ABSTRACT
Biological activity of Habanero pepper is principally due to capsaicinoids, flavonoids, carotenoids and peptides. However, the volatile fraction of this fruit has not been analysed earlier. Isolation of the volatile compounds from two varieties of Habanero pepper was performed by simultaneous distillation-extraction method. Gas chromatography-mass spectrometry analysis revealed 109 and 110 compounds in Mayapan and Jaguar varieties, respectively. The main compounds found were hexyl 3-methylbutanoate, 3,3-dimethylcyclohexanol, hexyl 3-methylbutanoate, (Z)-3-hexenyl 3-methylbutanoate and heptyl 3-methylbutanoate. Highest antioxidant activity was achieved from extracts of the Jaguar variety by means of the ABTS•+ method. Minimum inhibitory concentration analysis showed that V. cholerae was the most sensitive bacteria to the volatile extracts from both varieties.
Introduction
Pepper fruits (Capsicum spp.) are a significant source of bioactive compounds such as vitamins C and A, carotenoids, phenolic compounds, terpenoids, steroids and alkaloids known for their health promoting effect against degenerative diseases.[Citation1] The genus Capsicum comprises more than 200 varieties; the fruits vary widely in size, shape, flavour and sensory heat. The five main species of Capsicum cited in the literature are as follows: C. annuum, C. baccatum, C. chinense, C. frutescens and C. pubescens.[Citation2] The Yucatan is one of the main producers of C. chinense Jacq. In Mexico, also known as Yucatan Habanero pepper. Due to its designation of origin acquired in 2010 as well as to its cultural, culinary and economic value, this is one of the main horticultural crops in the area with high potential for industrialisation and exportation.[Citation3]
Habanero pepper is a highly aromatic fruit and is the hottest chili pepper in the world. The typical aroma is one of its most attractive properties, representing a quality parameter for the consumer.[Citation4,Citation5] Previous works have found 102 and 53 volatile compounds (GC-MS) in Habanero pepper from the Yucatan by utilising simultaneous steam distillation-solvent extraction and headspace solid-phase microextraction, respectively.[Citation3,Citation4] Depending on the ripeness of each species and the isolation method, the aroma of pepper fruits was varied due to differences in the volatile compounds profiles.[Citation6] The volatile fraction is generally constituted by low molecular weight compounds and a class of lipophilic secondary metabolites with high vapour pressure. These constituents have been shown to exhibit antimicrobial[Citation7–Citation9], antioxidant[Citation10], anticancer[Citation11], antidiabetic, and antithrombotic properties.[Citation12] Several scientific studies have reported biological activity of the C. chinense as antimicrobial[Citation13], anti-inflammatory[Citation14], antioxidant[Citation15] and anticancer.[Citation16] This effect is mainly due to the presence of capsaicinoids, flavonoids, and carotenoids.[Citation17,Citation18] However, the volatile fraction from this fruit has never been analysed in regards to the active function.
The aim of this study was to analyse the chemical composition of the volatile fraction of two varieties (Mayapan and Jaguar) of Habanero pepper and to investigate other properties of volatile extracts, such as antioxidant and antibacterial activity in vitro, in order to focus on the benefits of this fruit as a functional food in the human diet.
Material and methods
Chemicals and reagents
Pure chemicals were obtained from Aldrich (St. Louis, MO, USA) as follows: n-alkane solution (C8-C32), solution, a mixture of C5-C7, methyl nonanoate, chemical standards, dichloromethane, 2,2ʹ-azinobis-3-ethylbenzothiazoline-6-sulphonate (ABTS), potassium persulphate, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), p-iodo-nitrotetrazolium violet (INT) and dimethylsulfoxide (DMSO).
Samples
Fresh Habanero pepper fruits on mature stage () were provided by the National Institute for Forestry Agriculture and Livestock (INIFAP) from the Yucatan, Mexico. Jaguar and Mayapan varieties of Habanero pepper have been registered as CHL-008–101109 and CHL-009–170908 respectively by National Service Seed Inspection and Certification (SNICS).
Table 1. Characteristics of the two varieties of Habanero pepper.
Extraction of volatiles
Fresh fruits (200 g) were cut and homogenise with distilled water (600 mL) in a commercial blender for 1 min. The resultant puree was immediately transferred into 2 L-flask of an apparatus for simultaneous steam distillation–solvent extraction (SDE) and 500 µL (35.7 mg/mL) of methyl nonanoate as internal standard was added. Dichloromethane (40 mL) was utilised as the extracting solvent and the condenser was kept to 5°C. The SDE system was operated for 1 h as reported previously.[Citation19] The volatile extract was dried over anhydrous sodium sulphate and concentrated on a Kuderna-Danish evapourator until nearly 1 mL was obtained. The final volume of 0.2 mL was obtained by means of a gentle nitrogen stream. Extractions were done in triplicate.
Gas chromatography-mass spectrometry
GC-MS analyses were performed on a Perkin Elmer Clarus 500 (Shelton, CT, USA) gas chromatograph coupled to a MSD (Shelton, CT, USA). Injection was on splitless mode (ratio 1:10) at 250°C. Separation was performed on an AT-5 MS column (30 m x 0.25 mm x 0.50 μm film thickness) (Alltech, IL, USA). The column temperature was programmed as follows: 50 °C for 2 min then increased to 250°C at a rate of 4°C/min, held at 250°C for 10 min. The gas carrier (helium at 99.99% of purity) flow rate was 1 mL/min and detector temperature was at 250°C. Mass spectrometer parameters were as follows: electron impact ionisation mode at 70 eV; acquisition range, m/z 35–400; interface and source temperatures were 250°C and 250°C, respectively.
The retention times of n-alkanes mixture were used to calculate lineal retention indexes (LRI) for all identified compounds and for reference standards. Identification of volatile compounds was performed comparing their LRI and mass spectra with the authentic standards from Sigma-Aldrich. Tentative identification of compounds, for which it was not possible to find reference compounds, was performed by comparison of their mass spectra with those from various libraries (NIST 02, Wiley 275, Palisade 600 and our specific library for volatile compounds, Flavourlib) and experimental LRIs with those reported in the literature.[Citation20] Concentrations were expressed as mg methyl nonanoate equivalents kg of fresh weight, response factors being taken as 1.0 for all compounds with reference to the internal standard and a recovery factor of 70% was considered.
Antioxidant activity
ABTS•+ radical-scavenging assay
The ABTS•+ assay was based on the method described[Citation21] with slight modifications. The ABTS radical cation (ABTS•+ radical dot) was generated by mixing a 7 mm ABTS solution in a 2.45 mm solution of potassium persulphate. The ABTS•+ solution was diluted in ethanol until it reached an absorbance of 0.70 ± 0.02 at 740 nm. Different concentrations (0 to 40 mg/mL) were made by diluting the volatile extract in pure ethanol. A 50 µL aliquot of the volatile extract solution was added in 150 µL of the radical ABTS•+ solution. Absorbance was measured at the time of 8 min in a microtiter plate reader (Thermo Science, Finland) at 740 nm. Measurements were performed in triplicate. A control experiment was conducted by using a solution without the test material. Free radical scavenging activity of each solution was calculated as percentage of inhibition, according to the following equation:
Where Ao: Absorbance of control, A1: Absorbance of sample.
DPPH radical-scavenging assay
The free radical scavenging activity of the extracts of Habanero pepper was measured by using the modified method described early[Citation22] as follows: volumes of 50 μL of the samples (0–40 mg/mL) were mixed with 150 μL of DPPH solution (0.1 mM in ethanol) in a 96-well microtiter plate. After incubation for 30 min in the dark at room temperature, the reduction of the absorbance was measured at 540 nm in a microtiter plate reader (Thermo Science, Finland). All measurements were carried out in triplicate. A pure DPPH solution was used as control.
Antibacterial activity
Bacterial cultures
The antimicrobial activity of the volatile extract was tested against different microorganisms, including Gram-positive bacteria: Staphylococcus aureus (ATCC 25923) and Enterococcus faecalis (ATCC 29212); Gram negatives: Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Vibrio cholera (ATCC 14033). LB (Luria-Bertani) agar was utilised for cultivating bacteria and LB broth medium for MIC test. Inoculum was prepared by making a dilution of the bacterial suspension in sterile NaCl solution until absorbance of 0.1 at 620 nm (1 × 108 cfu/mL) and then (100 μL) a further mixing with 9.9 mL NaCl solution (1 × 106 cfu/mL).
Determination of minimum inhibitory concentrations
A two-fold broth microdilution method was utilised to determine the MIC of the volatile extracts.[Citation23] The volatile extracts were dissolved in DMSO (5%) and in LB broth medium, in order to prepare a stock solution (20 mg/mL). LB broth medium (100 µL) was poured into each of the 96 wells of a microplate and then 100 µL of the stock solution (volatile extracts) was added into the first well, then a serial dilution was carried out by dispensing previous solution into the following well and so on, in order to obtain concentrations from 10 to 0.02 mg/mL (wells 1 to 10). A bacterial suspension of 100 µL was inoculated into the wells. The same test was performed simultaneously for growth control (LB + DMSO) and negative control (LB + DMSO + microorganism). Ampicillin (1 mg/mL) was utilised as a positive control. The plate was incubated at 37°C for 24 h and immediately after, 40 µL of INT solution (0.5 mg/mL) was added to each well. The plates were then incubated for another 60 min to ensure adequate color development. The MIC was determined as the lowest concentration at which it did not reveal any visual growth of microorganisms.
Statistical analysis
The data were analysed statistically by means of the Student’s t-test and analysis of variance for individual parameters was performed by Duncan’s test on the basis of mean values to find the significance at p ≤ 0.05. Statgraphics 5.0 software was utilised for statistical analysis.
Results and discussion
Volatile components analysis
GC-MS profile of the volatile constituents of the Habanero pepper varieties Mayapan and Jaguar demonstrated the presence of a wide range of compounds, including esters, followed by terpenes, long-chain hydrocarbons, alcohols, aldehydes, ketones and acids. In total, 109 and 110 constituents were identified and quantified from Mayapan and Jaguar varieties, respectively (). This finding was similar to those previously reported for other varieties of C. chinense, such as Creole (102 components) from the Yucatan[Citation4] and Cachucha (134 components) from Cuba.[Citation24] The results of this work demonstrated that esters were the predominant compounds in both varieties (29.4 and 30% Mayapan and Jaguar, respectively). The abundance of esters in C. chinense has been determined in the volatile fraction through different extraction methods, such as 35–58% in HS-SPME[Citation5,Citation25–Citation27] and 30–43% in SDE.[Citation4] The most important esters, in terms of their concentration, were hexyl pentanoate, hexyl 3-methylbutanoate and heptyl 3-methylbutanoate. Citronellyl 3-methylbutanoate is reported here for the first time in Habanero peppers from the Yucatan.
Table 2. Volatile compounds (mg/kg) in Habanero pepper cultivated varieties.
The second most important chemical group in both varieties were the terpenes (19%), particularly mono- and sesquiterpene hydrocarbons. The sesquiterpenes γ-himachalene (20.14 and 23.24 mg/mL from Mayapan and Jaguar varieties, respectively), α-copaene (3.0 and 4.28 mg/mL), β-cubebene (6.32 and 1.35 mg/mL) and (Z)-β-farnesene (3.40 and 2.96 mg/mL) were quantitatively predominant, in contrast to Pino et al.[Citation4,Citation19], who found β-caryophyllene and germacrene D as the most abundant sesquiterpenes in a creole variety of Habanero pepper from the Yucatan.
Moreover, a higher amount of cubenenes was determined in fruits of C. chinense from Ecuador.[Citation26] According to a previous study[Citation25] himachalene is one of the most characteristic compounds of the more pungent genotypes of Habanero pepper. Other compounds such as aliphatic alcohols, aldehydes, ketones and acids were qualitatively minor classes in Mayapan and Jaguar varieties. However, 3,3-dimethylcyclohexanol (83.74 and 38.69 mg/mL from Mayapan and Jaguar varieties, respectively) was quantitatively the major alcohol present in both varieties.
Branched hydrocarbons such as 2-methyl-1-tetradecene (6.57 and 7.70 mg/mL from Mayapan and Jaguar varieties, respectively) and 2-methyltetradecane (9.15 and 10.47 mg/mL from Mayapan and Jaguar varieties, respectively) were predominant. The methyl-branched hydrocarbons are thought to be related to capsaicin biosynthesis.[Citation25] The main components in both volatile extracts from Mayapan and Jaguar varieties were hexyl 3-methylbutanoate (84.08 and 140.71 mg/mL from Mayapan and Jaguar varieties, respectively), hexyl pentanoate (90.09 and 34.87 mg/mL), 3,3-dimethylcyclohexanol (83.74 and 38.69 mg/mL) and heptyl 3-methylbutanoate (54.69 and 16.75 mg/mL).
Total yield of volatiles, determined by an internal standard addition, was 539.12 and 357.89 mg/mL for Mayapan and Jaguar varieties, respectively. Similar concentration was reported (110.66–302.53 mg/mL) for Cachucha peppers from Cuba.[Citation24] Lesser concentrations were determined (103–228 mg/mL) in commercial fruits[Citation4] and (1.37–11.84 mg/mL) in experimental varieties of Habanero peppers from the Yucatan.[Citation19] According to the species and level of ripeness, the composition varies due to differences in volatile compound profile.[Citation5,Citation19]
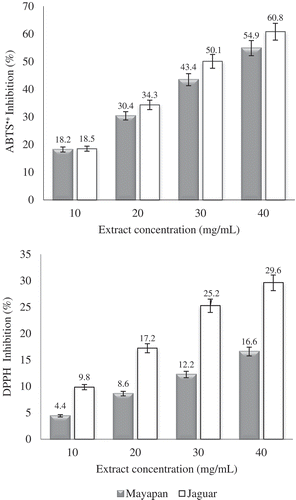
Antioxidant activity
illustrates how the extracts reduce the radicals DPPH and ABTS•+ in a dose-dependent manner. A strong inhibition was observed at 40 mg/mL. However, the values of ABTS•+ were significantly higher than the DPPH values. Certain factors, such as stereo selectivity of the radicals or the solubility of the extract in different testing systems, may affect the capacity of the extracts to react and quench different radicals.[Citation28] On the other hand, inhibition of the extract from the variety Jaguar was significantly higher than the extract of Mayapan variety by the DPPH method. This could be due to mechanisms of the reaction of DPPH with the structural conformations of antioxidant compounds. According to Prior et al.[Citation29] small molecules have facilitated access to the radical site. The volatile extract from Jaguar variety exhibited a higher antioxidant activity in both radicals than Mayapan variety. This is probably due to compounds such as (Z)-3-hexen-1-ol, α-pinene, p-cymene, limonene and γ-terpinene[Citation10,Citation30], whose concentration is greater than in Mayapan variety. Antioxidant activity of ethanolic extracts, hexane extracts and methanolic extracts of C. chinense has been previously reported.[Citation17] However, there are no studies concerning antioxidant activity from volatile extracts. Phenolic compounds, carotenoids, ascorbic acid and capsaicin are principally responsible for antioxidant activity in Capsicum species.[Citation15] However, due to their high boiling points, these organic compounds are not present in the volatile extracts, which explains in part the difference in activity found. According to the results elucidated in the present study, antioxidant activity of volatile fraction of the varieties Mayapan and Jaguar of Habanero peppers depends mainly on concentration of the volatile compounds present in each one.
Antibacterial activity
The results () present effective antimicrobial activity against all microorganisms tested, except for E. coli and E. faecalis (Mayapan volatile extract). The strongest antimicrobial activity was observed with the extract from Jaguar variety. Thus, growing inhibition of S. aureus and P. aeruginosa with Jaguar volatile extract (5 mg/mL) requires less concentration than required with the Mayapan volatile extract (10 mg/mL). Differences may be associated to a higher content of (Z)-3-hexen-1-ol, α-pinene, p-cimene and γ-terpinene in the volatile extract from Jaguar variety. According to other authors[Citation7,Citation30] these components have shown significant antimicrobial activity on S. aureus and P. aeruginosa. Other compounds such as α-terpineol[Citation7], linalool[Citation8] and (E)-β-caryophyllene[Citation31] (some minor constituents in the volatile extracts) are also known to have effective antimicrobial properties. E. faecalis showed inhibition only to extracts of the Jaguar variety (10 mg/mL), which can be attributed to antibacterial properties of high concentrations of γ-terpinene[Citation7,Citation32], α-pinene[Citation7,Citation8], (Z)-3-hexen-1-ol[Citation8] and p-cymene.[Citation7,Citation32] On the other hand, Elaissi et al.[Citation33] suggested that Gram-positive bacteria E. faecalis is more sensitive to p-cymene. The lowest MIC found against V. cholerae, using extracts from Jaguar and Mayapan varieties, were 2.5 and 5 mg/mL, respectively. It is known that antimicrobial activity of essential oils and their components are more pronounced against Gram-positive than against Gram-negative bacteria, since they possess an outer membrane surrounding the cell wall, which inhibits diffusion of hydrophobic compounds through the lipopolysaccharide cover.[Citation9] However, cyclic terpenes such as α-pinene, limonene, p-cymene and γ-terpinene may become accumulated in the lipid bilayer and distort the lipid-protein interaction; thus, the membrane can lose integrity and increase permeability to protons and ions.
Table 3. Minimum inhibitory concentration (MIC) of the volatile extracts from Habanero pepper varieties.
Conclusion
A total of 109 and 110 compounds were identified in the volatile extracts of Mayapan and Jaguar Habanero peppers. Both varieties exhibited the same prevalence of volatile compounds. Nevertheless, the differences between both varieties, by means of the volatile compounds identified by GC-MS, were mainly quantitative rather than qualitative. The volatile extract from the Jaguar variety showed higher antioxidant and antibacterial activity than those from the Mayapan variety. According to the findings of this study, antioxidant and antibacterial activity of volatile extracts of Mayapan and Jaguar Habanero pepper are most probably due to the presence of minor components rather than compounds present in larger quantities.
Additional information
Funding
References
- Silva, L. R.; Azevedo, J.; Pereira, M. J.; Valentão, P.; Andrade, P. B. Chemical Assessment and Antioxidant Capacity of Pepper (Capsicum Annuum L.) Seeds. Food Chem. Toxicol. 2013, 53, 240–248.
- Zimmer, A. R.; Leonardi, B.; Miron, D.; Schapoval, E.; Oliveira, J. R.; Gosmann, G. Antioxidant and Anti-Inflammatory Properties of Capsicum Baccatum: From Traditional Use to Scientific Approach. J. Ethnopharmacol. 2012, 139, 228–233.
- Cuevas-Glory, L. F.; Sosa-Moguel, O.; Pino, J.; Sauri-Duch, E. GC-MS Characterization of Volatile Compounds in Habanero Pepper (Capsicum Chinense Jacq.) By Optimization of Headspace Solid-Phase Microextraction Conditions. Food Analytical Methods 2015, 8, 1005–1013.
- Pino, J.; Sauri, E.; Marbot, R. Changes in Volatile Compounds of Habanero Chile Pepper (Capsicum Chinense Jack. Cv. Habanero) at Two Ripening Stages. Food Chem 2006, 9, 394–398.
- Sousa, E. T.; Rodrigues, F. D. M.; Martins, C. C.; De Oliveira, F. S.; Pereira, P. A. D. P.; De Andrade, J. B. Multivariate Optimization and HS-SPME/GC-MS Analysis of VOCs in Red, Yellow and Purple Varieties of Capsicum Chinense Sp. Peppers. Microchemical J. 2006, 82 (2): 142–149.
- Mazida, M. M.; Salleh, M. M.; Osmanet, H. Analysis of Volatile Aroma Compounds of Fresh Chilli (Capsicum Annuum) during Stages of Maturity Using Solid Phase Microextraction (SPME). J. Food Composition Anal. 2005, 18 (5): 427–437.
- Cosentino, S.; Tuberoso, C. I. G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In Vitro Antimicrobial Activity and Chemical Composition of Sardinian Thymus Essential Oils. Lett. Appl. Microbiol. 1999, 29 (2): 130–135.
- Dorman, H. J. D.; Deans, S. G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316.
- Burt, S.;. Essential Oils: Their Antibacterial Properties and Potential Applications in foods-A Review. Int. J. Food Microbiol. 2004, 94 (3): 223–253.
- Ruberto, G.; Baratta, M. T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chem 2000, 69, 167–174.
- Jia, S. S.; Xi, G. P.; Zhang, M.; Chen, Y. B.; Lei, B.; Dong, X. S.; Yang, Y. M. Induction of Apoptosis by D-Limonene Is Mediated by Inactivation of Akt in LS174T Human Colon Cancer Cells. Oncol. Rep. 2013, 29 (1): 349–354.
- Edris, A. E.;. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile constituents-A Review. Phytotherapy Res. 2007, 21 (4): 308–323.
- Brito-Argáez, L.; Moguel-Salazar, F.; Zamudio, F.; González-Estrada, T.; Islas-Flores, I. Characterization of a Capsicum Chinense Seed Peptide Fraction with Broad Antibacterial Activity. Asian J. Bioche. 2009, 4 (3): 77–87.
- Liu, Y.; Nair, M. G. Capsaicinoids in the Hottest Pepper Bhut Jolokia and Its Antioxidant and Antiinflammatory Activities. Nat. Prod. Commun. 2010, 5, 91–94.
- Rodríguez-Maturino, A.; Valenzuela-Solorio, A.; Troncoso-Rojas, R.; González-Mendoza, D.; Grimaldo-Juarez, O.; Aviles-Marin, M.; Cervantes-Diaz, L. Antioxidant Activity and Bioactive Compounds of Chiltepin (Capsicum Annuum Var. Glabriusculum) and Habanero (Capsicum Chinense): A Comparative Study. Med. J. Plant Res. 2012, 6 (9): 1758–1763.
- Amruthraj, N. J.; Preetham Raj, J. P.; Saravanan, S.; Lebel, L. A. Polar Aprotic Extraction of Capsaicinoids from Capsicum Chinense Bhut Jolokia Fruit for Antimicrobial Activity. Int. J. Biol. Pharm. Res. 2013, 4 (12): 959–964.
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M. R.; Conforti, F.; Statti, G.; Menichini, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum Chinense Jacq. 3cv Habanero. Food Chem 2009, 114 (2): 553–560.
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-Inflammatory Activity of Extracts from Fruits, Herbs and Spices. Food Chem 2010, 122, 987–996.
- Pino, J.; Gonzalez, M.; Ceballos, L.; Centurion-Yah, A. R.; Trujillo-Aguirre, J.; Latournerie-Moreno, L.; Sauri-Duch, E. Characterization of Total Capsaicinoids, Colour and Volatile Compounds of Habanero Chilli Pepper (Capsicum Chinense Jack.) Cultivars Grown in Yucatan. Food Chem 2007, 104, 1682–1686.
- Adams, R. P.;. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: IL, 2001.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1996, 26 (9–10): 1231–1237.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT- Food Sci. Technol. 1995, 28 (1): 25–30.
- Eloff, J. N.;. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Medica 1998, 64, 711–713.
- Pino, J.; Fuentes, V.; Barrios, O. Volatile Constituents of Cachucha Peppers (Capsicum Chinense Jacq.) Grown in Cuba. Food Chem 2011, 125, 860–864.
- Rodríguez-Borruezo, A.; Kollmannserber, S.; González-Mas, C.; Nitz, S.; Nuez, F. HS-SPME Comparative Analysis of Genotypic Diversity in the Volatile Fraction and Aroma-Contributing Compounds of Capsicum Fruits from the Annuum-Chinense-Frutescens Complex. J. Agric. Food Chem. 2010, 58, 4388–4400.
- Kollmannsberger, H.; Rodrıguez-Burruezo, A.; Nitz, S.; Nuez, F. Volatile and Capsaicinoid Composition of Aji (Capsicum Baccatum) and Rocoto (Capsicum Pubescens), Two Andean Species of Chile Peppers. J. Sci. Food Agric. 2011, 91, 1598–1611.
- Bogusz Junior, S.; Melo, A. M. T.; Zini, C. A.; Godoy, H. T. Optimization of the Extraction Conditions of the Volatile Compounds from Chili Peppers by Headspace Solid Phase Micro-Extraction. J. Chromatogr. 2011, 1218 (21): 3345–3350.
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free Radical Scavenging Properties of Wheat Extracts. J. Agric. Food Chem. 2002, 50, 1619–1624.
- Prior, R. L.; Wu, X.; Schaich, K. Standardized Methods for the Determination Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302.
- Dorman, H. J. D.; Surai, P.; Deans, S. G. In Vitro Antioxidant Activity of a Number of Plant Essential Oils and Phytoconstituents. J. Essential Oil Res. 2000, 12 (2): 241–248.
- Del-Vechio-Vieira, G.; Sousa, O. V.; Yamamoto, C. H.; Kaplan, M. A. C. Chemical Composition and Antimicrobial Activity of the Essential Oils of Ageratum Fastigiatum (Asteraceae). Records Nat. Prod. 2009, 3, 52–57.
- Sonboli, A.; Mirjalili, M. H.; Hadian, J.; Ebrahimi, S. N.; Yousefzadi, M. Antibacterial Activity and Composition of the Essential Oil of Ziziphora Clinopodioides Subsp. Bungeana (Juz.) Rech. F. From Iran. Z Naturforsch 2006, 61, 677–680.
- Elaissi, A.; Rouis, Z.; Mabrouk, S.; Salah, K. B. H.; Aouni, M.; Khouja, M. L.; Harzallah-Skhiri, F. Correlation between Chemical Composition and Antibacterial Activity of Essential Oils from Fifteen Eucalyptus Species Growing in the Korbous and Jbel Abderrahman Arboreta (North East Tunisia). Molecules 2012, 17 (3): 3044–3057.