ABSTRACT
The effect of chitosan (CH) coating combined with electrolyzed oxidizing water (EOW) on microbiological and physicochemical of hairtail (Trichiutus haumela) was evaluated during 15 days of storage at −3°C. Hairtail samples treated by EOW combined with 1.5% (w/v) CH were compared with samples soaked in single material. For storage of 15 days, the thiobarbituric acid value of hairtails increased steadily to 1.79, 1.52, 1.48 mg/100g (p < 0.05) in the EOW, CH, and EOW+CH group, respectively. All of these were significantly lower than that of the control group (2.01 ± 0.04 mg malonaldehyde/100g; p < 0.05). Microbiological results, total volatile basic nitrogen, pH, and peroxide value also revealed that EOW and CH significantly retarded hairtail spoilage. The sodium dodecyl sulfate–polyacrylamide gel electrophoresis pattern of sarcoplasmic proteins revealed CH+EOW could slow down the protein deterioration of fillet. In this study, CH coating combined with EOW effectively extended the shelf life of hairtail to 6–7 days.
Introduction
Hairtail (Trichiutus haumela) is one of the most abundant commercial marine fish which is widely distributed in the East China Sea and Sea of Japan.[Citation1] Because of the high amount of unsaturated fatty acid, hairtail is extremely perishable during the storage and transportation. To avoid this undesirable economic loss, chilled storage is adopted to deal with the problem of aquatic logistics. It contributes to the commercialization of hairtail as a fresh product.
Electrolyzed oxidizing water (EOW), also known as acidic electrolyzed water, has been regarded as a novel and effective antimicrobial agent in recent years. It is easily (and thus usually) generated by taking from the anode side of the electrolysis of a dilute NaCl solution.[Citation2] The resultant EOW has lower pH values and higher oxidation reduction potential (ORP) values and contains available chlorine. While neutral electrolyzed water (NEW) are referred to as mixed oxidants which are commonly produced by two different types of NEW generators. EOW has been found to be effective in inhibiting microbial growth, protein decomposition, and lipid oxidation of American shad.[Citation3] Xuan et al.[Citation4] found that EOW acted on Listeria monocytogenes by partly disrupting the cytoplasm of cell, inhibiting cellular respiration, changing the permeability of cell membrane, inducing intracellular macromolecules leakage, and eventually leading to cell death. Chen et al.[Citation5] found that slightly acidic EOW and EOW were effective against Vibrio parahaemolyticus due to changes they caused in cell membrane permeability, protein synthesis activity, and adenosine triphosphate biosynthesis pathways. It was also confirmed Wang et al.[Citation6]that acidic electrolyzed water treatment could completely suppress the proliferation of Vibrio parahaemolyticus on shrimp in combination with refrigeration temperature.Recently, Mansur and Oh[Citation7] also observed that the microbial populations on kale treated with slightly acidic EOW were much lower compared to those on untreated control. The available chlorine in EOW may play the key role in bactericidal activity according to Cui et al.[Citation2] Although EOW has been confirmed to effectively inactivate several food-borne microorganisms, little information is available on the application of EOW on hairtail preservation.
Chitosan (CH), a partially deacetylated chitin (poly-β-(1→4)N-acetyl-D-glucosamine), is a biocompatible polysaccharide with strong antimicrobial activities, which has been employed in the preservation of seafood products.[Citation8] CH coating has been shown to significantly improve the texture parameters and sensory scores of shrimp compared with a control sample.[Citation9] The antimicrobial properties of CH have been reported by Genskowsky et al.[Citation10] that films containing only CH were effective against some Gram-positive bacteria. It also has been found to be effective for preservation of silver carp fillets during refrigerated storage.[Citation11] However, the influence of the combined use of EOW and CH coating on quality of hairtail has never been studied. Therefore, the aim of the present study was to investigate if the combined treatment of EOW and CH could extend the shelf life of hairtail in chilled storage, from both microbiological and physicochemical aspects.
Materials and methods
Fish sample preparation
Hairtails were purchased from a local market in Zhoushan, China. Then, fish were kept in ice and transported to laboratory within 30 min. Hairtails were de-headed, gutted, and washed before being cut into fillets of 7 cm to 10 cm. The fish samples were randomly assigned into four groups: an uncoated control group, an EOW-treated group, a CH coating group, and CH+EOW group. The hairtails in the EOW group and CH group were soaked in the pH 4.5 of EOW and 1.5% CH solutions at a fillet/solution ratio of 1:2 (w/v) at 4°C for 15 min, respectively. The hairtails in EOW+CH group were immersed into the pH 6.1 EOW at a fillet/solution ratio of 1:2 (w/v) for 15 min followed by soaking in 1.5% of CH solutions at a fillet/solution ratio of 1:2 (w/v) for 15 min. All samples were drained, packed in air-proof polyethylene pouches, and stored at −3°C. The dorsal meat was taken from hairtail fillets for the quality assessment tests.
Preparation of EOW and chitosan
EOW was generated in a slightly acidic electrolyzed water generator (Beijing Zhouji Environmental Science & Technology Co Ltd., China), by electrolysis of a dilute HCl solution (1%) and NaCl (0.03%) for 1 min. The obtained EOW was then diluted in deionized water to produce EOW with an available chlorine concentration (ACC) of 30 mg/L which was determined by using an available chlorine analyzer (Palintest Co Ltd., England). The ORP and pH were determined immediately before treatment with a dual-scale pH meter (PB-10 pH-ORP, Sartorius Co Ltd., Germany). The EOW had a pH of 4.5 and an ORP of 867.4 mV. Food grade CH (molecular weight: 100,000–150,000) was purchased from Qingdao BZ Oligo Biotech Company Ltd. (Qingdao, China). CH solution was prepared with 1.5% (w/v) CH in 1% (v/v) acetic acid.
Quality assessment
Total viable count (TVC), total volatile basic nitrogen (TVB-N), pH, thiobarbituric acid (TBA), peroxide value (POV), and drop loss of samples were evaluated at day 0, 1, 3, 6, 9, 12, and 15, respectively, during storage.
Microbiological analyses: Determination of TVC in a food product is one of the simplest and widely used microbiological techniques.[Citation12] The TVC assessment was done by taking fillet samples of 5.0 g, homogenizing these in 45 mL sterile saline for 1 min and then transfering 1 ml of this homogeneous liquid to the agar plate. Plates were incubated at 37°C for 48 h and then TVC was measured. All samples were carried out in triplicate.
Assay of TVB-N: The determination of TVB-N was performed following the method of Khalafalla et al.[Citation13] The analysis included a perchloric acid extraction followed by alkalinization and steam distillation. Five grams of fish fillet was mixed with 45 mL of perchloric acid and homogenized for 2 min at 15,000 rpm. Then, the solution was centrifuged for 10 min with the revolving speed of 4000 r/min. After that, the supernatant was stored at 2°C–6°C. Total volatile bases were finally titrated with hydrochloric acid in a boric acid solution using a kjeldahl distillation unit (K9860, Jinan Hanon Co. Ltd., China). The concentration of samples was expressed as N mg/100 g. According to Ruiz-Capillas and Moral (2001), the maximal recommended limit is 40 mg/100 g for TVB-N.
Determination of TBA: TBA of hairtail was measured through a modified version of Lee and Yoon[Citation14] and Schmidt et al.[Citation15] Ten grams of hairtail fillet muscle was shredded and then mixed with 50 mL of trichloroacetic acid (0.1% ethylenediaminetetraacetic acid (EDTA)) for 30 s to produce a homogenous output. After filtration, the solution was centrifuged for 4 min with the revolving speed of 4000 rpm by centrifuge. The mixture of 5 mL of supernatant and 5 mL of TBA (0.2 M) was heated in a boiling water bath (100°C) for 30 min. The absorbance of the cooled solution was measured at 532 nm by a spectrophotometer (UV-255, Shimadzu, Instruments of Mfg. Co. Ltd.). The standard curve was prepared using 1,1,3,3-tetraethoxypropane at the volume ranging from 0.04 to 0.28 mL (equivalent to 0.4–0.24 mg of malondialdehyde), which dissolved in 3.86% perchloric acid. The concentration of TBA in hairtail was expressed as mg of malonaldehyde (MDA)/kg sample.
Assay of pH value: The pH value was evaluated by a pH meter (model pH 430, Corning Inc., Corning, NY, USA) which was calibrated with a standard buffer solution. Two grams of the samples was minced and mixed with 20 mL of deionized water. Each sample was detected for three times.
Assay of POV: The measurement of POV used a modified colorimetric method.[Citation16] Five grams of hairtail fillets was mixed with dichloromethane-methanol (7:3, v/v) and diluted with deionized water to 25mL. Then, 9 mL of dichloromethane-methanol and 1 mL of the solution was added with a drop of ferrous chloride (3.5 g/L) which was incubated with potassium thiocyanate at room temperature. Finally, the absorbance of sample was determined at 500 nm against a blank that contained all the reagents except the sample by using a spectrophotometer.
Assay of drop loss: The drop loss of samples was measured according to Boonsumrej et al.[Citation17] The hairtail fillets were weighed before and after freezing. The results were expressed as:
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE): The sarcoplasmic proteins were extracted by a modification of the method described in Teixeira et al.[Citation18] Hairtail (20 g) was added to cold buffer (200 mL, 100mmol/L sodium chloride, 1 mmol/L EDTA, 20 mmol/L sodium dihydrogen phosphate, pH 7.0; 4°C), then homogenized and centrifuged (10,000 g, 4°C, 10 min). The supernatant was frozen at −80°C before use. Sarcoplasmic protein solutions were diluted 4:1 in loading buffer (0.5 mmol/L Tris–HCl, 10% glycerol, 20 mg/mL SDS, 0.25% (v/v)β-mercaptoethanol, and 0.1%(w/v) bromophenol blue at pH 6.8) and heated for 10 min at 100°C before gel running. For SDS-PAGE, 10 μL of the sample was loaded in per well on the polyacrylamide gel made of 5% stacking gel and 12% separating gel, and then subjected to electrophoresis at 100 V for 80 min (Tanon EPS300, Tanon Inc., Beijing, China). After that, the gels were stained using 0.05% (w/v) Coomassie Brilliant Blue R-250 in 50% (v/v) methanol and 9.2% (v/v) glacial acetic acid, then destained using 10% (v/v) glacial acetic acid and 10% (v/v) methanol.
Statistical analysis
Data were expressed as mean ± SD. Significant differences were evaluated at p < 0.05 by analysis of variance and statistical analysis was performed using Origin version 8.5 (Origin Lab Corp., Northampton, MA, USA).
Results and discussion
Assay of TVC
According to Ko et al.[Citation19], the level of spoilage of aquatic products can be directly reflected by microbial proliferation, regarding the maximal limit of 7 log10 CFU/g for TVC as spoilage. Thus, VC typically is conducted to obtain information about the microbiological quality of seafood, which is an elementary proxy for fish freshness.
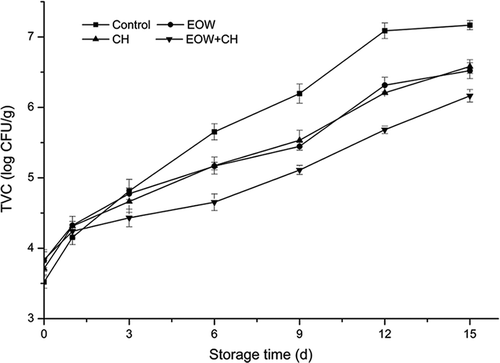
The TVC of the fillet samples from day 0 to day 15 is shown in . Values of TVC increased gradually with the storage time during the whole test period in ice. Compared to the control group, the microbial growth rate of the sample groups was markedly lower, indicating inhibition by 1.5% CH and EOW (pH 4.5). The TVCs of control, CH, EOW, and CH+EOW group on day 6 were 5.65 ± 0.12, 5.17 ± 0.11, 5.17 ± 0.09 4.65 ± 0.11 log10 CFU/g, respectively. On day 9, the TVC value of control group reached 6.24 log10 CFU/g, indicating a severe reduction in the edible quality of the fish. On day 12, it reached 7 log10 CFU/g which is considered as spoilage of raw fish.[Citation19] Fillets treated by EOW or CH individually showed the similar preservation effect. However, a CH coating combined with EOW could extend the shelf life of samples to three more days by retarding the enhancement of the TVCs, which is in agreement with that of Moreno-Vásquez et al.[Citation20] Their results suggested that CH functionalized with epigallocatechin gallate has potential antibacterial activity. CH mainly attack on the outer surface of the bacteria where the positively charged NH3+ group interacts with the negatively charged bacterial cell membranes, leading to the leakage of proteins and constituents of the bacteria and cell agglutination.[Citation21] Blank CH showed great bacterial growth inhibition against Staphylococcus aureus and Pseudomonas sp. Meanwhile, Li et al.[Citation22] confirmed that EOW was an effective disinfectant, even at low ACC.
Assay of TVB-N value
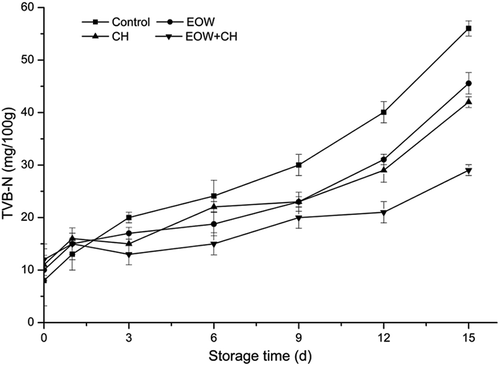
The changes of TVB-N values in hairtail stored at chilled temperatures are illustrated in . A value of 40 mg /100g is considered the start of spoilage.[Citation19] At day 0, TVB-N values of all groups were 8.012, 10.02, 11.01, and 11.99 mg N/100g, respectively, similar to the results of Khalafalla et al.[Citation13] The difference in the TVB-N value of the samples at day 0 may be due to the individual differences of hairtail meat and is not significant. On day 6, TVB-N value of the control group increased, whereas it was steady from day 0 to day 6 in experimental groups. On day 9, the TVB-N of the control group reached 30.01 mg N/100 mg – exceeding the 12-day reading of the EOW group and CH group. On day 12, the value of this group reached the value for decomposition. In EOW+CH group, TVB-N value remained 29 mg/100g on the fifteenth day, remaining acceptable for consumption throughout the entire storage period.
As shown in , TVB-N values increased slowly at the first 9 days in all the samples. The results were in agreement with the findings of Briones-Labarca et al.[Citation23] Thereafter, TVB-N increased rapidly due to protein autolytic deamination as well as the adenosine monophosphate (AMP) which released ammonia nitrogen via deamination.[Citation24] The reaction accumulated the dimethylamine and trimethylamine, leading to TVB-N value rise accordingly. A large amount of amino acids were decomposed by microorganisms and intensified deamination which resulted in a rapid increase in TVB-N value.
Compared to , the changes of TVB-N value had great correlation with the total bacterial count, possibly because that the protein decomposion by bacteria increased the content of volatile basic nitrogen directly. The results showed that EOW+CH could slow down the bacterial decomposition of protein. Moreover, the 1.5% CH group and EOW group prolonged the shelf life of hairtail by 2–3 days. On the other hand, the increase of TVB-N value of samples treated by CH coating+EOW was significantly less than those treated by EOW or CH alone, suggesting that CH coating maintained freshness 6 or 7 more days and demonstrated synergism in retarding of TVB-N value when used as a combination with EOW.
Assay of pH
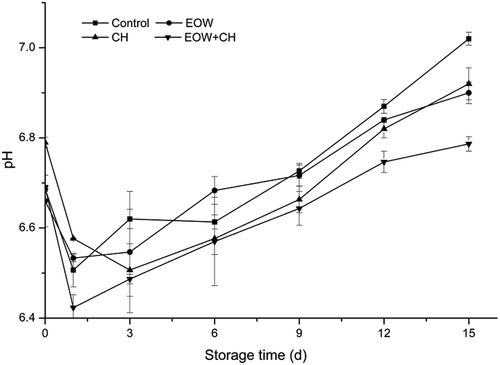
The detection of pH is one of the most frequently used methods to evaluate the quality of seafood products. The pH changes of the hairtail fish muscle during the 15 days at refrigerated temperature were showed in . The initial pH values of fillet samples in experimental groups and the control group were about 6.70, which was higher than that (6.5 and 6.6) reported by Xu et al.[Citation3] Variations amongst the initial values of pH may be linked to the harvesting season, fishing methods, transport, and the position of fish muscle. pH decreased slightly on day 1 versus day 0 in all sample groups and then increased rapidly thereafter. The decreasing pH values might be due to the lactic acid produced by glycogen decomposition.[Citation25] Moderate increases in pH happened from day 1, along with protein decomposition and accumulation of alkaline substance. The results were in accordance with Santiago et al.,[Citation26] who reported similar changes of pH values in Cyprinus carpio steaks stored in modified atmosphere packaging. While the pH of all samples increased after day 1, the pH value of fillets treated by EOW, CH, and CH +EOW was comparatively lower (p < 0.05) than the control group on the 3rd, 9th, 12th, and 15th days of storage. As proteins were decomposed into amino acids and other basic compounds, amino acids were transferred into ammonia and amine by bacteria simultaneously. It took a longer time to reach the minimum and peak of rigor stage for the experimental groups. Moreover, the combination of EOW and CH was more effective in inhibiting microbial activity than either treatment alone, resulting in a lower pH (p < 0.05), reaching a value of 6.78 ± 0.1 on day 15. The pH values of CH group and EOW group were similar at the end of storage, which were markedly higher than EOW+CH group. According to the information above and based on the results obtained in this study, the fillet samples treated by EOW+CH kept the lowest pH level for at least 15 days compared to other groups.
Assay of TBA value
The hairtail meat in this study is rich in proteins and fat, especially unsaturated fatty acids, which is susceptible to the pro-oxidants and oxygen attack and form hydroperoxides that easily decompose to secondary products, including MDA. Thus, TBA value was commonly used to judge the content of secondary peroxidation product malondialdehyde and the degree of fat oxidation.[Citation27]
As shown in , the TBA values were unstable during storage and showed an overall increasing trend for all sample groups. It was reported that MDA combined with amidogen of fish can decrease the TBA value over a short time interval.[Citation28] However, peroxidation would lead to the increasing of MDA and result in a rise of TBA value.
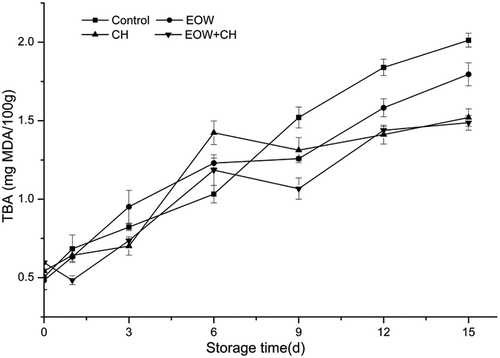
The hairtail is a fatty variety of fish, which is susceptible to lipid oxidation. The TBA values in four treatment group were in range of 0.5–1.0 mg MDA/100g from day 0 to day 3. After 15 days of storage, TBA was 1.79 ± 0.07 mg MDA/100g in EOW group, 1.52 ± 0.05 mg MDA/100g in CH group, and 1.48 ± 0.04 mg MDA/100g in EOW+CH group, respectively. All of these were significantly lower than that of the control group (2.01 ± 0.04 mg MDA/100g; p < 0.05). The reduction in lipid oxidation by EOW+CH could be contributed to the inhibition of microbial activity. Due to the thick CH coating cutoff contact with oxygen, the TBA values of the CH group and CH+EOW showed the slowest increase. TBA value of EOW group increased at the end of storage, which probably caused decomposition of EOW. While TBA values were shown to be slightly higher in EOW group, the effect CH+EOW was significantly different from CH alone. The results observed in this study showed that CH coating combined with EOW could be applied to antioxidant ability.
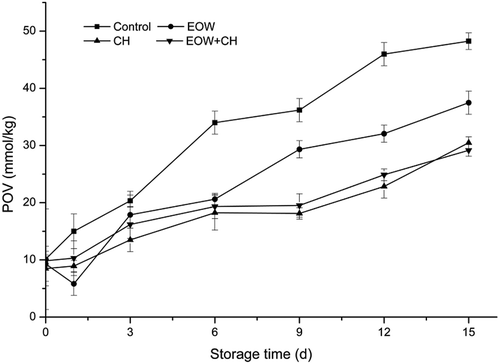
POV
POV is another important index of lipid and fatty acid oxidation. High POV values generally correspond with a high degree of oxidative rancidity in fish. Changes in the POV of the control group and treated hairtail fillets were presented in . Initial POV of all groups were in the range of 8.5–10 mmol/kg. An acceleration of lipid oxidation was observed in all groups, but this increase was relatively higher in the control group compared to other experimental groups. The POV increased significantly (p < 0.05) on day 6 of storage and reached a maximum of 48.24 ± 1.44 mmol/kg on day 15 in the group without any treatment. Although the POV of EOW group declined slightly on day 1, it increased to 37.48 ± 2.02 mmol/kg after 14 days. The sample became oxidized and sensorially unacceptable similar to the control group.
The CH group and CH+EOW group also saw the POV increased progressively throughout storage. Samples coated with CH stored at chilled temperature for up to 15 days increased in the POV from 8.48 mmol/kg to 30.49 mmol/kg fish muscle. Likewise, the POV of EOW+CH group increased from 9.85 mmol/kg to 29.16 mmol/kg. This finding was related to the change of TBA value, indicating that antioxidant capacity of EOW was time limited. These results show that CH+EOW can delay lipid oxidation effectively. In addition to other aspects influencing fish sensory quality, fillet samples coated with CH alone could be taken into consideration for prolonging shelf life as well.
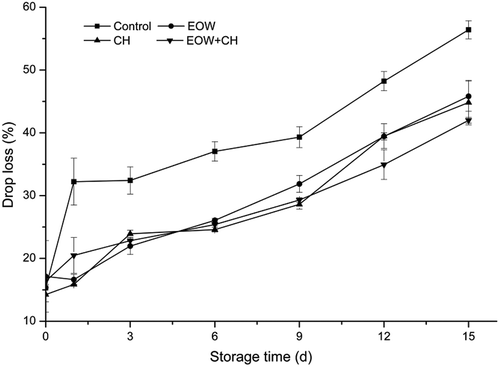
Drop loss
Drop loss of fish meat is closely related to the composition of the fish muscle. In addition to fish species, growth environment greatly influences the muscle composition of a fish. Drop loss was influenced by many factors, such as protein denaturation, lipid oxidation, and even the freshness of the fish meat itself.
A significant (p < 0.05) difference was observed for drop loss in the control group during storage period, which increased from 15.31 ± 0.8% on day 0 to 56.41 ± 1.4% on day 15 as shown in . It is confirmed that fish meat becomes tougher with a progressive loss of fluid.[Citation29] Without any treatment, fish muscle in control group underwent deterioration and its water-holding capacity reduced rapidly. The drop loss of EOW group decreased to 16.63 ± 0.8% on day 1, possibly resulting from changes in membrane permeability of the fish muscle cell. Then, the drop loss increased gradually at the following storage period. Drop loss of EOW+CH group exhibited a continuous upward trend in accordance with CH group. The results indicated that 1.5% CH and EOW (pH = 4.5) had similar preservation effect and delayed fillet drop loss effectively. Different from those microindexes mentioned above, drop loss is a macroindex to evaluate the quality of hairtail fish meat, whereas its decline is bound to affect fish muscle texture and other indexes. In this study, CH+EOW could inhibit microbial growth, slow down the speed of fatty oxidation and pH increase, and lower protein denaturation, thus reducing the drop loss of fish to a certain extent.
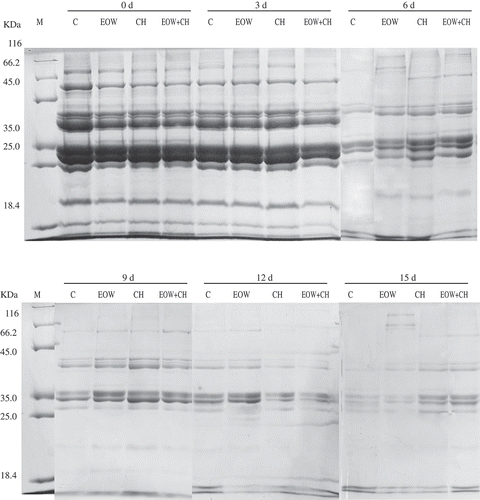
SDS–PAGE patterns
The first changes occurring in postmortem fish muscle are hydrolytic reaction of muscle proteins and connective tissue.[Citation30] Protein solubility and stability can be influenced by many different factors such as species, refrigerated condition, and time span of storage. Thus, the amount and type of proteins bands can be used as a proxy for fish freshness and quality.
The SDS–PAGE patterns of hairtail sarcoplasmic proteins treated with CH, CH+EOW, and CH, respectively, are depicted in , and the molecular weight was in the range of 18.4–116 kDa. On day 1 and day 3, there were nine SDS-PAGE bands. The pattern indicated the bands with molecular weights between 116kDa and 66.2 kDa disappeared after 6 days of storage, with an obvious decrease in the intensity of the bands (25.0–18.4 kDa). The bands of control sample showed a general degradation on day 12 and almost entirely disappeared on day 15. However, the bands of EOW group, CH group, and EOW+CH group were dimly visible even at the end of storage period. Whilst, bands of CH group had demonstrated that CH had effective function on maintaining the quality of sarcoplasmic protein until day 12. This can be explained as CH delaying molecular changes of protein molecules. [Citation31] Although some changes of protein bands in EOW group seemed to have no enhancement of protein stability at the decomposition stage, EOW would not produce adverse effect on the quality of sarcoplasmic proteins based on the research of Wang et al.[Citation32] Besides, sarcoplasmic proteins in EOW and EOW+CS group were at a lower degradation level on day 15. To the best of our knowledge, very few studies relate to the changes in sarcoplasmic proteins during postmortem storage. This study primarily demonstrated that the combination of EOW and CH was effective in maintaining the quality of sarcoplasmic protein and inhibiting its hydrolytic reaction, thus preserving the quality of hairtail.
Figure 7. SDS–PAGE patterns of sarcoplasmic protein from hairtail muscle treated with EOW and chitosan alone or in combination. M: marker; C: untreated control; EOW: hairtail fillets treated with electrolyzed oxidizing water alone; CH: hairtail fillets treated with chitosan alone; EOW+CH: hairtail fillets treated with EOW and chitosan.
Conclusion
The results of TVC, TVB-N value, TBA value, pH value, POV, and drop loss revealed that 1.5% CH or EOW treatment would extend the freshness of the hairtail by 2–3 days compared to the control group. CH coating combined with EOW effectively inhibited microbial growth, slowed the increase of TVB-N and lipid oxidation, and thus could prolong the shelf life by 6 to 7 days. Sarcoplasmic proteins in EOW and EOW+CH group were less degraded at the end of storage. In general, EOW+CH had better preservation effect on hairtail than either treatment alone. Therefore, CH coating combined with EOW has a great potential for hairtail preservation. The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Zhao, X.;. In Situ Target-Strength Measurement of Young Hairtail (Trichiurus haumela) in the Yellow Sea. ICES J Marine Sci. J Du Conseil, 2006, 63, 46–51.
- Cui, X. D.; Shang, Y. C.; Shi, Z. G.; Xin, H. W.; Cao, W. Physicochemical Properties and Bactericidal Efficiency of Neutral and Acidic Electrolyzed Water under Different Storage Conditions. J Food Eng, 2009, 91, 582–586.
- Xu, G. C.; Tang, X.; Tang, S. H.; You, H. B.; Shi, H. W. Combined Effect of Electrolyzed Oxidizing Water and Chitosan on the Microbiological, Physicochemical, and Sensory Attributes of American Shad (Alosa sapidissima) during Refrigerated Storage. Food Control, 2014, 46, 397–402.
- Xuan, X. T.; Liu, D. H.; Ding, T. Lethal Injury Mechanism of Slightly Acidic Electrolyzed Water on Listeria Monocytogenes. J. Food Saf. Qual., 2015, 6, 245–251.
- Chen, T. Y.; Kuo, S. H.; Chen, S. T.; Wang, D. F. Differential Proteomics to Explore the Inhibitory Effects of Acidic, Slightly Acidic Electrolysed Water and Sodium Hypochlorite Solution on Vibrio Parahaemolyticus. Food Chem, 2016, 194, 529–537.
- Wang, J. J.; Sun, W. S.; Jin, M. T. Fate of Vibrio Parahaemolyticus on Shrimp after Acidic Electrolyzed Water Treatment. Int. J. Food Microbiol., 2014, 179, 50–56.
- Mansur, A. R.; Oh, D. H. Combined Effects of Thermosonication and Slightly Acidic Electrolyzed Water on the Microbial Quality and Shelf Life Extension of Fresh-Cut Kale during Refrigeration Storage. Food Microbiol., 2015, 51, 154–162.
- Chang, S. H.; Lin, H. T.; Wu, G. J.; Tsai, G. J. pH Effects on Solubility, Zeta Potential, and Correlation between Antibacterial Activity and Molecular Weight of Chitosan. Carbohydr Polym, 2015, 134, 74–81.
- Yuan, G. F.; Lv, H.; Tang, W. Y.; Zhang, X. J.; Sun, H. Y. Effect of Chitosan Coating Combined with Pomegranate Peel Extract on the Quality of Pacific White Shrimp during Iced Storage. Food Control, 2016, 59, 818–823.
- Genskowsky, E.; Puente, L. A.; Pérez-Álvarez, J. A.; Fernandez-Lopez, J.; Muñoz, L. A.; Viuda-Martos, M. Assessment of Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Maqui Berry (Aristotelia chilensis). LWT Food Sci. Technol., 2015, 64, 1057–1062.
- Ramezani, Z.; Zarei, M.; Raminnejad, N. Comparing the Effectiveness of Chitosan and Nanochitosan Coatings on the Quality of Refrigerated Silver Carp Fillets. Food Control, 2015, 51, 43–48.
- Diez-Gonzalez, F. Total Viable Counts | Specific Techniques. Encyclopedia Food Microbiol, 2014, 3, 630–635.
- Khalafalla, F. A.; Ali, F. H.; Hassan, A. R. Quality Improvement and Shelf-Life Extension of Refrigerated Nile Tilapia (Oreochromis niloticus) Fillets Using Natural Herbs. Beni-Suef Univ. J. Basic Appl. Sci., 2015, 4, 33–40.
- Lee, Y. J.; Yoon, W. B. Effects of Particle Size and Heating Time on Thiobarbituric Acid (TBA) Test of Soybean Powder. Food Chem, 2013, 138, 841–850.
- Schmidt, R.; Klemme, D. A.; Scow, K.; Hristova, K. Microbial Biosafety of Pilot-Scale Bioreactor Treating MTBE and TBA-contaminated Drinking Water Supply. J. Hazard. Mater., 2012, 210, 524–528.
- Mehta, B. M.; Darji, V. B.; Aparnathi, K. D. Comparison of Five Analytical Methods for the Determination of Peroxide Value in Oxidized Ghee. Food Chem, 2015, 185, 449–453.
- Boonsumrej, S.; Chaiwanichsiri, S.; Tantratian, S.; Suzuki, T.; Takai, R. Effects of Freezing and Thawing on the Quality Changes of Tiger Shrimp (Penaeus monodon) Frozen by Air-Blast and Cryogenic Freezing. J Food Eng, 2007, 80, 292–299.
- Teixeira, B.; Fidalgo, L.; Mendes, R.; Costa, G.; Cordeiro, C. Changes of Enzymes Activity and Protein Profiles Caused by High-Pressure Processing in Sea Bass (Dicentrarchus labrax) Fillets. J. Agric. Food Chem., 2013, 61, 2851–2860.
- Ko, W. C.; Yang, S. Y.; Chang, C. K.; Chang, W. Effects of Adjustable Parallel High Voltage Electrostatic Field on the Freshness of Tilapia (Orechromis niloticus) during Refrigeration. LWT Food Sci. Technol., 2016, 66, 151–157.
- Moreno-Vásquez, M. J.; Valenzuela-Buitimea, E. L.; Plascencia-Jatomea, M. Functionalization of Chitosan by a Free Radical Reaction: Characterization, Antioxidant and Antibacterial Potential. Carbohydr Polym, 2017, 155, 117–127.
- Shahidi, F.; Arachchi, J. K. V.; Jeon, Y. J. Food Application of Chitin and Chitosans. Trends Food Sci. Technol., 1999, 10, 37–51.
- Li, J.; Ding, T.; Liu, X. Y. Synergetic Effects of Ultrasound and Slightly Acidic Electrolyzed Water against Staphylococcus Aureus Evaluated by Flow Cytometry and Electron Microscopy. Ultrason Sonochem, 2016, 38, 711–719.
- Briones-Labarca, V.; Perez-Won, M.; Zamarca, M.; Aguilera-Radic, J. M.; Tabilo-Munizaga, G. Effects of High Hydrostatic Pressure on Microstructure, Texture, Colour and Biochemical Changes of Red Abalone (Haliotis rufecens) during Cold Storage Time. Innovative Food Science Emerging Technologies, 2012, 13, 42–50.
- Graciela, B.; Isabel, E.; Nuria, M.; Maria, D.; Amparo, C. Influence of Storage Conditions on Some Physical and Chemical Properties of Smoked Salmon (Salmo salar) Processed by Vacuum Impregnation Techniques. Food Chem, 2003, 81, 85–90.
- Bahmani, Z. A.; Rezai, M.; Hosseini, S. V.; Regenstein, J. M.; Böhme, K.; Alishahi, A.; Yadollahi, F. Chilled Storage of Golden Gray Mullet (Liza aurata). LWT Food Sci. Technol., 2011, 44, 1894–1900.
- Santiago, P. A.; Carmen, G. S.; Ricardo, P. M. Assessment of Quality Changes in Frozen Sardine (Sardina pilchardus) by Fluorescence Detection. J. Am. Oil Chemists’ Soc., 1998, 75, 575–561.
- Xu, J. L.; Riccioli, C.; Sun, D. W. Efficient Integration of Particle Analysis in Hyperspectral Imaging for Rapid Assessment of Oxidative Degradation in Salmon Fillet. J Food Eng, 2016, 169, 259–271.
- Woodward, B.;. Dietary Vitamin Requirements of Cultured Young Fish with Emphasis on Quantitative Estimates for Salmonids. Aquaculture, 1994, 124, 133–168.
- Ocaño-Higuera, V. M.; Marquez-Ríos, E.; Canizales-Dávila, M.; Castillo-Yáñez, F. J.; Pacheco-Aguilar, R. Postmortem Changes in Cazon Fish Muscle Stored on Ice. Food Chem, 2009, 116, 933–938.
- Li, T. T.; Li, J. R.; Hu, W. Z.; Chen, J. R.; Li, H. J. Protein Changes in Post Mortem Large Yellow Croaker (Pseudosciaena crocea) Monitored by SDS-PAGE and Proteome Analysis. Food Control, 2014, 41, 49–55.
- Feng, X.; Bansal, N.; Yang, H. Fish Gelatin Combined with Chitosan Coating Inhibits Myofibril Degradation of Golden Pomfret (Trachinotus blochii) Fillet during Cold Storage. Food Chem, 2016, 200, 283–292.
- Wang, M.; Wang, J. J.; Sun, X. H.; Pan, Y. J.; Zhao, Y. Preliminary Mechanism of Acidic Electrolyzed Water Ice on Improving the Quality and Safety of Shrimp. Food Chem, 2015, 176, 333–341.