; Sentandeu, M. A.; Toldrá, F. Identification of Small Troponin T Peptides Generated in Dry-Cured Ham. Food Chem. 2010, 123, 691–697. DOI: 10.1016/j.foodchem.2010.05.035.
- Escudero, E.; Mora, L.; Aristory, M. C.; Toldrá, F. Possible Biological Markers of the Time of Processing of Dry-Cured Ham. Meat Sci. 2011, 89, 536–539. DOI: 10.1016/j.meatsci.2011.05.002.
- Mora, L.; Valero, M. L.; Sánchez del Pino, M. M.; Sentandreu, M. A.; Toldrá, F. Small Peptides Released from Muscle Glycolytic Enzymes during Dry-Cured Ham Processing. J. Proteomics. 2011, 74, 442–450. DOI: 10.1016/j.jprot.2010.12.008.
- Bellati, M.; Dazzi, G.; Chizzolini, R.; Palmia, F.; Parolari, G. Physical and Chemical Changes in Proteins during the Maturation of Parma Ham. Viandes Et Produits Carnes. 1985, 6(4), 142–145.
- Larrea, V.; Hernando, I.; Quiles, A.; Lluch, M. A.; Pérez-Munuera, I. Changes in Proteins during Teruel Dry-Cured Ham Processing. Meat Sci. 2006, 74, 586–593. DOI: 10.1016/j.meatsci.2006.05.009.
- Toldrá, F.; Flores, M. The Role of Muscle Proteases and Lipases in Flavor Development during the Processing of Dry-Cured Ham. Crit. Rev. Food Sci. 1998, 38(4), 331–352. DOI: 10.1080/10408699891274237.
- Martín, L.; Antequera, T.; Ventanas, J.; Benítez-Donoso, R.; Córdoba, J. J. Free Amino Acids and Other Non-Volatile Compounds Formed during Processing of Iberian Ham. Meat Sci. 2001, 59, 363–367. DOI: 10.1016/S0309-1740(01)00088-2.
- Petrova, I.; Tolstorebrov, I.; Mora, L.; Toldrá, F.; Eikevik, T. M. Evolution of Proteolytic and Physico-Chemical Characteristics of Norwegian Dry-Cured Ham during Its Processing. Meat Sci. 2016, 121, 243–249. DOI: 10.1016/j.meatsci.2016.06.023.
- Mora, L.; Toldrá, F. Proteomic Identification of Small (B2000 Da) Myoglobin Peptides Generated in Dry-Cured Ham. Food Technol. Biotechnol. 2012, 50(3), 343–349.
- Mora, L.; Gallego, M.; Aristoy, M. C.; Fraser, P. D.; Toldrá, F. Peptides Naturally Generated from Ubiquitin-60S Ribosomal Protein as Potential Biomarkers of Dry-Cured Ham Processing Time. Food Control. 2015, 48, 102–107. DOI: 10.1016/j.foodcont.2013.12.029.
- Gallego, M.; Mora, L.; Aristoy, M. C.; Toldrá, F. The Use of Label-Free Mass Spectrometry for Relative Quantification of Sarcoplasmic Proteins during the Processing of Dry-Cured Ham. Food Chem. 2016, 196, 437–444. DOI: 10.1016/j.foodchem.2015.09.062.
- Hansen-Moller, J.; Hinrichsen, L.; Jacobscn, T. Evaluation of Peptides Generated in Italian-Style Dry-Cured Ham during Processing. J. Agric. Food Chem. 1997, 45(8), 3123–3128. DOI: 10.1021/jf960743x.
- Rodríguez-Nuñez, E.; Aristoy, M. C.; Toldrá, F. Peptide Generation in the Processing of Dry-Cured Ham. Food Chem. 1995, 53, 187–190. DOI: 10.1016/0308-8146(95)90786-7.
- Mora, L.; Escudero, E.; Fraser, P. D.; Aristoy, M. C.; Toldrá, F. Proteomic Identification of Antioxidant Peptides from 400 to 2500 Da Generated in Spanish Dry-Cured Ham Contained in a Size-Exclusion Chromatography Fraction. Food Res Int. 2014, 56, 68–76. DOI: 10.1016/j.foodres.2013.12.001.
- Escudero, E.; Mora, L.; Fraser, P. D.; Aristoy, M. C.; Toldrá, F. Identification of Novel Antioxidant Peptides Generated in Spanish Dry-Cured Ham. Food Chem. 2013, 138, 1282–1288. DOI: 10.1016/j.foodchem.2012.10.133.
- Mora, L.; Calvo, L.; Escudero, E.; Toldrá, F. Differences in Pig Genotypes Influence the Generation of Peptides in Dry-Cured Ham Processing. Food Res. Int. 2016, 86, 74–82. DOI: 10.1016/j.foodres.2016.04.023.
- Mora, L.; Escudero, E.; Arihara, K.; Toldrá, F. Antihypertensive Effect of Peptides Naturally Generated during Iberian Dry-Cured Ham Processing. Food Res. Int. 2015, 78, 71–78. DOI: 10.1016/j.foodres.2015.11.005.
- Sentandreu, M. A.; Toldrá, F. Biochemical Properties of Dipeptidyl Peptidase III Purified from Porcine Skeletal Mucsle. J. Agric. Food Chem. 1998, 46, 3977–3984. DOI: 10.1021/jf980356i.
- Sentandreu, M. A.; Toldrá, F. Purification and Biochemical Properties of Dipeptidyl Peptidase I Purified from Porcine Skeletal Mucsle. J. Agric. Food Chem. 2000, 48, 5014–5022. DOI: 10.1021/jf990892q.
- Sentandreu, M. A.; Toldrá, F. Dipeptidyl Peptidase Activities along the Bradikinin Processing of Serrano Dry-Cured Ham. Europe Food Res. Technol. 2001, 213, 83–87. DOI: 10.1007/s002170100355.
- Sentandreu, M. A.; Toldrá, F. Partial Purification and Characterization of Dipeptidyl Peptidase II from Porcine Skeletal Muscle. Meat Sci. 2001, 57, 93–103. DOI: 10.1016/S0309-1740(00)00082-6.
- Zhao, G. M.; Zhou, G. H.; Xu, X. L.; Peng, Z. Q.; Huan, Y. J.; Jin, Z. M. Studies on Time-Related Changes of Dipeptidyl Peptidase during Processing of Jinhua Ham Using Response Surface Methodology. Meat Sci. 2005, 69(1), 165–174. DOI: 10.1016/j.meatsci.2004.06.018.
- Flores, M.; Marina, M.; Toldrá, F. Purification and Characterization of a Soluble Methionyl Aminopeptidase from Porcine Skeletal Muscle. Meat Sci. 2000, 56, 247–253. DOI: 10.1016/S0309-1740(00)00049-8.
- Toldrá, F.; Aristory, M. C.; Flores, M. Contribution of Muscle Aminopeptidases to Flavor Development in Dry-Cured Ham. Food Res. Int. 2000, 33, 181–185. DOI: 10.1016/S0963-9969(00)00032-6.
- Pshezhetsky, A. V.; Rawling, N. D.; Barrett, A. J.; Woessner, J. F. Lysosomal Carboxypeptidase A. Handbook of Proteomin Enzymes. 2004. 1923–1928.
