ABSTRACT
β-Lactoglobulin (β-Lg) was treated with ultraviolet light-C (UV-C) at 11.8 W m–2 or ultrasound at 900 W for 15 or 30 min. All treatments increased the β-structure, free sulfhydryl groups (FSH), dityrosine, and ABTS radical scavenging activity, but decreased the α-helicity, random coils, disulfide bonds (S–S), and intrinsic fluorescence intensity. UV-C treatment produced higher surface hydrophobicity (Ho), carbonyl, dityrosine, and antioxidant activity, and lower fluorescence intensity, total sulfhydryl group (TSH), and S–S compared with ultrasound treatment. UV-C treatment for 30 min gave the maximum Ho, carbonyl, dityrosine, and antioxidant activity, but the minimum TSH and S–S contents from β-Lg. Ultrasound treatment for 15 min produced the maximum β-sheet, TSH, and S–S contents, while treatment for 30 min gave the minimum carbonyl, dityrosine, and antioxidant activity. Data obtained in this study suggest that ultrasound and UV-C treatments demonstrated the potential for improving antioxidant activity in β-Lg, especially UV-C treatment.
Introduction
β-Lactoglobulin (β-Lg) is a major whey protein that has wide ranging nutritional, functional, and biological value.[Citation1,Citation2] β-Lg is a small, soluble, and globular protein consisting of 162 amino acids in a single peptide chain with a relative molecular mass of 18.4 kDa.[Citation3] Bioactive peptides derived from β-Lg have antioxidant, antihypertensive, and antimicrobial activities.[Citation4] The presence and position of amino acids in β-Lg are responsible for its antioxidant activity, and the peptidic bond have no positive influence on antioxidant capacity.[Citation5] The antioxidant power of a specific protein depends on the source, type, and structural properties of the protein, and the treatment conditions.
At present, there is growing interest in “green” quality improvements and shelf-life extension technologies in the dairy industry, such as ultraviolet (UV) radiation and ultrasound. UV-C germicidal wavelengths of 200–280 nm can inactivate bacteria and viruses, and prelong the shelf-life of milk or milk-based products.[Citation6–Citation9] UV-C (2.34 × 1019 quanta s–1) irradiation has been shown to cause the loss of native polypeptides, Trp disappearance, and changes in the primary structures of β-Lg.[Citation10] Our previous studies have indicated the effects of high hydrostatic pressure, UV-C (11.8 W m–2), and far-infrared treatments on the allergenicity, digestibility, and antioxidant and antihypertensive activities of α-casein.[Citation11,Citation12] Changes in the primary structure, resulting in dityrosine, N-formylkynurenine, and carbonylation products, have been observed on β-Lg, β-casein or α-lactalbumin after 24 h of UV-C (2.34 × 1019 quanta s–1) exposure.[Citation10] UV-C exposure (10.6 and 63.7 kJ m–2) has also induced the development of large protein aggregates by disulfide exchange and protein backbone cleavage in egg white.[Citation13] Individual proteins can exhibit different degrees of response to UV-C radiation depending on amino acid compositions, molecular structures, and treatment conditions. However, how UV-C affects intra- and intermolecular bonds has not been well-established, and the antioxidant property–structure relationships of β-Lg in milk after UV-C treatments have not been studied.
High-intensity ultrasound has also been used in the dairy industry for emulsification, sterilisation, extraction, degassing, filtration, homogenisation, and enhancing oxidation.[Citation1,Citation14,Citation15] The effect of ultrasound is associated with cavitation, heating, dynamic agitation, shear stresses, and turbulence.[Citation16] Previous research has indicated that ultrasound has a minimal effect on the secondary and tertiary structures of proteins and did not disrupt covalent bonds, and no change in secondary structure was detected in whey protein isolates treated with ultrasonication (24 kHz, 300 W cm–2).[Citation17] Similar results were obtained by another report, which showed that ultrasound caused no change in sulfhydryl content.[Citation1] In contrast, ultrasound can modify the secondary structure of β-Lg and lead to increases in surface hydrophobicity[Citation15] and the propensity of whey protein concentrate to aggregate.[Citation18] However, the free sulfhydryl content, surface hydrophobicity, and ultraviolet absorption of ovalbumin were gradually increased when the ultrasound power was increased from 200 to 600 W.[Citation19] These different results are due to the different protein compositions, pH, and ultrasonication conditions. Nevertheless, the relationship between the potential antioxidant activity and the conformational modification of ultrasound-treated β-Lg has yet to be explained.
A better understanding of the relationship between the molecular level and oxidative/antioxidant activity of β-Lg after treating with different processing methods is important for monitoring the bioactive activity of proteins. Therefore, this study aimed to (i) investigate and compare the structure and conformation of β-Lg molecules treated with ultrasound and UV-C, (ii) evaluate the effects of these two treatments on the oxidative and antioxidant properties of β-Lg, and (iii) correlate the structure and oxidative/antioxidant activity of β-Lg.
Materials and methods
Materials
Fresh cow milk was obtained from Shanghai Bright Hoistan pasture and aseptically transferred to the laboratory at Shanghai Jiao Tong University under refrigerated conditions. β-Lg was isolated from fresh bovine milk using a one-step method[Citation20], and protein concentrations were determined using a BCA kit (Beyotime Institute of Biotechnology, Jiangsu, China).
UV-C treatment
UV-C treatment was carried out using UV-C equipment (UVH30DC3, Shanghai Guoda UV Equipment Co., Ltd, Shanghai, China) at 254 nm with a radiation dose of 11.8 Wm–2. Ten milliliters of protein solutions with concentration of 2.0 mg/mL were placed in a Petri dish (90 mm diameter, 15 mm height), giving a depth of ~1 mm, and exposed to UV-C light for 15 or 30 min.[Citation12]
Ultrasound treatment
Ultrasound treatment was carried out using a high-intensity ultrasound liquid processor (Zealway, China) operating at 40 kHz with an input power of 900 W. A 50 mL (2 mg/mL) of protein solutions in a 100-mL beaker were placed into the ultrasonic tank and treated for 15 or 30 min.[Citation21] Water temperature fluctuation inside the ultrasonic bath was lower than 2°C during the treatment. After treatment, all samples were stored at –20°C until analysis.
SDS-PAGE analysis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed following a previous method using 12% polyacrylamide slab gels.[Citation11] β-Lg samples (80 mg mL–1) were mixed with loading buffer (5% 2-β-mercaptoethanol, 2.5% sodium dodecyl sulfate (SDS), 10 mM Tris-HCl, 2.5 mL glycerol and 0.002% bromophenol blue). All samples were boiled for 5 min before electrophoresis. A 5-μL sample and marker (Spectra Multicolour High Range Protein Ladder, Thermo Scientific, MA, USA) were added to the electrophoretic lane. The gels were stained with 0.1% Coomassie Brilliant Blue R-250.
Intrinsic tryptophan fluorescence spectra
Intrinsic tryptophan fluorescence spectra were obtained using a F4500 fluorescence spectrophotometer (Hitachi High-Tech, Tokyo, Japan) using an excitation wavelength of 295 nm and observing an emission wavelength of 350 nm with a scan rate of 1200 nm min–1. Measurements were performed on pre-prepared β-Lg samples (0.1 mg mL–1) using a slit width of 5.0 nm. All measurements were performed in triplicate and corrected for the corresponding blank sample. β-Lg protein sample concentrations were determined using a BCA kit (Beyotime Institute of Biotechnology, Jiangsu, China).
Fourier transform infrared spectrum (FTIR) analysis
FTIR spectra of β-Lg were recorded using an IRPrestige-21 spectrometer (Nicolet 6700, Thermo Fisher, Waltham, MA, USA). The procedures for sample preparation and data processing were based on a previous method with slight modifications.[Citation19] β-Lg powder (2 mg) was mixed with KBr, ground, and then pressed into a pellet. Spectral measurements were carried out with a resolution of 4 cm–1 in the wavenumber region of 4,000–400 cm–1. Deconvolved spectra were then iteratively curve-fitted with Gaussian band shapes. Bands at 1654 ± 4 cm–1, 1620 ± 20 cm–1, 1680 ± 20 cm–1, and 1645 ± 5 cm–1 corresponded to α-helix, β-sheet, β-turn, and random coils, respectively.[Citation11,Citation22,Citation23] Measurements were performed six times.
Sulfhydryl (SH) and disulfide bond contents
The free SH group (FSH), total SH group (TSH), and disulfide bond (S–S) contents in the β-Lg samples were determined using the following methods. TSH content was determined using a total SH assay kit (Jiancheng Bioengineering Institute, Nanjing, China) according to manufacturer instructions. For SH content determination, 50 µL of 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman’s reagent) was added to 100 μL (1 mg/mL) of β-Lg sample, and the solution was mixed. After binding for 10 min at 25°C, the absorbance at 412 nm was measured. FSH content was determined using a Multiskan GO Microplate spectrophotometer (Thermo Scientific, MA, USA) at 412 nmand calculated as follows: μmol FSH g–1 = 73.53 × A412 × D/C, where A412 is the absorbance at 412 nm, C is the protein concentration (mg mL–1), D is the dilution factor, 3.02, and 73.53 is derived from 106/(1.36 × 104), where 1.36 × 104 is the molar absorptivity of Ellman’s reagent. All measurements were performed in triplicate. The S–S contents were half the difference between TSH and FSH contents.[Citation23]
Surface hydrophobicity (ho)
The Ho of β-Lg sample was determined following a modified previously reported method.[Citation24] A stock solution of 1-aniline 8-naphthalene sulfonate (ANS, 8 mM) was prepared in phosphate buffer (0.01 M, pH 7.6). The protein solutions were diluted with the phosphate buffer (pH 7.6) to a final protein concentration in the range 0.05–0.2 mg mL–1. The fluorescence intensity was recorded at wavelengths of 390 nm (excitation wavelength) and 470 nm (emission wavelength) using an F4500 FL spectrophotometer (Hitachi Co., Japan). The concentrations of the β-Lg samples were determined using a BCA kit (Beyotime Institute of Biotechnology, Jiangsu, China). The initial slope of the fluorescence intensity vs. protein concentration plot was used as the index of Ho.
Carbonyl and dityrosine contents
Carbonyl contents were determined using a previously reported method with slight modifications.[Citation25] The protein carbonyl concentration was measured using a protein carbonyl assay kit (Jiancheng Bioengineering Institute, Nanjing, China) based on the reaction of protein carbonyls with 2,4-dinitrophenylhydrazine (DNPH), which afforded a maroon dye. The samples were measured at 370 nm using a spectrophotometer (Multiskan1510, Thermo Fisher Scientific, USA) and the results are expressed in nmol mg–1 protein.
To determine the dityrosine content, a 2 mL (2 mg/mL) of β-Lg solution was first dissolved in PBS (2 mL, pH 6.7, 0.01 M, containing 0.15 M NaCl) and then analysed using an F4500 FL spectrophotometer (Hitachi, Japan). The fluorescence intensity was recorded at 325 nm excitation and 420 nm emission with a gap width of 10 nm. Protein concentrations were determined using a BCA kit (Beyotime Institute of Biotechnology, Jiangsu, China). Corrected fluorescence was obtained by dividing measured fluorescence by protein concentration (mg/mL). The results were expressed in arbitrary units (A.U.).
Antioxidant activities
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and2,2′-azino-bis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) radical scavenging activity were estimated according to the previous methods [Citation26], with some modifications.
DPPH: Briefly, a 0.8 mL (2 mg/mL) of β-Lg sample or ascorbic acid was added to DPPH solution (1.5 mL, 0.2 mM) freshly prepared in ethanol. The solution was then kept in dark for 30 min. The absorbance at 517 nm was recorded using a microplate reader. Background interferences from absolute ethanol and water were deducted before calculating radical-scavenging activity. Ascorbic acid was used for comparison, and the results are reported as the percentage DPPH radical scavenging activity (%) = (Ac–As)/Ac × 100, where Ac and As are the absorbances of the control and sample, respectively.
ABTS: ABTS stock solution (7.4 mM) was added to potassium persulfate solution (2.6 mM) at a ratio of 1:1 (v/v) and kept in dark for at least 12 h at room temperature. This ABTS•+ solution was then diluted with 20 mL (1:20) of ethanol to an absorbance of 0.7 (±0.02) at 734 nm. A 0.2 mL (2 mg/mL) of β-Lg sample was added to the diluted ABTS•+ solution (0.8 mL). After incubation for 6 min at room temperature, the absorbance at 734 nm was recorded using a microplate reader. The ABTS radical scavenging activity was calculated in the same as described for DPPH.
Reducing power (RP) of β-Lg were investigated as previously described with minor modifications.[Citation12] A 1 mL (2 mg/mL) of β-Lg sample was added to sodium phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 mL, 1% solution), incubated at 50°C for 20 min, and then trichloroacetic acid (2.5 mL, 10%) was added. After centrifugation at 3000 rpm for 10 min (Centorion K24OR-2003 refrigerated centrifuge), 2.5 mL of the upper layer was mixed with 2.5 mL deionised water and 0.5 mL ferric chloride (0.1% solution). Results are expressed as the absorbance values at 700 nm after allowing the solution to stand for 10 min. A high absorbance indicated an increase in the RP.
Statistical analysis
Statistical analysis of variance was performed using SPSS 19.0 statistical data analytical software (SPSS Inc., Chicago, IL, USA). Significant differences between the means were determined using a least significant difference test at p < 0.05. Three replicates were performed for each treatment.
Results and discussion
SDS-PAGE of β-lg
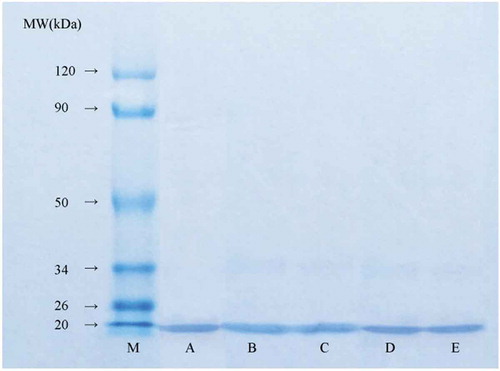
SDS-PAGE was used to produce the electrophoretic profiles of β-Lg shown in . The band of β-Lg was located at approximately 18.4 kDa, which was in agreement with previous reports.[Citation3,Citation10,Citation15] As shown in , no significant changes were observed in the electrophoretic behaviour of β-Lg after ultrasound treatment compared with the control, indicating that β-Lg was still present after exposure to ultrasound without a change in molecular weight induced by protein degradation. Similar results have been obtained in the SDS-PAGE studies of ultrasonication-treated β-Lg,[Citation21] ovalbumin,[Citation19] and black bean protein isolate.[Citation27] The band intensity of β-Lg had slightly decreased after UV-C treatment, especially the 30-min UV-C treatment (). Similarly, UV-C treatment for 15 min resulted in reduced β-Lg band intensities, which reduced more with increasing treatment time.[Citation21] A previous study also reported the removal of α-casein bands after 180 s of UV-light treatment due to the slight breakdown of polypeptide chains.[Citation28] These differences were related to the spectral range, light intensity, sample depth, amino acid composition, and molecular structure.
Secondary structural analysis
FTIR analysis of the amide I band showed that UV-C and ultrasound treatments changed the secondary structural components of β-Lg (). Both treatments significantly decreased the α-helix and random coil contents, while increasing the β-sheet and β-turn contents, compared with the control. The α-helix structure is mainly stabilised by hydrogen bonds between the carbonyl oxygen and amino hydrogen of a polypeptide chain, and these hydrogen bonds may be disturbed by physical effects, such as protein damage, during processing.[Citation29] In addition, protein oxidation might be another cause of the disruptions of α- helical structure, which has been confirmed by previous study that protein oxidation decreased the percentage of α-helix conformations while increasing the β-sheet and random coil content. [Citation30]
Table 1. Secondary structure, SH and S–S contents, and Ho values of the untreated control and treated β-Lg samples.
The α-helix and random coil contents increased with treatment time for ultrasound (from 15 to 30 min), while the β-sheet and β-turn contents increased with treatment time for UV-C (from 15 to 30 min). However, the α-helix and β-structure contents decreased with treatment time for UV-C and ultrasound, respectively. Among the treatments, ultrasound for 15 min generated the highest β-sheet content, and the lowest α-helix and random contents. Moreover, UV-C treatment for 15 min gave the highest α-helix and lowest β-sheet contents. A previous study indicated that UV-C treatment significantly decreased the contents of β-sheet and random structures and increased the content of α-helix and β-turn structures in α-casein.[Citation11] In contrast, an increase in the β-sheet structure with a concomitant decrease in α-helix structure has been observed in black bean protein isolates under sonication.[Citation27] However, ultrasound did not lead to significant changes in the secondary conformation, except for increasing strand 1.[Citation1] These different changes to the secondary structure under the different treatments might be due to different treatment conditions, protein type, dominant secondary structure type, and degree of ordered structure.[Citation18]
Intrinsic tryptophan fluorescence spectra
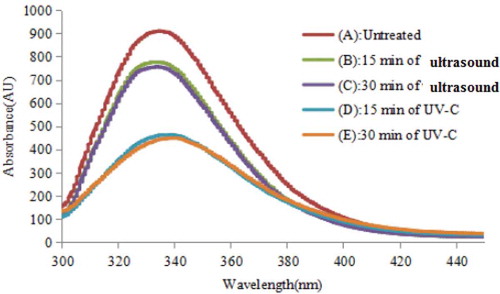
UV-C and ultrasound treatments cause conformational changes in the tertiary structure of proteins, which can be measured using intrinsic fluorescence spectroscopy. The intrinsic fluorescence of protein can be considered, to a first approximation, as that of tryptophan. Therefore, the change in the tertiary structure of β-Lg under different treatments can be monitored using intrinsic emission spectroscopy and fluorescence intensity. shows that UV-C and ultrasound treatments caused the most significant decrease in the intrinsic tryptophan fluorescence absorbance compared with the control. This decrease in fluorescence intensity in the treated samples could be attributed to the quenching of tryptophan fluorescence, or to the oxidation of tryptophan and formation of N’-formylkynurenine.[Citation31] The intrinsic fluorescence emission of the UV-C-treated sample was lower than that of the ultrasound-treated sample, because the tryptophan residues were exposed to the environment of different polarity.[Citation11,Citation24] However, no significant difference was observed between 15-min and 30-min treatments for both UV-C and ultrasound.
Sulfhydryl (SH) and disulfide bond (S–S) contents
It is widely accepted that, a variety of intracellular and extracellular oxidants can transform SH groups into disulfide (S-S) bonds, which can connect residues on the same protein or between two different proteins, causing changes in protein conformation. [Citation32] As a result of these changes, some hydrophobic groups can be exposed on the surface of the protein and can participate in inter- or intra-molecular interactions that can induce aggregation. [Citation33] As shown in , the TSH, FSH, and S–S contents of the control samples were 6.77 ± 0.24, 0.68 ± 0.01, and 3.04 ± 0.21 (×10–2) mmol mg–1 protein, respectively. Compared with the control, both treatments decreased the TSH and S–S contents, and increased the FSH contents. Increased FSH contents were also reported in α-casein after UV-C treatment and in ovalbumin treated with ultrasound. [Citation12,Citation19] Like pulsed light or heat denaturation process, UV-C or ultrasound may induce structure modifications including protein unfolding, which leads to an increase of the exposed hydrophobic groups and a reduction of S-S bonds. [Citation34] The decrease in TSH suggested that β-Lg became progressively denatured and unfolded as heating time increased. The formation of disulfide linkages was evidenced by the reduction in TSH content, which was due to the formation of disulfide bonds through SH oxidation or disulfide interchanges.[Citation32] The S–S contents of β-Lg treated with ultrasound were markedly higher than those treated with UV-C, which can be attributed to the exposure of buried groups during protein unfolding. When β-Lg was treated with UV-C and ultrasound, the contents of TSH and S–S decreased with increasing treatment time. Moreover, UV-C treatment afforded the largest increase in FSH content (after 15 min) and the smallest reduction in TSH and S–S contents (after 30 min) among all treatments (). These changes might be attributed to S–H/S–S interchange reactions, which rearranged the disulfide (S–S) bonds between the intramolecular and intermolecular protein chains during different treatments.[Citation13] Further research is needed in order to investigate the mechanism of protein unfolding and subsequent aggregation of unfolded molecules under UV-C or ultrasound treatment.
Surface hydrophobicity (ho)
The Ho value was used to evaluate the possible exposure of hydrophobic regions, or the conformational difference in the tertiary structures of proteins.[Citation22,Citation24,Citation35] The Ho values of the UV-C treated samples were significantly higher than those of the control and ultrasound treated samples (). An increase in Ho was also observed in α-casein after UV-C treatment for 5 min.[Citation11] This indicated that the proteins unfolded after UV-C treatments, exposing the hydrophobic regions of the protein molecules. Furthermore, the extent of protein unfolding increased with increasing treatment time, as indicated by increased Ho values of 9.64 × 103 (after 15 min) and 11.41 × 103 (after 30 min). However, no statistical difference in Ho values was observed between the control and ultrasound-treated samples (). Previous studies have shown that the surface hydrophobicities of egg white proteins decreased after sonication for more than 5 min.[Citation36] However, ultrasound treatment also causes an increase in the surface hydrophobicity for soy protein isolate (SPI) and ovalbumin.[Citation19,Citation27] The Ho of the ultrasound-treated samples increased due to protein unfolding or decreased due to protein aggregation/partial denaturation of proteins,[Citation27] which was closely related to the protein type and composition, as well as the ultrasound conditions.
Carbonyl and dityrosine contents
Protein carbonyls undergo a noticeable change in oxidised proteins and their concentration is highly indicative of protein oxidation.[Citation37] Carbonyl groups are generated during protein oxidation using hydroxyl radicals. The carbonyl content of the control sample was 1.00 ± 0.04 nmol mg–1 protein (). Both treatments caused a significant increase in the carbonyl content compared with the control. For the 30-min treatments, UV-C produced the highest carbonyl content in β-Lg, reaching 16.73 ± 0.27 nmol mg–1 protein, but ultrasound produced the smallest carbonyl content. This was consistent with a previous report, in which the carbonyl content of raw milk significantly increased after UV-C irradiation.[Citation25] Ultrasound treatment produced significant increase in the carbonyl content compared with the control sample (). This increase might be caused directly by the treatments on amino acid residues such as Trp, Tyr, and Cys, which are involved in protein oxidation.[Citation37] Extensive studies should be conducted to evaluate protein oxidation mechanism under UV-C or ultrasound treatments.
Table 2. Carbonyl and bityrosine content, and antioxidant activities of the untreated control and treated β-Lg samples.
Dityrosine production was used as a useful marker for protein modification by hydroxyl radicals. As shown in , the dityrosine contents of β-Lg after ultrasound and UV-C treatments were increased compared with the control sample (403 AU). Moreover, the UV-C treatments gave higher dityrosine contents than the ultrasound treatments. The results after ultrasound for 15 and 30 min ultrasound showed no significant differences. UV-C treatment for 30 min resulted in a higher dityrosine content than UV-C treatment for 15 min (). Lipid and protein oxidation caused by UV-C treatments in meat and milk have been observed previously.[Citation25]
Antioxidant activity
DPPH is a stable free radical that can accept an electron or hydrogen radical, and is often used as a substrate to evaluate antioxidant activity of peptides and protein hydrolysates.[Citation12] Higher DPPH radical scavenging activities indicate a higher antioxidant activity. As shown in , ultrasound treatments significantly decreased the DPPH radical scavenging activity compared with the control. In contrast, the DPPH radical scavenging activity of ovalbumin has been enhanced using ultrasonic treatment.[Citation38] These conflicting results could be due to different ultrasound conditions (intensity, time, and temperature) and proteins (type, composition, and concentration). In this study, UV-C treatment increased the DPPH radical scavenging activity compared with the control. Similar results have been reported for the change in DPPH radical scavenging activity for α-casein treated with UV-C for 15 min.[Citation12] UV-C treatment gave a higher DPPH radical scavenging activity than ultrasound treatment. No statistical difference in DPPH radical scavenging activities was observed between 15-min and 30-min treatments, irrespective of the treatment. The highest DPPH radical scavenging activity was observed for UV-C treatment (). In this study, no accurate explanations were provided on the mechanisms of DPPH radical scavenging activity change induced by UV-C or ultrasound treatment, which should be investigated in future study.
ABTS radical scavenging activity can be used to evaluate the ability of a sample to convert ABTS•+ to a colourless product through hydrogen-donating or chain-breaking properties. Both treatments significantly increased the antioxidant activity of β-Lg in the ABTS•+ assay, but to different degrees (). Ultrasound treatment gave a lower maximum ABTS radical scavenging activity than UV-C treatment. The ABTS radical scavenging activity increased with exposure time (from 15 to 30 min) for UV-C treatment, but the opposite trend was observed for ultrasound treatment. No reasonable explanation has been determined for these phenomena, which should be investigated further. The 30-min ultrasound and the 30-min UV-C treatments gave the lowest and highest ABTS radical scavenging activity, respectively. Another study reported that UV-C treatment for both 5 min and 15 min caused a slight decrease in the ABTS radical scavenging activity of α-casein.[Citation12] The difference in the antioxidant activity indicated by ABTS radical scavenging activity might be related to the protein type and treatment conditions.
RP assays are often used to evaluate the ability of a natural antioxidant to donate electrons or hydrogen. A higher absorbance at 700 nm indicated a higher antioxidant activity. UV-C treatment afforded a higher antioxidant activity for β-Lg compared with the control and ultrasound treatment. However, no significant change in the RP value was observed for the ultrasound treatment compared with the control. For each treatment, the treatment time did not noticeably affect the RP values. UV-C treatment also increased the RP value, while UV-C treatment for 15 min has been shown to give a higher RP for α-casein than treatment for 5 min.[Citation10] A previous study showed that the RP value was higher in ultrasound-treated ovalbumin compared with the control.[Citation38] This was probably related to the structural changes in the protein under the UV-C and ultrasound treatments, which caused protein unfolding and exposed active groups with antioxidant activities. The tertiary structure and conformational changes in the protein exposed the hidden peptide sequences and therefore affected the antioxidant activity.
Correlation analysis showed that the ultrasound and UV-C treatments had a significantly positive correlation with oxidant degree or antioxidant activity (P < 0.001). Different treatments also had a significantly correlation with tertiary structure (P < 0.001). Among secondary structure characteristics, β-turn had the highest correlation with different treatments (P < 0.01). UV-C treated samples also exhibited the more distinct content decline of S-S and increase of the value Ho than ultrasound treated samples, corresponding to the value in . Moreover, the data also exhibited great correlation between oxidant/antioxidant activity and tertiary structure (P < 0.01). For example, the correlation coefficients between oxidant and antioxidant capacities and Ho value were greater than 0.927(P < 0.001). When compared with DPPH scavenging activity or reducing power, ABTS scavenging activity showed better correlation with tertiary structure. The relationship between ABTS and Ho had the strongest correlation (r = 0.981, (P < 0.001).
Conclusion
UV-C and ultrasound treatments increased protein oxidation in β-Lg. Secondary and tertiary structures varied to different degrees, which affected the oxidation and antioxidant activities. Among all treatments, ultrasound for 30 min gave the lowest protein oxidation and DPPH radical scavenging activity, and an insignificant change in RP, while UV-C irradiation for 30 min led to more significant protein oxidation and increased the antioxidant activity.
Additional information
Funding
References
- Arzeni, C.; Martinez, K.; Zema, P.; Arias, A.; Perez, O. E.; Pilosof, A. M. R. Comparative Study of High Intensity Ultrasound Effects on Food Proteins Functionality. J. Food Eng. 2012, 108(3), 463–472.
- Rafe, A.; Razavi, S. M. A. Effect of Thermal Treatment on Chemical Structure of Β-Lactoglobulin and Basil Seed Gum Mixture at Different States by ATR-FTIR Spectroscopy. Int. J. Food Properties. 2015, 18(12), 2652–2664. DOI: 10.1080/10942912.2014.999864.
- Hernández-Ledesma, B.; Recio, I.; Amigo, L. β-Lactoglobulin as Source of Bioactive Peptides. Amino Acids. 2008, 35(2), 257–265. DOI: 10.1007/s00726-007-0585-1.
- Pihlanto, A.;. Antioxidative Peptides Derived from Milk Proteins. Int. Dairy J. 2006, 16(11), 1306–1314. DOI: 10.1016/j.idairyj.2006.06.005.
- Hernández-Ledesma, B.; Amigo, L.; Recio, I.; Bartolomé, B. ACE-inhibitory and Radical-Scavenging Activity of Peptides Derived from β-lactoglobulin F (19−25). Interactions with Ascorbic Acid. J. Agric. Food Chem. 2007, 55(9), 3392–3397. DOI: 10.1021/jf063427j.
- Marchesini, G.; Balzan, S.; Montemurro, F.; Fasolato, F.; Andrighetto, I.; Segato, S.; Novelli, E. Effect of Ultrasound Alone or Ultrasound Coupled with CO2 on the Chemical Composition, Cheese-Making Properties and Sensory Traits of Raw Milk. Innov. Food Sci. & Emerg. Technol. 2012, 16, 391–397. DOI: 10.1016/j.ifset.2012.09.003.
- Falguera, V.; Pagan, J.; Garza, S.; Garvín, A.; Ibarz, A. Ultraviolet Processing of Liquid Food: A Review: Part 2: Effects on Microorganisms and on Food Components and Properties. Food Res. Int. 2011, 44, 1580–1588. DOI: 10.1016/j.foodres.2011.03.025.
- Semagoto, H. M.; Liu, D.; Koboyatau, K.; Hu, J.; Lu, N.; Liu, X.; Regenstein, J. M.; Zhou, P. Effects of UV Induced Photo-Oxidation on the Physicochemical Properties of Milk Protein Concentrate. Food Res. Int. 2014, 62, 580–588. DOI: 10.1016/j.foodres.2014.04.012.
- Hu, G.; Zheng, Y.; Liu, Z.; Deng, Y. Effects of UV-C and Single-And Multiple-Cycle High Hydrostatic Pressure Treatments on Flavor Evolution of Cow Milk: Gas Chromatography-Mass Spectrometry, Electronic Nose, and Electronic Tongue Analyses. Int. J. Food Properties. 2017, 20(7), 1677–1688. DOI: 10.1080/10942912.2016.1217876.
- Scheidegger, D.; Larsen, G.; Kivatinitz, S. C. Oxidative Consequences of UV Irradiation on Isolated Milk Proteins: Effects of Hydrogen Peroxide and Bivalent Metal Ions. Int. Dairy J. 2016, 55, 64–71. DOI: 10.1016/j.idairyj.2015.12.005.
- Hu, G.; Zheng, Y.; Liu, Z.; Deng, Y.; Zhao, Y. Structure and IgE-binding Properties of α-casein Treated by High Hydrostatic Pressure, UV-C, and far-IR Radiations. Food Chem. 2016, 204, 46–55. DOI: 10.1016/j.foodchem.2016.02.113.
- Hu, G.; Zheng, Y.; Liu, Z.; Deng, Y.; Zhao, Y. Effects of High Hydrostatic Pressure, Ultraviolet light-C, and Far-Infrared Treatments on the Digestibility, Antioxidant and Antihypertensive Activity of α-casein. Food Chem. 2017, 221, 1860–1866.
- Manzocco, L.; Panozzo, A.; Nicoli, M. C. Effect of Ultraviolet Processing on Selected Properties of Egg White. Food Chem. 2012, 135(2), 522–527.
- Mohammadi, V.; Ghasemi-Varnamkhasti, M.; Ebrahimi, R.; Abbasvali, M. Ultrasonic Techniques for the Milk Production Industry. Meas. 2014, 58, 93–102. DOI: 10.1016/j.measurement.2014.08.022.
- Stanic-Vucinic, D.; Prodic, I.; Apostolovic, D.; Nikolic, M.; Velickovic, T. C. Structure and Antioxidant Activity of β-lactoglobulin-glycoconjugates Obtained by High-Intensity-Ultrasound-Induced Maillard Reaction in Aqueous Model Systems under Neutral Conditions. Food Chem. 2013, 138(1), 590–599. DOI: 10.1016/j.foodchem.2012.10.087.
- Deng, Y.; Zhao, Y. Effect of Pulsed Vacuum and Ultrasound Osmopretreatments on Glass Transition Temperature, Texture, Microstructure and Calcium Penetration of Dried Apples (Fuji). LWT-Food Sci. Technol. 2008, 41(9), 1575–1585. DOI: 10.1016/j.lwt.2007.10.018.
- Frydenberg, R. P.; Hammershøj, M.; Andersen, U.; Greve, M. T.; Wiking, L. Protein Denaturation of Whey Protein Isolates (Wpis) Induced by High Intensity Ultrasound during Heat Gelation. Food Chem. 2016, 192, 415–423. DOI: 10.1016/j.foodchem.2015.07.037.
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of Ultrasound on the Thermal and Structural Characteristics of Proteins in Reconstituted Whey Protein Concentrate. Ultrason. Sonochem. 2011, 18(5), 951–957. DOI: 10.1016/j.ultsonch.2010.12.016.
- Yang, W. H.; Tu, Z. C.; Wang, H.; Li, X.; Tian, M. High-Intensity Ultrasound Enhances the Immunoglobulin (Ig) G and IgE Binding of Ovalbumin. J. Sci. Food Agric. 2017, 97(7), 2714–2720. DOI: 10.1002/jsfa.8095.
- Stojadinovic, M.; Burazer, L.; Ercili-Cura, D.; Sancho, A.; Buchert, J.; Velickovic, T. C.; Stanic-Vucinic, D. One-Step Method for Isolation and Purification of Native Betalactoglobulin from Bovine Whey. J. Sci. Food Agric. 2012, 92(7), 1432–1440. DOI: 10.1002/jsfa.4722.
- Tammineedi, C. V.; Choudhary, R.; Perez-Alvarado, G. C.; Watson, D. G. Determining the Effect of UV-C, High Intensity Ultrasound and Nonthermal Atmospheric Plasma Treatments on Reducing the Allergenicity of α-casein and Whey Proteins. LWT-Food Sci. Technol. 2013, 54(1), 35–41. DOI: 10.1016/j.lwt.2013.05.020.
- Zhang, Y.; Deng, Y.; Zhao, Y. Structure-Based Modelling of Hemocyanin Allergenicity in Squid and Its Response to High Hydrostatic Pressure. Sci. Rep. 2017, 7, 40021. DOI: 10.1038/srep40021.
- Wang, T.; Liu, F.; Wang, R.; Wang, L.; Zhang, H.; Chen, Z. Solubilization by Freeze-Milling of Water-Insoluble Subunits in Rice Proteins. Food Funct. 2015, 6(2), 423–430. DOI: 10.1039/C4FO00828F.
- Jin, Y.; Deng, Y.; Qian, B.; Zhang, Y.; Liu, Z.; Zhao, Y. Allergenic Response to Squid (Todarodes Pacificus) Tropomyosin Tod P1 Structure Modifications Induced by High Hydrostatic Pressure. Food Chem. Toxicol. 2015, 76, 86–93. DOI: 10.1016/j.fct.2014.12.002.
- Hu, G.; Zheng, Y.; Wang, D.; Zha, B.; Liu, Z.; Deng, Y. Comparison of Microbiological Loads and Physicochemical Properties of Raw Milk Treated with Single-/Multiple-Cycle High Hydrostatic Pressure and ultraviolet-C Light. High Press. Res. 2015, 35(3), 330–338. DOI: 10.1080/08957959.2015.1063626.
- Li, Z.; Jiang, A.; Yue, T.; Wang, J.; Wang, Y.; Su, J. Purification and Identification of Five Novel Antioxidant Peptides from Goat Milk Casein Hydrolysates. J. Dairy Sci. 2013, 96(7), 4242–4251. DOI: 10.3168/jds.2012-6511.
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of Ultrasound on the Structure and Physical Properties of Black Bean Protein Isolates. Food Res. Int. 2014, 62, 595–601.
- Anugu, A. K. Effect of Pulsed UV Lights and Pulsed Electric Fields on Selected Isolated Milk Proteins and their Allergenic Properties. (Doctoral dissertation, Alabama A & M University), 2009.
- Zhao, J. Y.; Dong, F. J.; Li, Y. Y.; Kong, B. H.; Liu, Q. Effect of Freeze-Thaw Cycles on the Emulsion Activity and Structural Characteristics of Soy Protein Isolate. Process Biochem. 2015, 50, 1607–1613.
- Sun, W.; Zhou, F.; Sun, D. W.; Zhao, M. Effect of Oxidation on the Emulsifying Properties of MPs. Food Bioprocess Technol. 2013, 6, 1703–1712.
- Kristo, E.; Hazizaj, A.; Corredig, M. Structural Changes Imposed on Whey Proteins by UV Irradiation in a Continuous UV Light Reactor. J. Agric. Food Chem. 2012, 60(24), 6204–6209. DOI: 10.1021/jf300278k.
- Feng, D.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of Microwave Radiation and Water Bath Heating on the Physicochemical Properties of Actomyosin from Silver Carp (Hypophthalmichthys Molitrix) during Setting. J. Food Process. Preservation. 2017, 41(4), e13031. DOI: 10.1111/jfpp.13031.
- Yongsawatdigul, J.; Park, J. W. Thermal Denaturation and Aggregation of Threadfin Bream Actomyosin. Food Chem. 2003, 83(3), 409–416.
- Siddique, M. A. B.; Maresca, P.; Pataro, G.; Ferrari, G. Influence of Pulsed Light Treatment on the Aggregation of Whey Proteinisolate. Food Res. Int. 2017, 99, 419–425. DOI: 10.1016/j.foodres.2017.06.003.
- Jiang, L. Z.; Wang, Z. J.; Li., Y.; Meng, X. H.; Sui, X. N.; Qi, B. K.; Zhou, L. Y. Relationship between Surface Hydro-Phobicity and Structure of Soy Protein Isolate Subjected to Different Ionic Strength. Int. J. Food Properties. 2015, 18, 1059–1074. DOI: 10.1080/10942912.2013.865057.
- Arzeni, C.; Pérez, O. E.; Pilosof, A. M. Functionality of Egg White Proteins as Affected by High Intensity Ultrasound. Food Hydrocoll. 2012, 29(2), 308–316. DOI: 10.1016/j.foodhyd.2012.03.009.
- Pattison, D. I.; Rahmanto, A. S.; Davies, M. J. Photo-Oxidation Proteins. Photochemical Photobiol. Sci. 2012, 11(1), 38–53. DOI: 10.1039/C1PP05164D.
- Xie, H.; Tu, Z.; Wang, H.; Huang, T. Influence of Ultrasounic Treatment on the Antioxidant Activity of Ovalbumin. Chinese Science Paper Online, May 20, 2015. http://www.paper.edu.cn/releasepaper/content/201505-255.