ABSTRACT
In this study, the immunogenic and structural properties of ovalbumin (OVA) induced by pulsed electric fields (PEF) treatment were investigated. The immunogenic properties were estimated by the IgG and IgE binding abilities, which were determined by enzyme-linked immunosorbent assay (ELISA) using rabbit polyclonal antibodies and egg-allergy patients’ sera, respectively. The structural changes were monitored by circular dichroism (CD), ultraviolet absorption, and fluorescence spectroscopy. When the OVA samples were treated at low electric field intensity (below 25 kV/cm, for 180 μs) or for short time (less than 60 μs, at 35 kV/cm), the IgG and IgE binding capacities gradually increased due to the partial unfolding of OVA. This was reflected in the increase in free SH content, surface hydrophobicity, and UV absorption. However, when the OVA samples were treated at high electric field intensity (exceeding 25 kV/cm, for 180 μs) or for long time (more than 60 μs, at 35 kV/cm), the IgG and IgE binding abilities significantly reduced due to the aggregation of OVA. This was also reflected in the decrease in free SH content, surface hydrophobicity, and UV absorption. Moreover, high-intensity PEF treatment caused a loss of the α-helix structure. The results showed that the largest decrease in the immunogenic properties was observed at 35 kV/cm for 180 μs. Therefore, PEF processing has the potential for utilization as a method for egg desensitization.
Introduction
Hen eggs are one of the most essential daily sources of nutrition and ingredients for food processing. However, it is reported that eggs cause 1.6%−8.9% of food allergy in infants and young children and are the second most common food allergen.[Citation1] For patients with persistent egg allergy, the most effective measure to prevent it is to avoid the egg components completely. This is often very difficult due to the wide application of egg proteins as food ingredients.[Citation2] Therefore, the development of hypo-allergic egg-derived products is emphasized.
Reducing the immunogenic properties of egg allergens by processing methods has been determined by some researchers. Tong et al.[Citation3] showed that heating treatments at temperatures above 70°C led to the decrease of IgE binding of ovotransferrin. Ma et al.[Citation4] stated that glycation significantly reduced the potential allergenicity of ovalbumin (OVA) but increased the antigenicity of OVA. Lee et al.[Citation5] reported that the IgG and IgE bindings of OVA were significantly decreased by γ-irradiation treatment at 3−10 kGy. López-Expósito et al.[Citation6] described that enzymatic hydrolysis combined with high-pressure treatment could significantly reduce the IgG and IgE binding abilities of OVA. However, these treatments might degrade the quality of proteins and have a risk on food production, for example, enzymatic hydrolysis could produce unpleasant bitterness and might cause nutritional issues by metabolic disorders.[Citation7] Heating treatments could alter the nutritional and flavour characteristics of proteins by denaturation. Therefore, it is imperative to seek safe and fast methods to decrease the immunogenic properties of egg proteins with the aim of reducing allergenicity.
Pulsed electric fields (PEF) is an emerging, promising non-thermal technology for food preservation.[Citation8] During PEF, liquid foods are processed for a few milliseconds by the external electrical field of high intensity. Compared with conventional thermal processing methods, it has many advantages, including the ability to retain food-quality attributes that may be lost during conventional thermal processing.[Citation9] Extensive literature claim that PEF could be applied in sterilizing food, inactivating enzymes, extracting intracellular components, and degrading pesticide residues.[Citation10,Citation11] The main application of PEF in egg products is sterilization to extend shelf life.[Citation12] Recently, some researchers have studied the effect of PEF treatment on the structure and stability of egg white proteins.[Citation13,Citation14] However, little attention has been devoted to determining the influence of PEF on the immunogenic properties of egg proteins. Moreover, previous studies mainly focused on the structural and property alterations induced by PEF in the multi-protein egg system.[Citation15,Citation16] Studying a single-protein egg system contributes to understanding the mechanism of alteration in the structure and properties of proteins.
OVA accounts for about 54% of egg white protein and is considered as the dominant allergen in egg white.[Citation4] It is a glycoprotein (molecular weight of about 45 kDa) with 385 amino acids, of which about one-quarter are charged.[Citation17] Because of ready availability in quantities, it becomes one of the ideal experimental models in the study of protein structure and properties, such as foaming and immunogenic properties.[Citation18] It is reported that PEF treatments of 20−35 kV/cm for 800 µs led to the aggregation of OVA and bovine serum albumin,[Citation19] suggesting that PEF might have an effect on the immunogenicity of OVA. However, other physicochemical properties of OVA have not been studied. Further studies about the changes in structure and IgG and IgE bindings of OVA induced by PEF treatment are still necessary. Therefore, the purpose of this study was to investigate the immunogenic and structural property alterations induced by PEF in OVA and explore the relationship between them.
Materials and methods
Materials
According to the method of Yang et al.,[Citation1] OVA was isolated and purified from fresh hen eggs (purchased from Laonangou company, two weeks after laying, each egg weighs about 40 g) by ammonium sulphate precipitation and isoelectric precipitation, and the purity was more than 98%. Polyclonal antisera against OVA were obtained from young (three months old, about 2.0 kg) Japanese male rabbits. Human sera numbered P1−P4, whose specific IgE levels were 15.4, 22.5, 29.8, and 64.6 kU/L, were bought from PlasmaLab International (Everett, W.A., USA) and stored at −80°C until used. Goat anti-rabbit IgG-HRP conjugate and goat anti-human IgE-HRP conjugate were from Immunology Consultants Laboratory, Inc. (Oregon, Portland). Freund’s adjuvant, 3,3’,5,5’-tetramethylbenzidine (TMB), 5,5’-dithiobis-2-nitrobenzoic acid (DTNB), and 8-anilino-1-naphthalenesulfonate (ANS) were purchased from Sigma-Aldrich (St. Louis, Mo., U.S.A). Polystyrene microplate (flat-bottomed, 96 wells, 300 μL per well) was bought from Jet Bio-Filtration Products, Co., Ltd. (Guangzhou, China). All chemicals used were of analytical grade.
PEF treatment
A bench-scale pulse generator system (designed by Tsinghua University, Beijing, China) with a unipolar square wave[Citation20] was applied to process the OVA samples. The PEF apparatus has two co-field flow treatment chambers with a volume of 0.05 mL, electrode gap distance of 0.4 cm, and an inner diameter of 0.4 cm. The OVA solution, 10 mg/mL, dissolved in 10 mM phosphate buffer, pH 7.4, was treated for 180 μs at 20, 25, 30, and 35 kV/cm, and at 35 kV/cm for 60, 120, 180, and 240 μs. The electrical conductivity of the sample was 0.08 S/m. During processing, the temperature of the sample was kept below 20°C by a cooling coil submerged in an ice-water bath. After treating by PEF, all samples were stored at 4°C for less than 48 h. PEF treatment time (t) was calculated as follows:[Citation21]
where n is the number of treatment chambers, V is the volume of a chamber (mL), f is the pulse repetition rate (pulses per second, Hz), W is the pulse width (μs), and v is the flow rate (mL/s). In this study, the pulse repetition rate and pulse width were 20 Hz and 5 µs, respectively. The flow rate of the OVA sample was 0.33 mL/s.
Immunogenic property evaluation
The immunogenic properties were evaluated by the IgG and IgE binding abilities, which were determined by enzyme-linked immunosorbent assay (ELISA).[Citation1] IgG binding ability was measured by the indirect competitive ELISA with rabbit polyclonal antisera.[Citation22] First, the polystyrene microplate was coated with 100 μL of 2 μg/mL OVA and incubated overnight at 4°C. Then, it was blocked by 50 mg/mL skimmed milk for 1 h at 37°C. Subsequently, 50 μL of either standard OVA (0.5−32 μg/mL) or diluted OVA (dilution ratio 1:1,000) samples and 50 μL of IgG antisera (dilution ratio 1:12,800) were added and the samples were incubated for 30 min at 37°C. Then, 100 μL of goat anti-rabbit IgG-HRP conjugate (dilution ratio 1:10,000) was added and the samples were incubated for 30 min at 37°C. The microplate was washed five times with PBST (50 mmol/L PBS with 0.05% Tween 20, pH 7.4) after every step described above. It was then coloured in 100 μL of TMB solution, followed by incubation for 15 min at 37°C. The reaction was stopped by adding 50 μL of 2 M sulphuric acid. Finally, the absorbance was measured at 450 nm using an HF2000 microplate reader (Huaan Magnech, Beijing, China). The IgG binding ability was calculated from a standard curve of OVA with the linear logarithmic correlation in the range of 0.5−32 μg/mL. All analyses were carried out in triplicate, and the average values were converted to concentration equivalents in mg/mL through multiplication by the dilution factor.
The IgE binding ability was determined by indirect ELISA with egg-allergy patients’ sera.[Citation3] First, the microplate was coated with 100 μL of 2 μg/mL OVA samples (including untreated and PEF-treated OVA) and incubated overnight at 4°C. Then it was blocked with 50 mg/mL skimmed milk for 1 h at 37°C. Subsequently, 100 μL of IgE antisera (dilution ratio 1:32) was added and incubated for 30 min at 37°C. Then 100 μL of goat anti-human IgE-HRP conjugate (dilution ratio 1:5,000 in PBST) was added and the samples were incubated for 30 min at 37°C. The microplate was washed five times with PBST after every step described above. Colour development, reaction termination, and absorbance measurement steps were followed using protocol as described for IgG binding ability.
Determination of free SH content
Free sulphydryl content was determined by the modification of Ellman’s method.[Citation23] In this study, 0.4 mL of OVA sample was added to 0.6 mL phosphate buffer (0.1 mol/L, pH 8.0) containing 0.01 mL of Ellman’s reagent (4 mg of DTNB in Tris-glycine buffer). After incubation for 20 min at room temperature, the absorbance was measured at 412 nm against a reagent blank. The free sulphydryl content was calculated as follows:
where A412 is the absorbance at 412 nm against reagent blank, D is the dilution coefficient, and C is the protein concentration (mg/mL) in the tested sample.
Measurement of surface hydrophobicity of OVA
ANS fluorescence was used to measure the surface hydrophobicity of OVA, according to the method of Matulis and Lovrien.[Citation24] The ratio of sample (0.4 mg/mL) and ANS solution (8 mmol/L) was 400:1 (V/V). The relative fluorescence intensity was determined using an F-7000 spectrofluorimeter (Hitachi, Tokyo, Japan). The excitation wavelength was 390 nm and the emission spectrum was scanned from 420 to 600 nm at a speed of 800 nm/min. The photomultiplier voltage was 400 V. The bandwidth, excitation, and emission slits were all 5.0 nm in size.
UV absorption spectra analysis
UV absorption spectrum was performed by a UV-2910 spectrophotometer (Hitachi, Tokyo, Japan). The concentration of OVA was 0.4 mg/mL. The UV absorption spectra were scanned from 220 to 400 nm at a speed of 800 nm/min.
Secondary structure analysis
Far-UV circular dichroism (CD) spectroscopy was performed to define the secondary structure of OVA treated by PEF. The CD spectra of OVA (0.1 mg/mL) were measured by a MOS-450 CD spectrometer (French Bio-Logic SAS, Claix, French) at room temperature. The path length of the quartz cuvette used was 1 mm. The bandwidth and step resolution were 1.0 nm. The scan speed was 100 nm/min. The data were shown in terms of molar residue ellipticity ([θ]) in deg cm2/dmol. The contents of different secondary structures were analysed with DichroWeb.
Statistical analysis
All samples were prepared in triplicate and the experiments were repeated three times. Statistical analysis was measured by analysis of variance (ANOVA) with SPSS 19.0 (SPSS Inc., Chicago, USA). One-way ANOVA with a 95% confidence interval was used to assess the significance of the results obtained. The ANOVA data with p < 0.05 were considered statistically significant. Results were expressed as the mean value ± standard deviation.
Results and discussion
Effect of PEF treatment on the immunogenic properties of OVA
The immunogenic properties were evaluated by IgG and IgE binding abilities, which were assessed by ELISA with rabbit polyclonal antibodies and egg allergy patients’ sera, respectively. The IgG and IgE binding capacities of OVA induced by PEF treatment at various intensities and times are shown in and , respectively. ) shows a gradual increase in the IgG binding ability of OVA treated at 20−25 kV/cm, in comparison to untreated OVA. However, in the range of 30−35 kV/cm, the IgG binding of OVA was significantly decreased. The PEF intensity of 35 kV/cm gave the highest decline of the IgG binding ability, which was employed in subsequent experiments. ) illustrates a significant increase in IgG binding of OVA induced by PEF at 35 kV/cm for 60 µs, in comparison to that of untreated OVA. However, the IgG binding capacity of OVA was gradually decreased when the PEF treating time was above 60 µs, and it tended to be stable when the duration exceeded 180 µs.
Figure 1. Effect of PEF intensity and treatment time on IgG binding ability of OVA. A: PEF treatment time was 180 μs; B: PEF intensity was 35 kV/cm. Means with different letters (a−e) in the bars are significantly different (p < 0.05).
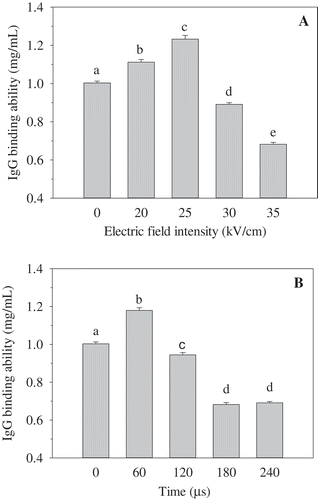
Figure 2. Effect of PEF treatment on IgE binding ability of OVA. A: PEF treatment time was 120 μs; B: PEF electric field intensity was 35 kV/cm. P1–P4 stand for the egg-allergy patients numbered 1, 2, 3, and 4, respectively. Means with different letters (a−e) in the bars are significantly different (p < 0.05).
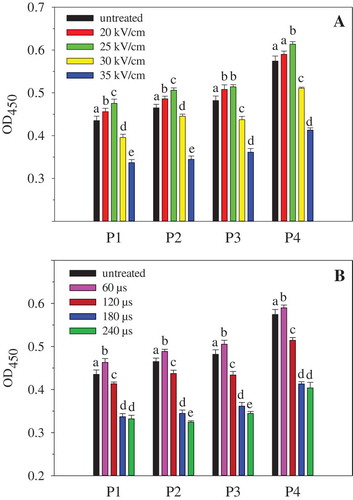
The IgE binding ability of OVA under different PEF processing conditions is shown in . The results were similar to those shown in . PEF treatment of lower intensity (20−25 kV/cm) or shorter time (60 µs) resulted in an increase in IgE binding of OVA, whereas higher intensity (30−35 kV/cm) or longer treatment time (120−240 µs) led to a significant decrease in IgE binding of OVA. The results in indicated that the sera of different patients had different IgE binding abilities to the same sample. However, the influence of PEF on the binding of IgE from different patients was similar.
According to some reports, PEF could change the structure of food proteins. Egg white proteins were partially unfolded and aggregated after PEF treatment at 25 kV/cm.[Citation14] The secondary and tertiary structures of whey protein were also changed by PEF processing.[Citation25] Alterations in the conformational structure of protein may result in changes of the IgG and IgE binding abilities,[Citation3,Citation22] which depend on the integrity of the IgG and IgE epitopes. Therefore, alteration of the immunogenic properties of OVA may be attributed to structural changes induced by PEF. To show the relationship between the immunogenic and structural properties of OVA induced by PEF, the structural changes were measured in subsequent experiments.
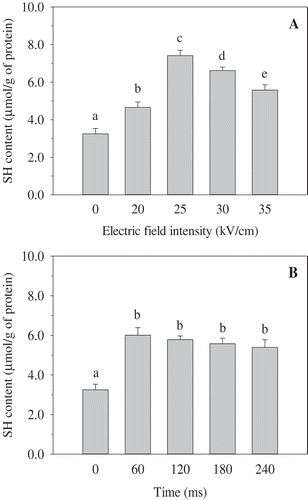
Effect of PEF on free SH content of OVA
Sulphydryl (-SH) and disulphide bonds (-S-S-) are related to protein functional properties, such as gelling and emulsifying. There are four free SH (C11, C30, C367, and C382) and one disulphide bond (C73−C120) in an OVA molecule.[Citation26] As shown in , PEF treatment had a significant influence on the free SH content of OVA. In the range 20−25 kV/cm, the free SH content of OVA was significantly increased. Meanwhile, at intensity above 25 kV/cm, the free SH content was progressively and significantly decreased ()). The intensity of 25 kV/cm resulted in the highest increase. At 35 kV/cm, a significant increase of the free SH content in the range of 0−60 μs was observed, whereas a slight decrease of the free SH content was found in the range of PEF treatment time 120−240 μs ()). The PEF treatment time of 60 μs gave the maximum free SH content. Moreover, the free SH content of OVA treated by PEF was higher than that of untreated OVA. The result suggested that PEF treatment induced unfolding of OVA and exposed internal sulphydryl bonds buried inside the molecule to the surface. PEF could make OVA become reactive with DTNB by partial unfolding protein or enhancing SH ionization.[Citation27] However, high strength or long duration of PEF processing could reduce the free sulphydryl groups by forming disulphide bridges. This indicated protein aggregation through the formation of intramolecular disulphide bonds.[Citation19] It is similar to the findings reported in a previous study,[Citation28] wherein they found that the increase of free sulphydryl was the result of partial unfolding of soybean protein isolate (SPI) under moderate PEF conditions, while the decrease resulted from the aggregation of protein by rigorous PEF conditions. However, Wu et al.[Citation14] observed that the free sulphydryl of egg white proteins progressively increased with treatment duration in the range of 200−800 μs at 25 kV/cm. The different results could be attributed to the different proteins under study and the un-identical PEF treatment conditions.
Effect of PEF on the surface hydrophobicity of OVA
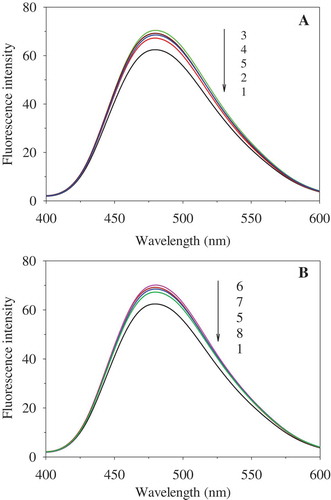
ANS has been widely used to measure protein surface hydrophobicity, which is one of the characteristics for evaluating conformational changes in proteins. The fluorescence spectra of PEF-treated OVA are depicted in . When the PEF treatment time was 180 μs, the fluorescence intensity of OVA progressively enhanced with increase in PEF intensity (0–25 kV/cm), with the maximum value observed at 25 kV/cm. Then it decreased after the PEF strength exceeded 25 kV/cm (30 and 35 kV/cm) ()). When the PEF intensity was 35 kV/cm, it increased with increasing treatment time, reached the maximum point at 60 μs, and then declined on further increasing the treatment duration ()). PEF could cause the movement of charged amino acid residues and destroy the intramolecular and intermolecular interactions, including hydrophobic interactions. The destruction of hydrophobic interactions caused by PEF treatment of low intensity or short time resulted in the exposure of hydrophobic groups and regions inside the molecules, thereby increasing the surface hydrophobicity of OVA. However, OVA molecules treated by PEF processing of high intensity or long time might have aggregated through hydrophobic interactions due to the so-called hydrophobic collapse.[Citation29] Self-association aggregation of protein may have occurred as a consequence of an increased protein–protein interaction through hydrophobic interactions. This could have led to the formation of dimers, trimers, or oligomers,[Citation30] resulting in the decrease of surface hydrophobicity. Similarly, the surface hydrophobicity of lysozyme was significantly improved with increase in PEF treatment time at 35 kV/cm and remarkably declined when the time exceeded 600 μs.[Citation21] In addition, the surface hydrophobicity of SPI was enhanced with increase in PEF strength (0–30 kV/cm) when treated for 288 μs, while it declined at 40 kV/cm.[Citation28]
Figure 4. Effect of PEF treatment on ANS-binding fluorescence of OVA. A: PEF treatment time was 180 μs; B: PEF electric field intensity was 35 kV/cm. 1−5 represent OVA treated for 180 μs at 0, 20, 25, 30, and 35 kV/cm, respectively. 6−8 represent the OVA treated at 35 kV/cm for 60, 120, and 240 μs, respectively. The concentration of OVA samples was 0.4 mg/mL.
Effect of PEF on UV absorption spectra of OVA
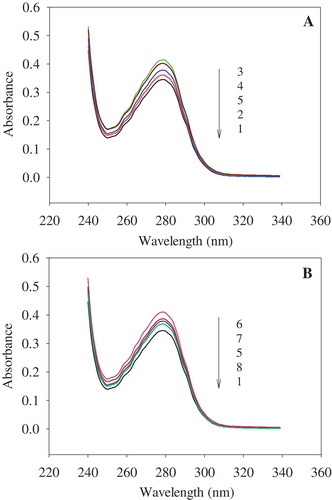
The UV absorbance of protein in the near-UV region depends on the residues of aromatic amino acids, including tryptophan, tyrosine, and phenylalanine. The residue of tryptophan is mainly responsible for the near-UV region.[Citation31] As shown in , the UV absorption intensity at 279 nm was enhanced after PEF processing. When treated for 180 µs, it increased gradually with increasing strength, reaching the maximum absorption at 25 kV/cm. When the PEF strength exceeded 25 kV/cm, absorption was reduced ()). When the PEF strength was increased to 35 kV/cm, the absorption intensity improved with increase of treatment time, reaching the maximum value at 60 μs. It then declined with further increase in PEF treatment time ()). The increase in absorption intensity indicated that tryptophan residues were exposed gradually, which might have been caused by the unfolding of OVA. PEF treatment could significantly affect the non-covalent bonds, such as electrostatic interactions, hydrogen bonds, and hydrophobic interactions.[Citation13] Some tryptophan, closed to the charged amino acids, are easily affected by PEF treatment. The exposure of these amino acids residues might lead to the baring of tryptophan residues. For instance, Trp184, closed to Glu185 (has a charged residue), might be exposed by PEF processing.[Citation17] However, increase in treatment intensity and duration resulted in the decrease in UV absorption intensity. It indicated that some tryptophan residues might have been reburied due to aggregates that formed through both covalent and non-covalent bonds induced by PEF treatment.[Citation14] The aggregation of OVA resulted in the decrease of UV absorption intensity.
Figure 5. Effect of PEF treatment on UV absorption of OVA. A: PEF treatment time was 180 μs; B: PEF electric field intensity was 35 kV/cm. 1−5 represent OVA treated for 180 μs at 0, 20, 25, 30, and 35 kV/cm, respectively. 6−8 represent the OVA treated at 35 kV/cm for 60, 120, and 240 μs, respectively. The concentration of OVA samples was 0.4 mg/mL.
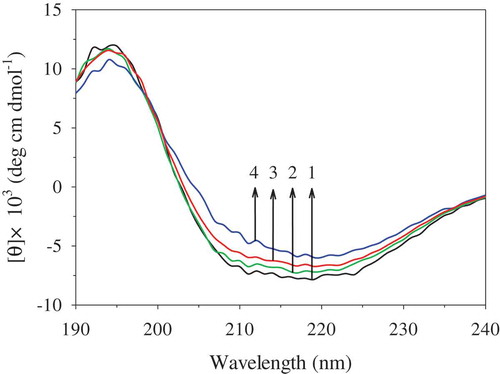
Effect of PEF on the far-UV CD spectra of OVA
shows that the far-UV CD spectra of OVA were significantly changed after PEF treatment. When the OVA samples were treated at 35 kV/cm for 60 µs, the intensities of positive and negative peaks were not significantly changed, compared to those of the untreated OVA. However, when the treatment time was prolonged to 120 µs and 180 µs, the intensities of the negative peaks at 208 nm and 222 nm were gradually decreased, indicating a loss of α-helix structure. The results shown in are consistent with this observation. It shows that PEF treatment at 35 kV/cm for 60 µs could not significantly change the secondary structure of OVA, while PEF processing for longer time (120−180 µs) significantly decreased the content of the α-helix structure and slightly increased the content of the β-sheet structure. Similar phenomena have also been found in peroxidase and lysozyme. The α-helix fractions of peroxidase were reduced by 55.4% after PEF treatment at 25 kV/cm for 124 µs[Citation32] and those of lysozyme were decreased after PEF treatment at 25−35 kV/cm for 1200 μs.[Citation30] However, PEF treatment at 35.6 kV/cm for 200−500 μs caused the disruption of β-sheet in the secondary structure of the pepsin.[Citation33] These different results might be caused by different proteins and PEF treatment conditions. The α-helix structure was susceptible to conformational changes by high electric field due to its dipole moment.[Citation34] The protein with a certain amount of α-helix has the potential to be affected by electric fields. Therefore, the effects of PEF treatment on OVA caused a conformational change of the protein, resulting in loss in stability of some amount of the α-helix structure.
Table 1. Effect of PEF treatment on the content (%) of secondary structures of OVA.
Relationship between the immunogenic and structural properties of OVA after PEF treatment
PEF treatment significantly influenced the conformational structure of OVA, which was closely related to IgG and IgE bindings of OVA. When the OVA samples were treated at low strength (below 25 kV/cm) for 180 µs or at 35 kV/cm for a short time (less than 60 µs), the free SH content, fluorescence intensity, and UV absorption were progressively increased, indicating the unfolding of the tertiary structure of OVA. Unfolding of the tertiary structure finally led to the increase of IgG and IgE binding abilities. The local electrostatic fields of protein solution play an essential role in proteins, including folding, molecular recognition, and catalytic functions.[Citation35] However, the external electric field could affect the local electrostatic fields in proteins and disrupt the electric interactions of peptide chains. PEF treatment could induce the movement of free electrons, ions, and other charged particles, polarization, and the displacement of bound charges, electrons in atoms, and atoms in molecules. It also could change the orientation of the molecular dipole moment and increase the dielectric constant of the molecules.[Citation14] These factors caused the dissociation of non-covalent bonds and led to the unfolding of polypeptides. Afterwards, the tertiary structure of OVA became loose and more allergenic and antigenic epitopes were exposed on the surface of OVA molecules. This could have caused more IgG and IgE antibodies to bind to epitopes, finally resulting in the increase in the IgG and IgE binding abilities of OVA. However, with further increase in PEF strength (above 25 kV/cm) and treatment duration (higher than 60 µs), the free SH, fluorescence intensity, and UV absorption of OVA gradually declined. The initial step for protein interaction and aggregation upon PEF treatment is the unfolding of the protein molecules. Partially unfolded protein intermediates could lead to protein aggregation. PEF treatment at higher intensity or longer duration resulted in the exposure of more hydrophobic and thiol groups forming protein aggregates through disulphide bonds and hydrophobic interactions. This might have buried some epitopes and eventually decreased the IgG and IgE binding abilities of OVA.
Moreover, the secondary structure of OVA was significantly affected by PEF treatment. The IgG and IgE epitopes were widely spread along the whole sequence of OVA and throughout its conformation. They were composed primarily of hydrophobic amino acids, followed by polar and charged residues, and comprised α-helix and β-sheet structures. The charged residues could be easily affected by electric fields produced by PEF. When treated at 35 kV/cm for 60 µs, the secondary structure of OVA remained stable. However, when treated at harsh conditions for a long time (more than 120 µs), the α-helix content of OVA was significantly decreased. This could have altered some epitopes and hence they were unable to bind to IgG or IgE antibody. Therefore, the alterations of secondary and tertiary structures induced by PEF treatment resulted in changes in the immunogenic properties of OVA.
Conclusion
PEF treatment significantly changed the conformational structure, which influenced the immunogenic properties of OVA. High strength and long duration of PEF processing reduced its immunogenic properties, with the largest decrease observed after treatment at 35 kV/cm for 180 μs. Therefore, PEF processing has the potential to decrease the allergenicity of OVA. In the future, the functional properties of OVA should be studied and PEF treatment conditions should be optimized. In addition, changes in the allergenicity of OVA during digestion in vitro should also be studied.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (NSFC) (No. 31360374), Implementation Plan of Science and Technology in Higher Education Institutions of Jiangxi Province (No. KJLDI3019), and Excellent Youth Foundation of Jiangxi Province (No. 20162BCB23017).
References
- Yang, W.-H.; Tu, Z.-C.; Wang, H.; Li, X.; Tian, M. High Intensity Ultrasound Enhances the IgG and IgE Binding of Ovalbumin. J. Sci. Food Agric. 2017, 97, 2714–2720. DOI: 10.1002/jsfa.2017.97.issue-9.
- Verhoeckx, K. C. M.; Vissers, Y. M.; Baumert, J. L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; Van Der Bolt, N.; Wichers, H.; Kimber, I. Food Processing and Allergenicity. Food Chem. Toxicol. 2015, 80, 223–240.
- Tong, P.; Gao, J.; Chen, H.; Li, X.; Zhang, Y.; Jian, S.; Wichers, H.; Wu, Z.; Yang, A.; Liu, F. Effect of Heat Treatment on the Potential Allergenicity and Conformational Structure of Egg Allergen Ovotransferrin. Food Chem. 2012, 131, 603–610.
- Ma, X. J.; Chen, H. B.; Gao, J. Y.; Hu, C. Q.; Li, X. Conformation Affects the Potential Allergenicity of Ovalbumin after Heating and Glycation. Food Addit. Contam. Part A. Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1684–1692.
- Lee, J.-W.; Seo, J.-H.; Kim, J.-H.; Lee, S.-Y.; Byun, M.-W. Comparison of the Changes of the Antigenicities of a Hen’s Egg Albumin by a Gamma and an Electron Beam Irradiation. Radiat. Phys. Chem. 2007, 76, 879–885.
- López-Expósito, I.; Chicón, R.; Belloque, J.; Recio, I.; Alonso, E.; López-Fandiño, R. Changes in the Ovalbumin Proteolysis Profile by High Pressure and Its Effect on IgG and IgE Binding. J. Agric. Food Chem. 2008, 56, 11809–11816.
- Chen, H.; Virk, M. S.; Chen, F. Phenolic Acids Inhibit the Formation of Advanced Glycation End Products in Food Simulation Systems Depending on Their Reducing Powers and Structures. Int. J. Food Sci. Nutr. 2016, 67, 400–411.
- Tao, X.; Chen, J.; Li, L.; Zhao, L.; Zhang, M.; Sun, A. Influence of Pulsed Electric Field on Escherichia Coli and Saccharomyces Cerevisiae. Int. J. Food Prop. 2015, 18, 1416–1427.
- Zhao, W.; Tang, Y.; Lu, L.; Chen, X.; Li, C. Review: Pulsed Electric Fields Processing of Protein-Based Foods. Food Bioprocess Technol. 2014, 7, 114–125.
- Puértolas, E.; Martínez De Marañón, I. Olive Oil Pilot-Production Assisted by Pulsed Electric Field: Impact on Extraction Yield, Chemical Parameters and Sensory Properties. Food Chem. 2015, 167, 497–502. DOI: 10.1016/j.foodchem.2014.07.029.
- Chen, J.; Tao, X.-Y.; Sun, A.-D.; Wang, Y.; Liao, X.-J.; Li, L.-N.; Zhang, S. Influence of Pulsed Electric Field and Thermal Treatments on the Quality of Blueberry Juice. Int. J. Food Prop. 2014, 17, 1419–1427. DOI: 10.1080/10942912.2012.713429.
- Yogesh, K. Pulsed Electric Field Processing of Egg Products: A Review. J. Food Sci. Technol. 2016, 53, 934–945.
- Wu, L.; Zhao, W.; Yang, R. J.; Yan, W. X. Pulsed Electric Field (Pef)-Induced Aggregation between Lysozyme, Ovalbumin and Ovotransferrin in Multi-Protein System. Food Chem. 2015, 175, 115–120.
- Wu, L.; Zhao, W.; Yang, R.; Chen, X. Effects of Pulsed Electric Fields Processing on Stability of Egg White Proteins. J. Food Eng. 2014, 139, 13–18.
- Wu, L.; Zhao, W.; Yang, R.; Yan, W.; Sun, Q. Aggregation of Egg White Proteins with Pulsed Electric Fields and Thermal Processes. J. Sci. Food Agric. 2016, 96, 3334–3341.
- Zhao, W.; Yang, R. J.; Tang, Y. L.; Zhang, W. B.; Hua, X. Investigation of the Protein-Protein Aggregation of Egg White Proteins under Pulsed Electric Fields. J. Agric. Food Chem. 2009, 57, 3571–3577.
- Nisbet, A. D.; Saundry, R. H.; Moir, A. J. G.; Fothergill, L. A.; Fothergill, J. E. The Complete Amino-Acid-Sequence of Hen Ovalbumin. Eur. J. Biochem. 1981, 115, 335–345.
- Yamasaki, M.; Takahashi, N.; Hirose, M. Crystal Structure of S-Ovalbumin as a Non-Loop-Inserted Thermostabilized Serpin Form. J. Biol. Chem. 2003, 278, 35524–35530.
- Zhao, W.; Yang, R. J. Pulsed Electric Field Induced Aggregation of Food Proteins: Ovalbumin and Bovine Serum Albumin. Food Bioprocess Technol. 2012, 5, 1706–1714.
- Bi, X.; Liu, F.; Rao, L.; Li, J.; Liu, B.; Liao, X.; Wu, J. Effects of Electric Field Strength and Pulse Rise Time on Physicochemical and Sensory Properties of Apple Juice by Pulsed Electric Field. Innov. Food Sci. Emerg. Technol. 2013, 17, 85–92.
- Zhao, W.; Yang, R.; Lu, R.; Tang, Y.; Zhang, W. Investigation of the Mechanisms of Pulsed Electric Fields on Inactivation of Enzyme: Lysozyme. J. Agric. Food Chem. 2007, 55, 9850–9858.
- Zhong, J. Z.; Liu, W.; Liu, C. M.; Wang, Q. H.; Li, T.; Tu, Z. C.; Luo, S. J.; Cai, X. F.; Xu, Y. J. Aggregation and Conformational Changes of Bovine β-lactoglobulin Subjected to Dynamic High-Pressure Microfluidization in Relation to Antigenicity. J. Dairy Sci. 2012, 95, 4237–4245.
- Beveridge, T.; Toma, S. J.; Nakai, S. Determination of SH- and SS- Groups in Some Food Proteins Using Ellman’s Reagent. J. Food Sci. 1974, 39, 49–51.
- Matulis, D.; Lovrien, R. 1-Anilino-8-Naphthalene Sulfonate Anion-Protein Binding Depends Primarily on Ion Pair Formation. Biophys. J. 1998, 74, 422–429.
- Xiang, B. Y.; Ngadi, M. O.; Ochoa-Martinez, L. A.; Simpson, M. V. Pulsed Electric Field-Induced Structural Modification of Whey Protein Isolate. Food Bioprocess Technol. 2011, 4, 1341–1348.
- Ishimaru, T.; Ito, K.; Tanaka, M.; Tanaka, S.; Matsudomi, N. The Role of the Disulfide Bridge in the Stability and Structural Integrity of Ovalbumin Evaluated by Site-Directed Mutagenesis. Biosci. Biotechnol. Biochem. 2011, 75, 544–549.
- Fernandez-Diaz, M. D.; Barsotti, L.; Dumay, E.; Cheftel, J. C. Effects of Pulsed Electric Fields on Ovalbumin Solutions and Dialyzed Egg White. J. Agric. Food Chem. 2000, 48, 2332–2339.
- Li, Y. Q.; Chen, Z. X.; Mo, H. Z. Effects of Pulsed Electric Fields on Physicochemical Properties of Soybean Protein Isolates. LWT Food Sci. Technol. 2007, 40, 1167–1175.
- Eyles, S. J.; Radford, S. E.; Robinson, C. V.; Dobson, C. M. Kinetic Consequences of the Removal of a Disulfide Bridge on the Folding of Hen Lysozyme. Biochemistry 1994, 33, 13038–13048.
- Zhao, W.; Yang, R. The Effect of Pulsed Electric Fields on the Inactivation and Structure of Lysozyme. Food Chem. 2008, 110, 334–343.
- Divsalar, A.; Saboury, A. A.; Ahmad, F.; Moosavi-Movahedi, A. A. Effects of Temperature and Chromium (III) Ion on the Structure of Bovine β-Lactoglobulin-A. J. Braz. Chem. Soc. 2009, 20, 1782–1789.
- Zhong, K.; Wu, J.; Wang, Z.; Chen, F.; Liao, X.; Hu, X.; Zhang, Z. Inactivation Kinetics and Secondary Structural Change of PEF-treated POD and PPO. Food Chem. 2007, 100, 115–123.
- Zhao, W.; Yang, R. Effect of High-Intensity Pulsed Electric Fields on the Activity, Conformation and Self-Aggregation of Pepsin. Food Chem. 2009, 114, 777–781.
- Budi, A.; Legge, F. S.; Treutlein, H.; Yarovsky, I. Effect of Frequency on Insulin Response to Electric Field Stress. J. Phys. Chem. B 2007, 111, 5748–5756.
- Park, E. S.; Boxer, S. G. Origins of the Sensitivity of Molecular Vibrations to Electric Fields: Carbonyl and Nitrosyl Stretches in Model Compounds and Proteins. J. Phys. Chem. B 2002, 106, 5800–5806.