ABSTRACT
This study used iron pot, aluminum alloy pot, and ceramic pot in exploring the migration law of lead and cadmium from pots to food during the cooking process. For this purpose, the pots were contacted with food simulation solutions such as distilled water, 4% acetic acid, 15% ethanol, and edible vegetable oil under normal and high temperatures. Results showed that the migration of lead and cadmium was significantly affected by high temperature, and the released amounts of lead and cadmium were increased with the cooking time. The pot material and the properties of food also affected the migration of lead and cadmium during the cooking process. When contacting with water, 4% acetic acid, and 15% ethanol simulation solutions, the released amounts of lead and cadmium from iron pot were increased more significantly than that in other pots (p < 0.05). Meanwhile, the released amounts of lead and cadmium from aluminum alloy pot and iron pot were firstly increased and then decreased over cooking time, although migration of lead and cadmium from ceramic pot is keeping increasing when contacting with vegetable oil. The migration of lead and cadmium from three different pots was positively correlated with the concentration of acetic acid solution. The migration of lead and cadmium was also affected by the concentration of ethanol. When the pots were contacted with 15% ethanol, the released amounts of lead and cadmium were the highest among those in other simulation solutions.
Introduction
Cooking is an important step in food processing. The quality of the pots that contact directly with food during the cooking process is closely related to food safety. Pots materials often contain two major elements, namely, lead and cadmium. Considerable literatures[Citation1,Citation2] report that lead and cadmium are toxic to human health, and evaluation of the released amounts of these elements from ports are the standard tests to guarantee the quality of pots.[Citation3–Citation5] The lead from pots may be released into food, especially when pots are worn or used under high temperature.[Citation6] The diffusion-controlled ion exchange reactions could result in the migration of lead from ceramic materials into water or acid solutions.[Citation7]
Some studies show that pH and ethanol content affect the migration of lead and cadmium. [Citation8,Citation9] Pots can be used for many purposes, such as frying, wine cooking, and soup making, and are part of the diverse cooking methods in China. When pots are contacted with acid and alcoholic food under high temperature, the lead and cadmium in them may be released into the food.
The migration of heavy metals from glass, ceramic package, and containers to food has been studied by scholars.[Citation10–Citation12] However, the migration of heavy metals in pots made of different materials has been rarely investigated, as well as the heavy metal safety issues of pots during the cooking process. This study used the common iron pot, aluminum alloy pot, and ceramic pot in exploring the effect of cooking temperature, pH, and ethanol concentration on the migration law of the lead and cadmium in these pots. For this purpose, the pots were contacted with different food simulation solutions under certain conditions.
Materials and methods
Materials and reagents
Ceramic pots, iron pots, and aluminum alloy pots (18 cm in diameter and 10 cm deep) were purchased from the supermarket and were selected as samples, and Jinlongyu soybean oil was purchased from the supermarket.
Instruments and equipment
The graphite furnace atomic absorption spectrometer FS90 with the automatic sampler FS95 (Thermo, USA). The lead and cadmium uncoded hollow cathode lamps (Thermo, USA). The microwave digestion instrument Mars5X (CEM, USA). The electric heating graphite digestion device ED25G (APL, China). The electronic analytical balance PSC30U-120 (METTLER TOLEDO, USA). The bottle top dispenser (Eppendorf, USA). The lead standard solution (1000 mg/L) and the cadmium standard solution (1000 mg/L) came from the National Testing Center of Nonferrous Metals and Electronic Materials Analysis. The nitric acid, 100% ethanol, and glacial acetic acid were all analytical reagents from Hangzhou Chemical Reagent Co., Ltd. Ultrapure water was also used in this experiment.
Sample preparation and determination
The pots used in the experiment were of the same capacity and bottom size. They have been tested according to the national specifications on the released amounts of lead and cadmium, and all the samples met the Chinese standards.[Citation13–Citation16]
Experiment on migration of lead and cadmium in pots during the cooking process
Distilled water, 15% ethanol, 4% acetic acid, and edible vegetable oil were selected as the food simulation solutions, and the initial concentration of lead and cadmium in the four types of food simulation solutions was determined. Each pot was poured with 1 L food simulation. A total of 25 mL solution was sampled after the pots were soaked in solution for 2 h at 25°C. Parallel sampling was conducted for three times. Then, each pot was poured with 1 L food simulation solution, and the solution was boiled for 2 h at 100–200°C. A total of 25 mL solution was sampled every 30 min, and 1 mL solution was sampled in case of oil. Each experiment was repeated three times with three pots and parallel sampling was conducted for three times. The food simulation solution in the pot was replenished to reach the same volume every 0.5 h with the initial value prior to each sampling.
Effect of pH
The acetic acid food simulation solutions with concentrations of 4%, 1%, and 0.1% were prepared. The pH value and initial lead and cadmium concentration of the three types of food simulation solutions and distilled water were measured. Each aluminum alloy pot was poured with 1 L food simulation solution and boiled under slow fire for 2 h. A total of 25 mL solution was sampled every 30 min, and each experiment was repeated three times with three pots, parallel sampling was conducted for three times. The food simulation solution in the pot was replenished to reach the same volume every 0.5 h with the initial value prior to each sampling. Considering the water evaporation during the boiling process, which could result in lead and cadmium ions increased, the boiling food simulation solution was added to keep the same volume of solution.
Effect of ethanol concentration
The ethanol food simulation solutions with concentrations of 10%, 15%, and 20% were prepared. The initial lead and cadmium concentration of the three types of food simulation solutions was measured. Each aluminum alloy pot was poured with 1 L food simulation solution and boiled under slow fire for 2 h. A total of 25 mL solution was sampled every 30 min, and each experiment was repeated three times with three pots, parallel sampling was conducted for three times. The food simulation solution in the pot was replenished to reach the same volume every 0.5 h with the initial value prior to each sampling.
Determination of lead and cadmium in food stimulation solution
A total of 1 mL oil and turbid solution was sampled and poured into the digestion tube, then 7 mL concentrated nitric acid was added. After 15 minutes at room temperature, the tube was sealed and digested by microwave. The microwave digestion process was created as follows. The initial temperature was raised to 120°C in 5 min before being held constant for 3 min; then, the temperature was raised to 160°C in 5 min before being held constant for 3 min; finally, the temperature was raised to 180°C for 8 min before being held constant for 20 min.[Citation17] After completing the process above, the digestion solution was cooled to room temperature and then transferred to a 25-mL volumetric flask and diluted with 0.5% nitric acid. Then 25 mL was sampled for direct assay. The test was conducted using graphite furnace atomic absorption spectrometry.
Instrument conditions
The detection wavelength of lead element was 283.3 nm, the lamp current was 5 mA, and the carrier gas flow was 2.0 L/min; the analytical wavelength of cadmium element was 228.8 nm, the lamp current was 6 mA, and the carrier gas flow was 2.0 L/min. The regression equation of lead standard solution was y = 0.00926x+0.0065, in which r2 = 0.9990, RSD was 3.19%, the quantitation limit was 0.021 μg/L, and the recovery rate was 86.05%–98.29%. The regression equation of cadmium standard solution was y = 0.15011x+0.0038, in which r2 = 0.9989, RSD was 1.77%, the quantitation limit was 0.006 μg/L, and the recovery rate was 87.33%–105.67%.
Statistical analyses
The results are presented as average values and standard deviation. The differences between means of the groups were compared by analysis of variance (F). Least significant difference (LSD) was used at the significance level of a (p ≤ 0.05) to compare a difference between groups. DPS statistical package was used to perform statistical analyses. In this study the pots were used from the same manufacturer of the same batch, one pot only for a single cooking experiment. Each experiment was repeated three times with three pots, and parallel sampling was conducted for three times.
Results and discussion
Effect of temperature on migration of lead and cadmium
The three pots were contacted with distilled water, 4% acetic acid, and 15% ethanol food simulation solutions under normal (25°C) and high temperature (100°C) for 2 h, and the migration results are presented in . shows that when the three types of pots were contacted with distilled water and 4% acetic acid the migration of lead increased significantly under high temperature (p < 0.05) but did not change remarkably under normal temperature (p > 0.05). When the three types of pots were contacted with 15% ethanol, the released amounts of lead under high temperature increased significantly (p < 0.05); the released amounts increased from ND to 0.034 in ceramic pot, from ND to 0.412 in iron pot and from ND to 0.125 in aluminum pot under normal temperature. When the ceramic, iron, and aluminum pots were contacted with distilled water under high temperature, the released amounts of lead increased by 0.117, 1.500, and 0.790 μg/L compared with those under normal temperature. When the three types of pots were contacted with 4% acetic acid under high temperature, the released amounts of lead increased by 1.796, 4.892, and 3.022 μg/L. When the three types of pots were contacted with 15% ethanol under high temperature, the released amounts of lead increased by 0.086, 2.425, and 1.014 μg/L.
Table 1. Migration result of lead and cadmium from pot into food simulation solution under normal and high temperatures, μg per 1000 mL.
With regard to the migration of cadmium, when the three types of pots were contacted with distilled water, the released amounts of cadmium under high temperature increased significantly (p < 0.05); by contrast, the released amounts did not change remarkably under normal temperature (p > 0.05). When the three types of pots were contacted with 15% ethanol, the released amounts of cadmium under high temperature increased significantly (p < 0.05); the released amounts increased from ND to 0.015 in ceramic pot, from ND to 0.093 in iron pot, and from ND to 0.024 in aluminum pot under normal temperature. When the three types of pots were contacted with 4% acetic acid, the released amounts of cadmium increased significantly (p < 0.05) under normal temperature and showed an extremely significant increase under high temperature (p < 0.01). When the ceramic, iron, and aluminum pots were contacted with distilled water under high temperature, the released amounts of cadmium increased by 0.022, 0.065, and 0.027 μg/L compared with those under normal temperature. When the three types of pots were contacted with 4% acetic acid under high temperature, the released amounts of cadmium increased by 0.282, 0.386, and 0.171 μg/L. When the three types of pots were contacted with 15% ethanol under high temperature, the released amounts of cadmium increased by 0.050, 0.531, and 0.070 μg/L.
The results showed that the released amounts of lead and cadmium ions increased with the higher temperature. This result could be explained by the increased kinetic energy of lead and cadmium ions, resulting into accelerating the diffusing rate of lead and cadmium ions in pots. In addition, the ion exchange reactions were accelerated with increased temperature, resulting in the released amounts of lead and cadmium ions increased.[Citation18]
Effect of food properties and pot materials on migration of lead and cadmium
Pots are often used to cook different food, are made of various materials, and are part of the many cooking methods. The cooking time is usually between 3 min and 3 h. This study used distilled water (water-based food), 4% acetic acid (acidic food), 15% ethanol (alcoholic food), and edible vegetable oil (fatty food) to simulate four different types of food. [Citation19] Pots made of ceramic, iron, and aluminum alloy were selected for cooking. The cooking time was 0–2 h, and the temperature was kept at 100–200°C. Under these conditions, the effect of different food properties and pot materials on the migration of lead and cadmium during the cooking process was investigated. The results are shown in .
Figure 1. Migration result of lead and cadmium from three pots into four food simulation solutions, a: Lead and cadmium content of water; b: Lead and cadmium content of 15% ethanol; c: Lead and cadmium content of 4% acetic acid; d: Lead and cadmium content of vegetable oil.
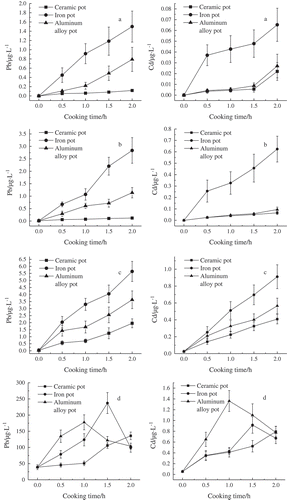
shows that the change in the released amounts of lead in the four food simulation solutions exhibited the same tendency with that of cadmium. When the pots were processed with distilled water, 15% ethanol, and 4% acetic acid, the released amounts of lead and cadmium from any of the three types of pots all increased with the cooking time. [Citation20] Notably, the released amounts of lead and cadmium were the highest from iron pot among the three types of pots. When pots were used for cooking with edible vegetable oil, the released amounts of lead and cadmium from iron pot and aluminum pot increased first and then declined.[Citation21] At the beginning, the lead and cadmium from pot materials were dissolved into oil, which caused the increase of lead and cadmium concentration in oil. Then when the oil temperature reached a critical point of smoking, lead, cadmium ions were taken away by smoke, which caused the decrease of lead and cadmium concentration in oil. Furthermore, the released amounts of lead and cadmium from ceramic pot increased along with the cooking time. The maximum released amounts of lead and cadmium from the three types of pots all exceeded the maximum released amounts of lead and cadmium from the pots filled with three other food simulation solutions at a significant level of (p < 0.05).
When the pots were processed with distilled water, the released amounts of lead and cadmium from iron pot were higher than that from ceramic pot and aluminum alloy pot at a significant level of (p < 0.05). The released amounts of lead and cadmium were 1.500 and 0.065 μg/L, respectively, after 2 h. The released amounts of lead from aluminum alloy pot were higher than that from ceramic pot, and the released amounts of lead were 6.752 times of that from ceramic pot after 2 h. However, the released amounts of cadmium did not differ significantly from aluminum alloy pot and ceramic pot (p > 0.05).
When the pots were processed with 15% ethanol, the released amounts of lead and cadmium from iron pot were much higher than that in ceramic pot and aluminum alloy pot at a significant level of (p < 0.05). The released amounts of the two elements were 2.837 and 0.624 μg/L, respectively, after 2 h. The released amounts of lead from aluminum alloy pot were higher than that from ceramic pot, and the released amounts of lead were 9.492 times of that from the ceramic pot after 2 h. Nevertheless, the released amounts of cadmium did not differ significantly from aluminum alloy pot and ceramic pot (p > 0.05).
When the pots were processed with 4% acetic acid, the released amounts of lead and cadmium from iron pot were higher than that from ceramic pot and aluminum alloy pot at a significant level of (P < 0.05). The released amounts of the two elements were 5.641 and 0.911 μg/L after 2 h. The released amounts of lead from aluminum alloy pot were higher than that from ceramic pot at a significant level of (p < 0.05). After 2 h, the released amounts of lead and cadmium were 1.854 and 1.385 times of those from the ceramic pot, respectively.
When the pots were processed with edible vegetable oil, the content of lead and cadmium in oil reached the maximum value when the aluminum alloy pot was used for cooking for 1 h, and the contents of lead and cadmium were 177.699 and 1.361 μg/L, respectively. However, the content of lead and cadmium decreased by 44.2% and 52.5% during the period from 1 h to 2 h. The contents of lead and cadmium in the oil reached the maximum value when the iron pot was used for cooking for 1.5 h, and the contents of the two elements were 237.178 and 0.916 μg/L. However, the contents of lead and cadmium decreased by 64.1% and 37.1% during the period from 1.5 h to 2 h. The released amounts of lead and cadmium from ceramic pot increased with the cooking time, and the released amounts of lead and cadmium were 135.910 and 0.794 μg/L, respectively.
In summary, the properties of food affected the migration of lead and cadmium in pots during the cooking process. Notably, grease presented a significant effect, whereas distilled water exhibited a small effect. The heat conductivity coefficient, metal content, and metal properties of pot material also affected the migration of lead and cadmium during the cooking process.
Effect of acetic acid concentration on migration of lead and cadmium
During the cooking process, the properties of condiments and food often lead pots to contact the acidic materials. When contacted with 4% acetic acid, the released amounts of lead and cadmium from iron pot and aluminum alloy pot were relatively high. However, in practice, a great amount of rust may be produced when iron pot is contacted with acid, thereby affecting the appearance of food. Therefore, iron pot should not be used to cook acidic food. In the present experiment, the aluminum alloy pot was selected for cooking the four food simulation solutions (i.e., distilled water and 0.1%, 1%, and 4% acetic acid). Accordingly, the effect of acetic acid concentration on the migration of lead and cadmium was determined. The results are shown in .
Figure 2. Migration result of lead and cadmium from aluminum alloy pot into different concentrations of acetic acid simulation solution and water.
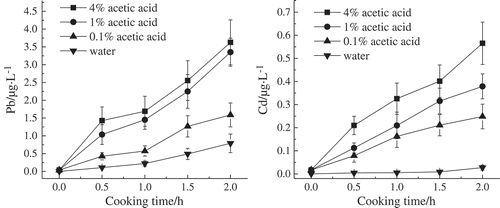
The pH values of 4%, 1%, and 0.1% acetic acid were 2.15, 2.8, and 4.27, respectively, during the experiment. The pH value of distilled water was 6.89. shows that the content of lead and cadmium in different concentrations of acetic acid simulation solution was all higher than that in the distilled water when the simulation was boiled in the aluminum pot for 2 h. This finding indicates that all acetic acid solutions of different concentrations affected the migration of lead and cadmium. The released amounts of lead was the lowest in the 0.1% acetic acid solution at 1.588 μg/L, and the highest released amounts of lead was found in the 4% acetic acid solution at 3.627 μg/L. The content of lead was lowest in the 0.1% acetic acid, followed by that in the 1% acetic acid and finally by that in the 4% acetic acid. The released amounts of cadmium at 0.249 μg/L were the least when the pot was processed with 0.1% acetic acid, whereas the released amounts of cadmium at 0.565 μg/L were the highest when the pot was processed with 4% acetic acid. The content of cadmium in different concentrations of acetic acid solution showed the same trend with that of lead.
In summary, acidity affected the migration of lead and cadmium, and the released amounts of lead and cadmium increased with the decrease in pH value; these results are consistent with the findings of considerable previous research. [Citation22,Citation23] The release of lead and cadmium were the highest when the pot was processed with the 4% acetic acid, such that the 4% acetic acid was often selected by various national standards across the world as the soak solution for checking whether the released amounts of lead and cadmium from pots meet the standards.
Effect of alcoholic strength on migration of lead and cadmium
In this experiment, 10, 15, and 20% ethanol solutions were selected as the simulation solutions based on the alcoholic strength scope of Chinese traditional rice wine. The effect of alcoholic strength on the migration of lead and cadmium during the cooking process was investigated using the same material pots as that in the experiment on the effect of pH value. The results are shown in .
Figure 3. Migration result of lead and cadmium from aluminum pot into different concentrations of ethanol simulation solution and water.
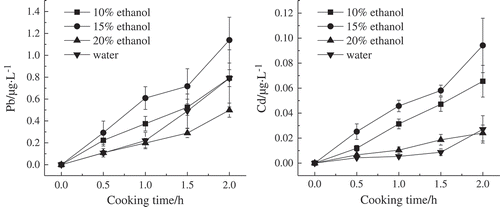
shows that the released amounts of lead at 1.139 μg/L was the highest at a significant level of p < 0.05 when the aluminum alloy pot was used to cook 15% ethanol. When the pot was contacted with 10% ethanol, no significant difference in the released amounts of lead was found from that in the distilled water (p > 0.05). The reason may be that low-concentration ethanol evaporated completely under high temperature to turn into aqueous solution. When the pot was contacted with 20% ethanol, the released amounts of lead were less than that in the 15% ethanol (i.e., 53.8% of that in the 15% ethanol). This result is attributed to that low-solubility salts were formed and deposited on the internal surface of ceramic pot when the ethanol concentration was high; as a consequence, the corrosion was mitigated and the released amounts of lead decreased accordingly.[Citation24]
The released amounts of cadmium were the highest when the aluminum alloy pot was used to cook 15% ethanol; the amount reached a significant level of p < 0.05 at 0.094 μg/L. The released amounts of cadmium in the 10% ethanol at 0.066 μg/L were significantly higher than that in the 20% ethanol and distilled water. The released amounts of cadmium in the 20% ethanol did not differ significantly from that in the distilled water. In general, the released amounts of cadmium were low when the pot was processed with ethanol solutions of different concentrations. The reason may be that the glass phase and crystalline phase existed in the cadmium in the pot and that the migration of cadmium was subject to many factors.[Citation25] This phenomenon therefore needs further investigation.
Conclusion
At present, studies on the migration of lead and cadmium focus mainly on the migration of heavy metals before food production and during food packaging process; on the contrary, studies on the migration of lead and cadmium during the cooking process are lacking. The present study investigated the migration of lead and cadmium. For this purpose, three types of pots were contacted with different food simulation solutions under different conditions, and the migration law of lead and cadmium during the cooking process was explored. The results show that the migration of lead and cadmium in iron pot was significantly affected by high temperature, and the migration of lead and cadmium increased with the cooking time. The material of pot and properties of food also affected the migration of lead and cadmium. Specifically, when contacted with water, 4% acetic acid, and 15% ethanol food simulation solutions, the released amounts of lead and cadmium from iron pot were higher than that from the two other types of pots at a significant level of p < 0.05. The released amounts of lead and cadmium were in the ranges of 1.500–5.641 and 0.065–0.911 μg/L, respectively. When contacted with edible vegetable oil, the released amounts of lead and cadmium from the three types of pots were higher than that when pots were contacted with the three other food simulation solutions at a significant level of (p < 0.05). The migration of lead and cadmium was also affected by acidity during the cooking process. In particular, the released amounts of lead and cadmium were high when the acidity was high. Alcohol strength also affected the migration of lead and cadmium during the cooking process. Specifically, the 15% ethanol showed the greatest effect. Moreover, when the ethanol concentration increased, the migration of lead and cadmium was inhibited. All the findings show that the migration of lead and cadmium in pots presented a regular law during the cooking process, and this migration was subject to many factors. The findings can provide theoretical basis and technical guidance for future in-depth study on the migration of heavy metals during the cooking process.
Additional information
Funding
References
- Papanikolaou, N. C.; Hatzidaki, E. G.; Belivanis, S.; Tzanakakis, G. N.; Tsatsakis, A. M. Lead Toxicity Update. A Brief Review. International. J. Clin. Exp. Med., 2005, 11, RA329–336.
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Croneberg, D. A. The Toxicity of Cadmium and Resulting Hazards for Human Health. J. Occup. Med. Toxicol., 2006, 1, 1–6.
- FDA. Imported and Domestic-Cadmium Contamination; CPG 7117.06 Sec.545.400 pottery (ceramics): U.S, 2005.
- FDA. Imported and Domestic-Lead Contamination; CPG 7117.07 Sec.545.450 pottery (ceramics): U.S, 2005.
- EC.. Council Directive 84/500/EEC of 15 October 1984 on the Approximation of the Laws of the Member States Relating to Ceramic Articles Intended to Come into Contact with Foodstuffs; Europe/European Commission, 1984. 84/500/EEC. E.U.
- Villalobos, M.; Merino-Sánchez, C.; Hall, C.; Grieshop, J.; Gutiérrez-Ruiz, M. E.; Handley, M. A. Lead (II) Detection and Contamination Routes in Environmental Sources, Cookware and Home-Prepared Foods from Zimatlán, Oaxaca. Sci. Total Environ., 2009, 407, 2836–2844.
- Dong, Z. H.; Lu, L. X.; Liu, Z. G.; Tang, Y. L.; Wang, J. Migration Model of Toxic Metals from Ceramic Food Contact Materials into Acid Food. Packaging Technol. Sci., 2015, 28, 545–556.
- Abou-Arab, A. A. K.;. Release of Lead from Glaze-Ceramicware into Foods Cooked by Open Flame and Microwave. Food Chem, 2001, 73, 163–168.
- Hashemi-Moghaddam, H.;. Investigation of Released Cadmium and Lead from Different Colors of over Glaze Designs to Food Stuff in Different Conditions. J. Chem. Health Risks, 2012, 2, 19–21.
- Sheets, R. W.;. Release of Heavy Metals from European and Asian Porcelain Dinnerware. Sci. Total Environ., 1998, 212, 107–113.
- Rebeniak, M.; Wojciechowskamazurek, M.; Mania, M.; Szynal, T.; Strzelecka, A.; Starska, K. Exposure to Lead and Cadmium Released from Ceramics and Glassware Intended to Come into Contact with Food. Roczniki Państwowego Zakadu Higieny, 2014, 65, 301–309.
- Elatrash, S.; Atoweir, N. Determination of Lead and Cadmium in Raw Cow’s Milk by Graphite Furnace Atomic Absorption Spectroscopy. Int J Quantum Chem, 2014, 12, 92–100.
- National Standard of the People’s Republic of China. Hygienic Standard of Ceramics for Food Containers; Chinese Ministry of Health, GB13121-1991. P. R. China, 1991.
- National Standard of the People’s Republic of China. Standard Permissible Limits and Testing Method for Release of Lead or Cadmium from Ceramic Cookware; Chinese Ministry of Health, GB8058-2003. P. R. China, 2003.
- National Standard of the People’s Republic of China. Hygienic Standard for Aluminum-Wares for Food Use; Chinese Ministry of Health, GB11333-89. P. R. China, 1989.
- National Standard of the People’s Republic of China. National Food Safety Standards for Stainless Steel Products; Chinese Ministry of Health, GB9684-2011.. P. R. China, 2011.
- Pavel, P. B.; Diacu, E.; Barbu, C. H. Microwave-Assisted Digestion Procedures for Total Lead and Cadmium Content Determination in Copsa Mica Soils. Revista De Chimie, 2013, 64, 22–26.
- Dong, Z. H.; Lu, L. X.; Liu, Z. G. Migration of Lead and Cadmium from Ceramic Food Packaging Materials into Foods. Sci. Technol. Food Ind., 2013, 34, 258–262.
- EC. On Plastic Materials and Articles Intended to Come into Contact with Foodstuffs; Commission Regulation No10/2011: E.U, 2011.
- Mohamed, N.; Chin, Y. M.; Pok, F. W. Leaching of Lead from Local Ceramic Tableware. Food Chem, 1995, 54, 245–249.
- Guo, D. M.; Cao, J. Y.; Wang, Y. Detection and Analysis of Harmful Composition of Cooking Oil Fume in the Kitchen. Chin. J. Dis. Control Prev., 2010, 14, 142–145.
- Demont, M.; Boutakhrit, K.; Fekete, V.; Bolle, F.; Loco, J. K. Migration of 18 Trace Elements from Ceramic Food Contact Material: Influence of Pigment, pH, Nature of Acid and Temperature. Food Chem. Toxicol., 2012, 50, 734–743.
- Perring, L.; Alonso, M. I.; Andrey, D.; Bourqui, B.; Zbinden, P. An Evaluation of Analytical Techniques for Determination of Lead, Cadmium, Chromium, and Mercury in Food-Packaging Materials. Fresenius J. Anal. Chem., 2001, 370, 76–81.
- Burger, J.; Gochfeld, M. Comparison of Arsenic, Cadmium, Chromium, Lead, Manganese, Mercury and Selenium in Feathers in Bald Eagle (Haliaeetus Leucocephalus), and Comparison with Common Eider (Somateria Mollissima), Glaucous-Winged Gull (Larus Glaucescens), Pigeon Guillemot (Cepphus Columba), and Tufted Puffin (Fratercula Cirrhata) from the Aleutian Chain of Alaska. Environ Monit Assess, 2009, 152, 357–367.
- Cabrera, C.; Lorenzo, M. L.; Lopez, M. C. Lead and Cadmium Contamination in Dairy Products and Its Repercussion on Total Dietary Intake. J. Agric. Food Chem., 2002, 43, 1605–1609.
