ABSTRACT
In this research, various concentrations of Kolkhoung (Pistacia khinjuk) hull oil (KHO), Beneh (Pistacia atlantica) hull oil (BHO), and Sesame (Sesamum indicume L. cv. Dezful) seed oil (1%, 2%, 4%, and 6%) as natural antioxidants were added to refined soybean oil (RSO) and antioxidant effect of these compounds was compared with that of tert-butyl hydroquinone (TBHQ) during 32 h frying process at 180°C. For this purpose, variation of acid value (AV), conjugated diene value (CDV), carbonyl value (CV), total polar compounds, and total tocol (TT) and total sterol contents of RSO was monitored during frying process. Results of oxidative stability assays revealed that 4%KHO had the best antioxidant effect which was fairly equal to that of TBHQ, followed by 4%BHO. It should be mentioned that in contrast to TBHQ, KHO and BHO are not pure antioxidant. The remarkable antioxidant effect of KHO and BHO during thermal process was due to high tocotrienol content and presence of suitable amount of Δ-7-avenasterol and Δ-5-avenasterol, respectively [TT content of KHO (2041 mg/kg) much higher than that of ordinary edible oils and 92.7% of TT content in KHO is formed by tocotrienols]. Antioxidant activity of Δ-7-avenasterol and Δ-5-avenasterol and tocotrienols during frying process was attributed to formation of stabilized tertiary alkyl radicals. These radicals cut down radical chains formed during frying process.
Introduction
Soybean oil is one of the most common oils across the world and has suitable nutritional value due to high content of linoleic acid, linolenic acid, and antioxidant compounds. Since this oil has high amount of linolenic acid, its oxidative stability, especially at high temperatures, is low and thus is not suitable for frying.[Citation1] Oxidative stability of this oil has been improved through development of low linolenic acid soybean varieties. Moreover, in oil-producing factories, oxidative stability of the soybean oil is enhanced by hydrogenation. However, use of hydrogenated oil causes various health-related problems such as the risk of cancer and cardiovascular diseases due to formation saturated and trans fatty acids during hydrogenation process.[Citation2] Addition of antioxidants to edible oils improves their oxidative stability. Use of some synthetic antioxidants in food and oils has been reduced in developed countries because they are suspected as causing cancer in the human body.[Citation3] In contrast, application of natural antioxidants has increased due to their positive effect on human health. Application of natural antioxidants also results in some problems. Most of these antioxidants have low oxidative stability at high temperature (frying). Moreover, they are usually found in small amount and their extraction is costly. Thus, researchers seek for suitable natural antioxidants with high thermal stability and low extraction cost that are available in sufficient amount. There are rich sources of natural antioxidants in Iran that have been investigated by many authors.[Citation4–Citation7] Fruits of two wild Pistacia species called Beneh (Pistacia atlantica) and Kolkhoung (Pistacia Khinjuk) represent a source of natural antioxidants that are available in high amount in Zagros forests in West and Southwest Iran.[Citation4,Citation5,Citation8,Citation9] Zagros forests cover an area of 5 million ha where Beneh, Kolkhoung, oak, and wild almond are found. Tavakoli et al. showed that Kolkhoung hull oil (KHO) is an oil containing tocopherols with unique oxidative stability during thermal process. Oxidative indices such as carbonyl value (CV), acid value (AV), conjugated diene value (CDV), and polar components of this oil show low increase. Moreover, it has been revealed in rancimat test that the effect of 100 ppm KHO on oxidative stability of olive oil was equal to that of 100 ppm tert-butyl hydroquinone (TBHQ).[Citation8] In another study, it was revealed that Beneh hull oil (BHO) is an oil with high oxidative stability during thermal process at 170°C.[Citation9] Also, Pazhouhanmehr et al. showed that addition of this oil increased oxidative stability of fish oil during thermal process.[Citation10] Based on previous researches, the present study was designed to evaluate the antioxidant effect of the addition of KHO into refined soybean oil (RSO) during the frying process, and then further measurement and comparison of the oxidative stability indexes with of the BHO and Sesame (Sesamum indicume L. cv. Dezful) seed oil (SSO) (as two oils containing high antioxidants) and TBHQ (as a typical synthetic antioxidant) added RSO.
Materials and methods
Materials
Kolkhoung and Beneh fruits (10 kg) were collected from forest located in Meimand, Iran (summer, 2016). Sesame seeds (S. indicume L. cv Dezful) were gathered from a farmland in Dezul, Khoozestan Province (summer, 2016). RSO (30 kg) without added antioxidants was supplied by the Narges Company (Shiraz, Iran). All samples were stored at 4°C until the initiation of the experiments (maximum 4 weeks). All chemicals and solvents used in this experiment had analytical reagent grade and were purchased from Merck and Sigma-Aldrich Chemical Companies (Darmstadt, Germany).
Oil extraction
The soft green hull of the Kolkhoung and Beneh fruits was removed using a dehuller device and, together with sesame seeds, was powdered in a mill (Mullinex Depose-Brevete S.G.C.G., France). The prepared powders were mixed with normal hexane (1:4) and placed on a shaker for 48 h in the dark condition. The mixtures were then placed under vacuum oven (OV11, Jeiotech, Korea) at 40°C for 6–12 h to remove the remained solvent.[Citation8,Citation9,Citation11]
Extraction of unsaponifiable matters
The saponification of the oils was carried out as following: 5 g of oil was mixed with 50 mL 1 N ethanolic KOH and subsequently heated at 95°C for 1 h. The mixture was cooled at ambient temperature and then 100 mL distilled water was added and completely mixed. The resulting solution was extracted twice using 100 mL diethyl ether in a decanter funnel. In each extraction step, the upper organic layer was collected and washed twice with 75 mL distilled water, once with 100 mL of 0.5 N ethanolic KOH and finally neutralized with 100 mL distilled water. Then, the organic layers were separated and dried using Na2SO4. The solution was filtered and evaporated to dryness in a vacuum oven (OV11, Jeiotech, Korea) at 45°C. For further purification of unsaponifiable compounds, they were dissolved in chloroform, filtered and finally evaporated at 45°C under vacuum conditions.[Citation4,Citation6,Citation12]
Preparation of various samples of RSO
To perform frying, different RSO samples containing 1%, 2%, 4%, and 6% KHO, BHO, SSO, and 100 ppm TBHQ were prepared to be compared with RSO without antioxidant as control.
Frying process
Potatoes were peeled and cut into 6 cm × 1 cm × 1 cm slices and submerged in water until needed and dried with a cotton cloth. Frying was performed using a conventional fryer (Tefal model 1250, Paris, France) equipped with a thermostat and stainless steel grid basket (in each replication, 2.5 L oil sample was used). Potato pieces were fried in 20-g batches at constant frying temperature (180°C) and were subjected to frying for 5 min. The rest time between frying periods was 15 min. The frying process was conducted for four consecutive days, 8 h per day. In 4 h intervals, about 10 g of oil was taken from the fryer, cooled at room temperature, and, after nitrogen injection, kept at −18°C until experiment time (maximum 1 week). The token volume of oil was not replenished during the frying process.[Citation5,Citation6,Citation13]
Fatty acid composition
Fatty acid composition of the oil samples was determined by a gas–liquid chromatography and reported in relative area percentages. Fatty acids were transesterified into their corresponding FAMEs (fatty acid methyl ester) by vigorous shaking of a solution of the oil in hexane (0.3 g in 7 mL) with 2 mL of 7 M methanolic potassium hydroxide at 50°C for 10 min. The FAMEs were identified using an HP-5890 gas chromatography (Agilent, Palo Alto, CA) equipped with a CP-FIL 88 (Supelco Inc., Bellefonte, PA) capillary fused silica column (60 m × 0.22 mm i.d., 0.2 mm film thickness) and the flame ionization detector. Nitrogen was used as a carrier gas at flow rate of 0.75 mL/min. The oven temperature was fixed at 198°C and that of the injector and the detector at 250°C.[Citation4,Citation5]
Gas chromatography analysis for sterol compounds of KHO, BHO, and SSO
To quantify sterol compounds of KHO, BHO, and SSO, a gas chromatography system using Betulin as a standard was applied. The compounds were separated by an SE 54 CB column (Macherey-Nagel, Duren, Germany; 50 m long, 0.25 mm ID, 0.25 µm film thickness). Further used parameters can be summarized as follows: hydrogen as a carrier gas, split ratio 1:20, injection and detection temperature adjusted to 320°C, temperature program, 240–255°C at 4°C/min.[Citation7,Citation8,Citation14]
HPLC analysis for tocol compounds of KHO, BHO, and SSO
Tocol compounds in the KHO were determined using a HPLC system (Waters ACQUITY UPLC® System, Milford, Massachusetts, USA) with a Spherisorb column (25 cm × 4 mm i.d., WATERS, USA) packed with silica (5 µm particle size) and a fluorescence detector operating at an excitation wavelength of 290 nm and an emission wavelength of 330 nm were utilized. The used mobile phase consisted of acetonitrile and water (9:1, v/v) at a flow rate of 0.5 mL/min. Tocopherols in test samples were verified by the comparison of retention time with reference standards.[Citation15]
Total sterol and total tocol contents of RSO samples
Total sterol (TS) content of RSO samples was quantified according to the Lieberman–Burchard color reaction.[Citation16] Total tocol (TT) content of RSO was also determined according to the colorimetric method described by Wong et al.[Citation4,Citation7,Citation17]
Acid value, conjugated diene value, and carbonyl value
AV was determined according to the AOCS Official Method Cd 3d-63.[Citation4,Citation7,Citation18] CDV was measured using the described procedure by Saguy et al.[Citation4,Citation7,Citation19] In this method, oil sample was mixed with HPLC grade hexane in 1:600 ratio and the absorption was determined spectroscopically (UV/Visible Philips Cambridge, UK) at 234 nm. CV of the oil samples was measured according to the developed method by Endo et al.[Citation4,Citation7,Citation20]
Total polar compounds content
The total polar compounds (TPC) content was determined according to the economical micro method described by Schulte with a slight modification on removing the solvent from the eluate.[Citation7,Citation21] The solvent was evaporated in a vacuum oven at 40°C for 25 min.
Statistical analysis
Initially, the data obtained from the experiments of chemical properties in the present investigation were analyzed in triplicate based on a randomized complete design (RCD). The subsequent tests of frying process of these oils were developed as factorial RCD with three replications (the variable in these experiments was oil samples and time of frying time). All data were analyzed using ANOVA. MStatC and SlideWrite software were used for ANOVA and regression analysis, respectively. The mean comparison was performed by Duncan multi-step test to determine significant differences (P < 0.05).
Result and discussion
Chemical properties
Chemical properties of KHO, BHO, and SSO are represented in . As seen, the dominant fatty acids of KHO and BHO and SSO were oleic acid (52–52.1%) and linoleic acid (47.1%), respectively. SFA value in KHO, BHO, and SSO was 25.8%, 25.6%, and 16.8%, indicating no significant difference (P > 0.05) between BHO and KHO. The dominant saturated fatty acid in the three oils was palmitic acid (10.2–23.4%), followed by stearic acid (2.4–5.7%). The content of linolenic acid as a highly oxidation-sensitive oil in KHO, BHO, and SSO was 1.5%, 1.2%, and 5%, suggesting that there is significant difference (P > 0.05) among the oils. Considering fatty acid composition of KHO, BHO, and SSO (ignoring other factors affecting oxidation stability of edible oils), it can be predicted that BHO is probably more stable than KHO and SSO.
Table 1. Chemical characteristics of KHO, BHO, and SSO.*
USM values of the studied oils are presented in . As can be observed, there is no significant difference (P > 0.05) among KHO, BHO, and SSO regarding USM value (0.64–0.78%). USM compounds can delay deterioration of food. The main components of USM compounds include hydrocarbons, terpene alcohols, esterols, and tocopherols. USM of edible oils varies between 0.5% and 2.5% and sometimes reaches 5–6%. The content of these compounds is highly affected by purification; thus, their value is extensively used as an index of purified oils’ quality.[Citation22] Sharayei et al. used USM of Beneh kernel oil as an antioxidant additive in canola oil and, based on frying process results, concluded that these compounds can be used as a stable natural antioxidant in edible oil.[Citation7]
Tocopherols are compounds with antioxidant and vitamin activities. Presence of these compounds is effective in improvement of oxidative stability of edible oils. TT content of KHO (2014 mg/kg) was significantly (P > 0.05) higher than that of other oils, followed by SSO (994 mg/kg) and BHO (703 mg/kg) (). TT content of KHO was apparently higher than that found in common plant oils.[Citation23] Tavakoli et al. reported that TT content of kernel oil from Kolkhoung was 619.4 mg/kg which is much lower than that measured in KHO.[Citation4] Also, amount of TT content of Beneh kernel oil was measured as 818.6 mg/kg.[Citation24]
Phytosterols as the one of the most important minor compounds of plant oils could play a critical role in food grading and are considered as an index for recognizing the fake and original edible oils. Most of plant oils have 1000–5000 ppm of esterol compounds.[Citation25,Citation26] As is evident in , the highest TS content was found in SSO (5041 mg/kg), followed by BHO and KHO (1649 and 1601 mg/kg).
As natural antioxidants, phenolic compounds represent a major factor for evaluation of edible oils’ quality. There is direct relation between oxidative stability and organoleptic properties of edible oils and their phenolics content. Moreover, phenolics play biological role in human body that prevents from diseases caused by formation of free radicals via improving antioxidation defense capacity of the body.[Citation27,Citation28] As can be seen from , there was significant difference (P > 0.05) among the three oils regarding TP value. The highest content of TP was found in SSO (994 mg/kg), followed by KHO (100 mg/kg) and BHO (82 mg/kg). Presence of large amount of antioxidant compounds in edible oils is a positive property; however, possessing high thermal stability especially during frying process is more important.
Thermal stability during frying process
CDV measurement as primary oxidation index is a suitable test for evaluating thermal stability of edible oils with high content of linoleic and linolenic acids including soybean oil. During oxidation of polyunsaturated fatty acids, diene and conjugated triene bonds are formed. By increase in the number of conjugated diene bonds, optical absorbance in 234 nm is enhanced which is used as an index of oxidation and reported as increase in oxygen absorption and formation hydroperoxides during first steps of oxidation.[Citation23,Citation29,Citation30] CDV variation in RSO treated with various concentrations of KHO, BHO, and SSO and 100 ppm TBHQ during 32 h frying process at 180°C is presented in . CDV increase for pure RSO and RSO samples containing 1%, 2%, 4%, and 6% KHO, BHO, and SSO and 100 ppm TBHQ was 885%, 867%, 725%, 769%, 793%, 862%, 721%, 779%, 869%, 846%, 822%, 780%, and 616%. As can be seen in , after 32 h of frying, the lowest CDV variation was observed in the sample containing 100 ppm TBHQ, followed by those containing 2% KHO, 4%BHO, 4% and 6% KHO, 1% and 6% BHO and 6%SSO, 4%SSO, 2%BHO and SSO, 1%KHO, 4%BHO, and pure RSO. KHO in level of 2% and BHO in level of 4% had antioxidant activity equal to 80% and 85% of TBHQ, respectively. Considering impurity of these oils compared to TBHQ, it is a remarkable result because some synthetic antioxidants cause cancer and cardiovascular diseases. 2%KHO was determined as the best sample because it is half of 4%BHO.
Table 2. Changes in CDV (mmol/L) of the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C.
Moreover, this assay showed that by increase of KHO, BHO, and SSO in RSO, CDV increase was not regularly changed. For example, 2%KHO and 4%BHO were the best sample and promoted RSO stability against CDV variation. This may be attributed to impurity of these oils. Besides antioxidants, there are also other compounds in KHO, BHO, and SSO that may cause unpredictable reactions in RSO. When concentration of these oils in RSO exceeds a threshold value, antioxidative state is replaced by prooxidative one. Such condition occurs when antioxidants’ concentration is increased in edible oils. Similar results have been reported by Sharayei et al. By increase in Beneh kernel oil content in canola oil, resistance against CDV variation during thermal process was improved and then reduced. Moreover, it was observed that 1% Beneh kernel oil had antioxidant activity equal to 65% of TBHQ.[Citation4] In another study, it was found that Beneh kernel oil at 1000 ppm concentration was not significantly different (P > 0.05) from TBHQ (100 ppm) in prevention of CDV increase in canola oil blended with palm olein and virgin olive oils.[Citation6]
Carbonyl compounds such as aldehydes and ketones are products of lipid secondary oxidation formed by decomposition of hydroperoxides and cause bad taste, deterioration, and reduced nutritional value of fried foods. They are more stable than peroxides during frying; thus, CV is a good index of oxidation variation of edible oils during thermal process.[Citation20] CV variations of different RSO samples during 32 h of frying process at 180°C are presented in . The highest increase of CV was observed in pure RSO (2051%), followed by 4%SSO (1445%), 6%SSO (1338%), 1%SSO (1263%), 1%KHO (1248%), 2%SSO (1177%), 6%KHO (1089%), 2%KHO (1078%), 2%BHO (995%), 1%BHO (929%), 4%BHO (867%), 100 ppm TBHQ (826%), and 4%KHO (580%). Based on these results, antioxidant effect of 1%, 2%, 4%, and 6% of KHO, BHO, and SSO was determined 66%, 77%, 142%, 76%, 89%, 83%, 95%, 86%, 65%, 70%, 57%, and 61% of that of TBHQ. Among various samples, 4%KHO had the best antioxidant activity in preventing from formation of carbonyl compounds in RSO which is even higher than that of TBHQ. The results of this assay showed that KHO and BHO can be used as substitutes for TBHQ to prevent the problems caused by this synthetic antioxidant. As observed in CDV test, by increase in KHO, BHO, and SSO in RSO, CV increase was not regularly changed. Evaluation of CV variation of canola oil blended with different concentrations of Beneh kernel oil indicated that by increase of this oil in canola oil, CV variation was not regularly changed so that among canola oils containing 0.05%, 0.1%, 0.2%, and 0.4% of Beneh kernel oil, the sample with 0.2% of this oil had the lowest antioxidant effect in preventing formation of carbonyl compounds, followed by samples containing 0.1%, 0.05%, and 0.4% of this oil. Moreover, it was observed that 0.4% Beneh kernel oil was better than 100 ppm TBHQ.[Citation5] Furthermore, Sharayei et al. confirmed that in CV assay, Beneh kernel oil at 1000 ppm concentration was not significantly different (P > 0.05) from TBHQ (100 ppm) in canola oil blended with palm olein and virgin olive oils.[Citation6]
Table 3. Changes in CV (µmol/g) of the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C.
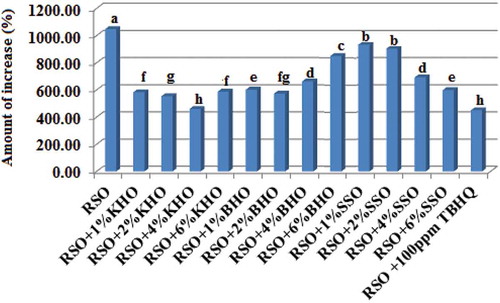
AV is known as the triglycerides hydrolysis index. Free fatty acids concentration is elevated during frying process which is due to hydrolysis of triglycerides or presence of carboxyl groups in polymeric or oxidative products. By hydrolysis of triglycerides and increase of free fatty acids, oxidation of edible oils is promoted, and thus, their shelf life is decreased.[Citation29] Parameters resulting from fitting of AV of various RSO samples during 32 h of frying process at 180°C are presented in . As can be seen, the highest AV variation slope was observed in pure RSO (a = 0.103), followed by samples containing 1%SSO (a = 0.099), 2%SSO (a = 0.098), 6%BHO (a = 0.086), 4%SSO (a = 0.072), 4%BHO (a = 0.067), 1%BHO (a = 0.065), 1%, 2%, and 6% KHO (a = 0.06), 6%SSO (a = 0.059), 2%BHO (a = 0.053), 100 ppm TBHQ (a = 0.05), and 4%KHO (a = 0.048). AV increase in pure RSO and RSO samples containing 1%, 2%, 4%, and 6% of KHO, BHO, and SSO and 100 ppm TBHQ was 1047%, 582%, 553%, 459%, 586%, 601%, 572%, 662%, 842%, 932%, 903%, 692%, 598%, and 450% (). [No significant difference (P > 0.05) was observed among RSO samples considering t0 AVs (0.32–0.34).] Thus, the most stable treatments against AV increase and triglycerides hydrolysis in RSO were 4%KHO and 100 ppm TBHQ [with no significant difference (P > 0.05)], followed by 2%KHO, 2%BHO, 6%KHO, 1%KHO, 1%BHO, 6%SSO, 4%BHO, 4%SSO, 6%SSO, 1% and 2% SSO, and pure RSO. Again, 4%KHO was determined as the best treatment. Antioxidant activity of the first six treatments (4%KHO, 2%KHO, 2%BHO, 6%KHO, 1%KHO, and 1%BHO) was 98%, 81%, 79%, 77%, 77%, and 75% of that of TBHQ which agrees with the results of CDV and CV. Regarding health-promoting effects of natural antioxidants, this is a remarkable finding. In AV test, different results were found in RSO by increase in KHO, BHO, and SSO, suggesting that by increase of these oils’ concentrations, their antioxidant activity was not necessarily increased regularly. As mentioned before, this effect is attributed to impurities of these oils as antioxidants. This result is in accordance with those reported by Sharayei et al. The authors found out that there was not linear relation between increased Beneh kernel oil concentration in canola oil and AV variation.[Citation5] In a similar work, it was revealed that during frying process, 100 ppm of Beneh kernel oil and 100 ppm TBHQ have similar antioxidant effect in canola oil blended with palm olein and virgin olive oils.[Citation6]
Table 4. The parameters calculated from the linear relationship between the AV (mg/mL) and frying time for the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C.*
Figure 1. Amount of increasing (%) in AV (mg/mL) of the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C. Means with the same lowercase letters are not significantly different at P < 0.05.
AV: acid value; BHO: beneh hull oil; KHO: kolkhoung hull oil; RSO: refined soybean oil; SSO: Seasame seed oil; TBHQ: tertiary butyl hydroquinone.
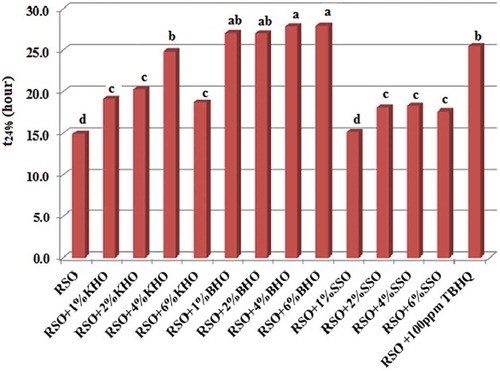
Measurement of polar compounds is a reliable method to evaluate fried oils’ quality and their reuse.[Citation31] In many European countries, it is recommended that edible oil containing 24–27% TPC should be abandoned.[Citation32] Assuming that acceptable TPC of the oils in this study is 24%, the time required for RSO samples to reach this point (t24) was calculated.[Citation7] Primary TPC of oil samples was determined between 4.7% and 4.9%, indicating no significant difference (P > 0.05). shows t24 values of RSO samples at 180°C. As seen, t24 values for pure RSO and samples containing 1%, 2%, 4%, and 6% of KHO, BHO, SSO, and 100 ppm TBHQ were 14.9, 19.2, 20.3, 24.9, 18.8, 27.1, 27, 27.9, 28, and 25.5 h. Statistically, RSO containing 4% and 6% BHO was determined as the best sample in polar compound assay, followed by those containing 1% and 2% BHO, 4%KHO, 100 ppm TBHQ, 1%, 2%, and 6%KHO, 2%, 4%, and 6% SSO, 2%SSO, and pure RSO. The results obtained in this assay indicated that all levels of BHO and 4%KHO had equal or better antioxidant activity than TBHQ in prevention of TPC increase during frying process. Sharayei et al. reported that by increase in Beneh kernel oil in canola oil, t24 value was decreased which can be due to occurrence of prooxidant state in canola oil. Moreover, the highest value of this factor was found in oil sample containing 100 ppm TBHQ.[Citation5] In another study, there was no significant difference (P > 0.05) between 100 ppm Beneh kernel oil and 100 ppm TBHQ in preventing formation of polar compounds in canola oil blended with palm olein and virgin olive oils.[Citation6] The results of polar compound assay indicated that hull oil of two wild Pistacia species can be ideal substitutes for TBHQ whose health problems are well documented. Contrary to the results of CDV, CV, and AV assay, that the best treatments were RSO samples containing KHO (4% and 2%), in this test, samples containing BHO were the most resistant treatments against formation of polar compounds.
Figure 2. The time required to TPC content of 24% (t24) for the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C. Means with the same lowercase letters are not significantly different at P < 0.05.
BHO: beneh hull oil; KHO: kolkhoung hull oil; RSO: refined soybean oil; SSO: Seasame seed oil; TBHQ: tertiary butyl hydroquinone; TPC: total polar compounds.
In the present study, there was no direct correlation between the increase in KHO and BHO concentrations in RSO and improving its oxidative stability but in a study conducted by Ramadan and Wahdan, it was found that with increasing concentrations of black cumin seed oil and coriander seed oil (10% and 20%) in corn oil, oxidative stability of oil blends improved, which was due to changes in the their fatty acid composition and increasing the their tocopherol content.[Citation33] In another study by Ramadan, it was found that increasing the amount of black cumin oil, cumin oil, coriander oil, and clove oil in high linoleic sunflower oil had the same effect in improving oxidative stability of oil blends, too.[Citation34]
Antioxidant compounds (TT and TS) changes
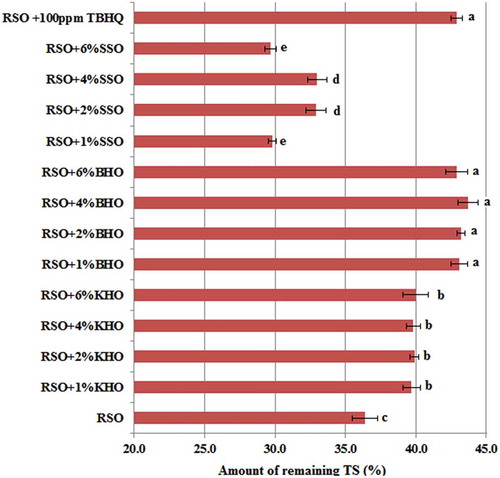
The results of oxidation stability tests indicated that hull oil of the two wild pistachio species can be regarded as good substitute for TBHQ. For better interpretation of oxidation stability assays (AV, CDV, CV, and TPC), variation trend in TT and TS contents of RSO was investigated under treatment with various concentrations of KHO, BHO, SSO, and 100 ppm TBHQ during 32 h frying process at 180°C. Primary values of TT in different RSO samples were estimated in the range of 1008 and 1104 mg/kg, showing that addition of various concentrations of KHO, BHO, and SSO into RSO had no significant effect (P > 0.05) on TT values at t0 (). As is evident from , TT content variation of different RSO samples showed a descending trend during frying process. The best sample in this assay was RSO containing 100 ppm TBHQ in which, TT content was reduced to 52.4% of the primary level, followed by RSO samples containing 4%KHO, pure RSO, 6%KHO, 1%KHO, 2%KHO, 2%SSO, 1%SSO, 1%BHO, 4%SSO, 2%BHO, 6%BHO, 6%SSO, and 4%BHO (45.1%, 38.7%, 34.7%, 34.1%, 34%, 32.2%, 30.1%, 29%, 28.1%, 27.7%, 27.7%, 25%, and 23.4%). Moreover, primary TS values of the samples were 2401 ± 36, 2394 ± 40, 2380 ± 40, 2368 ± 40, 2351 ± 40, 2399 ± 43, 2387 ± 42, 2370 ± 44, 2355 ± 43, 2431 ± 30, 2454 ± 33, 2525 ± 33, 2587 ± 30, and 2403 ± 41 mg/kg. Remaining TS value in virgin RSO and RSOs containing 1%, 2%, 4%, and 6% KHO, BHO, SSO, and 100 ppm TBHQ after 32 h of frying process was reduced to 36.4%, 39.7%, 39.9%, 39.8%, 40%, 43.1%, 43.2%, 43.7%, 42.9%, 29.8%, 32.9%, 33%, 29.7%, and 42.9% of primary values (). Based on primary TT and TS values, it was concluded that increase in TT and TS contents doesn’t account for suitable antioxidant effects of KHO and BHO, because RSO contains large amount of TT and TS and addition of such compounds can’t explain superiority of some samples. Therefore, tocopherol and esterol composition of KHO and BHO was qualitatively investigated to reveal the reason for their high antioxidant activity.
Table 5. Changes in TT content (mg/kg) of the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C.
Figure 3. Amount of remaining (%) TS content of the RSO as affected by the KHO, BHO, SSO, and TBHQ during frying process at 180°C. The columns showing the similar components with the same lowercase letters are not significantly different at P < 0.05. Error bars indicate standard deviations.
BHO: beneh hull oil; KHO: kolkhoung hull oil; RSO: refined soybean oil; SSO: seasame seed oil; TBHQ: tertiary butyl hydroquinone; TS: total sterol.
Tocopherol and esterol composition
Tocopherol and esterol composition of KHO, BHO, and SSO is represented in and . The tables show that tocol composition of the three oils is quite different from each other. In KHO, alpha tocotrienol (1077 mg/kg) and delta tocotrienol (815 mg/kg) were dominant tocols, followed by delta tocopherol, beta tocopherol, alpha tocopherol, and gamma tocopherol. The dominant tocol compound in BHO and SSO was beta tocopherol (485 mg/kg) and gamma tocopherol (935 mg/kg). Sterol composition of KHO, BHO, and SSO is represented in . The dominant esterol in the three oils was β-sitosterol. In KHO, the major sterols were β-sitosterol (1140 mg/kg), Δ-stigmastenol, campesterol, Δ-7-avenasterol, sitostanol, Δ5-avenasterol, stigmasterol, clerosterol, cholesterol, Δ5-24 stigmastadienol, and brassicasterol (202, 60, 33, 33, 29, 29, 28, 21, 17, and 10 mg/kg). In BHO, the major sterols were β-sitosterol (1014 mg/kg), Δ5-avenasterol, Δ-7-avenasterol, Δ-stigmastenol, Δ5-24 stigmastadienol, sitostanol, clerosterol, campesterol, brassicasterol, stigmasterol, and cholesterol (175, 172, 142, 67, 19, 18, 17, 9, 8, and 8 mg/kg). Similarly, β-sitosterol (3387 mg/kg) was the major sterol in SSO, followed by campesterol, Δ-stigmastenol, Sitostanol, stigmasterol, Δ5-avenasterol, Δ-7-avenasterol, Δ5-24 stigmastadienol, cholesterol, clerosterol, and brassicasterol (1134, 326, 230, 79, 103, 57, 28, 27, 18, and 12 mg/kg).
Table 6. The tocol composition of the KHO, BHO, and SSO.
Table 7. The sterol composition of the KHO, BHO, and SSO.
Tocols are classified into two groups, viz., tocopherols and tocotrienols. Tocopherols are good antioxidants in room temperature because they block the radical chain of lipid peroxidation by trapping lipid peroxyl radicals, thus extending the shelf life of fat-rich foods.[Citation35] However, in frying temperatures, tocopherols have lower efficiency and oil decomposition occurs in their presence. On the other hand, stability of frying oils is provided by non-classical antioxidants such as Δ-7-avenasterol and Δ-5-avenasterol because they possess ethylidene group in their structure. These compounds are ineffective at room temperature and their antioxidant activity during frying process is attributed to formation of stabilized tertiary alkyl radicals.[Citation36–Citation38] These radicals cut down radical chains formed during frying process. The highest Δ-7-avenasterol and Δ-5-avenasterol content was observed in BHO (347 mg/kg), followed by SSO and KHO (136 and 57 mg/kg). Thus, antioxidant activity of BHO compared to TBHQ is due to sufficient amount of Δ-7-avenasterol and Δ-5-avenasterol. However, another factor seems to be involved in suitable antioxidant activity of KHO. Analysis of KHO tocol structure indicated that this oil has a fairly different tocol composition compared to other edible oils. Tocotrienols constitute 92.7% of TT content of KHO. Among ordinary oils, palm oil has high tocotrienol content and, hence, possesses higher stability during frying process. It has been shown in recent studies that tocotrienols have better antioxidant activity against spontaneous oxidation of frying process than tocopherols. Tocotrienols have lipid tail three double bonds that act similar to Δ-7-avenasterol and Δ-5-avenasterol.[Citation39] In contrast to KHO, RSO lacks tocotrienols. Thus, by addition of these compounds to RSO via KHO, it is expected that they cause RSO stability during frying process. Presence of high tocotrienols in KHO can explain its suitable antioxidant effect during frying process.
Conclusion
This study was conducted to investigate the use of hull oil of two wild pistachio species found in Iran (1%, 2%, 4%, and 6%) as natural antioxidant for improving oxidative stability of soybean oil during frying process. Results of oxidation stability assays (TPC, AV, CV, and CDV) indicated that KHO in level of 4% had the best antioxidant effect in RSO compared to TBHQ, followed by 4%BHO. The importance of these results is more evident by noticing that KHO and BHO are not pure antioxidants. The remarkable antioxidant effect of KHO and BHO during thermal process is due to high tocotrienol content and presence of suitable amount of Δ-7-avenasterol and Δ-5-avenasterol, respectively. The results obtained in this study revealed that hull oil of the two wild Pistacia species can be ideal substitute for the synthetic antioxidant TBHQ. By this practice, not only oncogenic effect and cardiovascular diseases caused by synthetic antioxidants are prevented, but also import of synthetic antioxidants is avoided.
Additional information
Funding
References
- Hammond, E. G.; Johnson, L. A.; Su, C.; Wang, T.; White, P. J. Soybean Oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F.(Ed.); Wiley:New Jersey, 2005.
- Farr, W. E.;. Hydrogenation: Processing Technologies. In Bailey’s Industrial Oil and Fat Products; Shahidi, F.(EdEds.); Wiley:New Jersey, 2005.
- Gharavi, N.; Haggarty, S.; El-Kadi, A. Chemoprotective and Carcinogenic Effects of tert-Butylhydroquinone and Its Metabolites. Curr. Drug Metab. 2007, 8, 1–7. DOI: 10.2174/138920007779315035
- Tavakoli, J.; Hamedani, F.; Haddad Khodaparast, M. H. Investigating Chemical Properties and Oxidative Stability of Kernel Oil from Pistacia Khinjuk Growing Wild in Iran. J. Am. Oil Chem. Soc. 2016, 93, 681–687. DOI: 10.1007/s11746-016-2817-6
- Sharayei, P.; Farhoosh, R.; Poorazrang, H.; Haddad Khodaparast, M. H. Effect of Bene Kernel Oil on the Frying Stability of Canola Oil. J. Am. Oil Chem. Soc. 2011, 88, 648–654.
- Sharayei, P.; Farhoosh, R. Improved Frying Stability of Canola Oil Blended with Palm Olein and Virgin Olive Oils as Affected by Bene Kernel Oil and Its Unsaponifiable Matter. Eur. J. Lipid Sci. Technol. 2016, 118, 1495–1506. DOI: 10.1002/ejlt.v118.10
- Sharayei, P.; Farhoosh, R.; Poorazrang, H.; Haddad Khodaparast, M. H. Improvement of Canola Oil Frying Stability by Bene Kernel Oil’s Unsaponifiable Matter. J. Am. Oil Chem. Soc. 2011, 88, 993–1000. DOI: 10.1007/s11746-011-1764-5
- Tavakoli, J.; Haddad Khodaparast, M. H.; Esmaeilzadeh Kenari, R.; Aminlari, M.; Sharif, A. Introducing Pistacia Khinjuk (Kolkhoung) Fruit Hull Oil as a Vegetable Oil with Special Chemical Composition and Unique Oxidative Stability. J. Chem. Nat. Compounds. 2013, 5, 803–810. DOI: 10.1007/s10600-013-0752-4
- Farhoosh, R.; Haddad Khodaparast, M. H.; Sharif, A. Bene Hull Oil as a Highly Stable and Antioxidative Vegetable Oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1259–1265. DOI: 10.1002/ejlt.200900081
- Pazhouhanmehr, S.; Farhoosh, R.; Esmaeilzadeh Kenari, R.; Sharif, A. Oxidative Stability of Purified Common Kilka (Clupeonella Cultiventris Caspia) Oil as a Function of the Bene Kernel and Hull Oils. Int. J. Food Sci. Technol. 2014, 50, 396–403. DOI: 10.1111/ijfs.12609
- Tavakoli, J.; Brewer, M. S.; Zarei Jelyani, A.; Estakhr, P. Oxidative Stability of Olive Oil during Thermal Process: Effect of Pistacia Khinjuk Fruit Oil. Int. J. Food Properties. 2017, DOI: 10.1080/10942912 2017. 1285787
- Lozano, Y. F.; Dhuique Mayer, C.; Bannon, C.; Gaydou, E. M. Unsaponifiable Matter, Total Sterol and Tocopherol Contents of Avocado Oil Varieties. J. Am. Oil Chem. Soc. 1993, 70, 561–565. DOI: 10.1007/BF02545319
- Tyagi, V. K.; Vasisihtha, A. K. Changes in the Characteristics and Composition of Oils during Deep-Fat Frying. J. Am. Oil Chem. Soc. 1996, 73, 499–506. DOI: 10.1007/BF02523926
- ISO 12228-1. Determination of individual and total sterols contents - Gas chromatographic method - Part 1: Animal and vegetable fats and oils. International Organization for Standardization, 2014.
- Fu, J. Y.; Htar, T. T.; De Silva, L.; Tan, D. M. Y.; Chuah, L. H. Chromatographic Separation of Vitamin E Enantiomers. Molecules. 2017, 22, 233. DOI: 10.3390/molecules22020233
- Sabir, S. M.; Hayat, I.; Gardezi, S. D. A. Estimation of Sterols in Edible Fats and Oils. Pakistan J. Nutr. 2003, 2, 178–181. DOI: 10.3923/pjn.2003.178.181
- Wong, M. L.; Timms, R. E.; Goh, E. M. Colorimetric Determination of Total Tocopherols in Palm Oil, Olein and Stearin. J. Am. Oil Chem. Soc. 1988, 65, 258–261. DOI: 10.1007/BF02636412
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, 1993.
- Saguy, I. S.; Shani, A.; Weinberg, P.; Garti, N. Utilization of Jojoba Oil for Deep-Fat Frying of Foods. J. Lebenson. Wiss. Technol. 1996, 29, 573–577. DOI: 10.1006/fstl.1996.0088
- Endo, Y.; Li, C. M.; Tagiri-Endo, M.; Fugimoto, K. A. Modified Method for the Estimation of Total Carbonyl Compounds in Heated and Frying Oils Using 2-Propanol as a Solvent. J. Am. Oil Chem. Soc. 2001, 10, 1021–1024. DOI: 10.1007/s11746-001-0381-1
- Schulte, E.;. Economical Micromethod for Determination of Polar Components in Frying Fats. Eur. J. Lipid Sci. Technol. 2004, 106, 772–776. DOI: 10.1002/(ISSN)1438-9312
- Malecka, M.;. Antioxidant Properties of the Unsaponifiable Matter Isolated from Tomato Seeds, Oat Grains and Wheat Germ Oil. Food Chem. 2002, 79, 327–330. DOI: 10.1016/S0308-8146(02)00152-8
- Shahidi, F.;. Baile’s Industrial Oil and Fat Productions. 6th Ed. Wiley: New Jersey, 2005.
- Farhoosh, R.; Tavakoli, J.; Haddad Khodaparast, M. H. Chemical Composition and Oxidative Stability of Kernel Oils from Two Current Subspecies of Pistacia atlantica in Iran. J. Am. Oil Chem. Soc. 2008, 85, 723–729. DOI: 10.1007/s11746-008-1258-2
- Lagarda, M. J.; Garcia-Llatas, G.; Farre, R. Analysis of Phytosterols in Foods. J Pharm Biomed Anal. 2006, 41, 1486–1496. DOI: 10.1016/j.jpba.2006.02.052
- Crane, S.; Aurore, G.; Joseph, H.; Mouloungui, Z.; Bourgeois, P. Composition of Fatty Acids Triacylglycerols and Unsaponifiable Matter in Calophyllum Calaba L. Oil from Guadeloupe. Phytochemistry. 2005, 66, pp. 1825–1831. DOI: 10.1016/j.phytochem.2005.06.009
- Robards, K.; Prenzler, P. D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. DOI: 10.1016/S0308-8146(99)00093-X
- Ryan, D.; Robards, K. Phenolic Compounds in Olives. J. Analyst. 1998, 123, 31–44. DOI: 10.1039/a708920a
- Frankel, E. N.;. Lipid Oxidation; The Oily Press Ltd: Dundee, Scotland, 1998, pp. 79–98.
- Farmer, E. H.; Sutton, D. A. Peroxidation in Relation to Olefinic Structure. Trans. Faraday Soc. 1946, 42, 228–232. DOI: 10.1039/tf9464200228
- Pantzaris, T. P.;. Comparison of Monounsaturated and Polyunsaturated Oils in Continuous Frying. Grasas Y Aceites. 1998, 49, 319–352. DOI: 10.3989/gya.1998.v49.i3-4.733
- Paul, S.; Mittal, G. S. Regulating the Use of Degraded Oil Fat in Deep-Fat Oil Food Frying. Crit Rev Food Sci Nutr. 1997, 37, 635–662. DOI: 10.1080/10408399709527793
- Ramadan, M. F.; Wahdan, K. M. M. Blending of Corn Oil with Black Cumin (Nigella Sativa) and Coriander (Coriandrum Sativum) Seed Oils: Impact on Functionality, Stability and Radical Scavenging Activity. Food Chem. 2012, 132, 873–879. DOI: 10.1016/j.foodchem.2011.11.054
- Ramadan, M. F.;. Healthy Blends of High Linoleic Sunflower Oil with Selected Cold Pressed Oils: Functionality, Stability and Antioxidative Characteristics. Ind Crops Prod. 2013, 43, 65–72. DOI: 10.1016/j.indcrop.2012.07.013
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. DOI: 10.3109/10715762.2014.996146
- Kochhar, S. P.;. Stabilisation of Frying Oils with Natural Antioxidative Components. Eur. J. Lipid Sci. Technol. 2000, 102, 552–559. DOI: 10.1002/(ISSN)1438-9312
- Ahsan, H.; Ahad, A.; Siddiqui, W. A. A Review of Characterization of Tocotrienols from Plant Oils and Foods. Chem. Biol. 2015, 8, 45–59. DOI: 10.1007/s12154-014-0127-8
- Tasan, M.; Demirci, M. Total and Individual Tocopherol Contents of Sunflower Oil at Different Steps of Refining. Eur. Food Res. Technol. 2005, 220, 251–254. DOI: 10.1007/s00217-004-1045-8
- Wagner, K. H.; Wotruba, F.; Elmadfa, I. Antioxidative Potential of Tocotrienols and Tocopherols in Coconut Fat at Different Oxidation Temperatures. Eur. J. Lipid Sci. Technol. 2001, 103, 746–751. DOI: 10.1002/1438-9312(200111)103:11<746::AID-EJLT746>3.0.CO;2-P
