ABSTRACT
This paper aimed to develop a high-performance liquid chromatography (HPLC) method that can simultaneously detect ten biogenic amines (BAs) in commercially available soybean paste and to determine the contents of BAs in these products. Heptylamine (Hep) was used as the internal standard reagent and benzoyl chloride as the sample derivatization reagent; Inertsil ODS-3 chromatographic column was set as the stationary phase while the acetonitrile and 5 mmol/L ammonium acetate solution as the mobile phases; the samples were treated with gradient elution and detected at 254 nm (UV). Perchloric acid solution (0.4 mol/L) was employed as the sample extraction solvent to determine the contents of ten BAs in soybean paste products. All BAs were separated within 20 min. The methodological validation suggested good linearity, whereas the linear correlation coefficient of all standards was greater than 0.99 with the linear range of 0.1–80 μg/g, detection limits of 0.01–0.03 μg/g, and quantitation limits of 0.03–0.10 μg/g. For 17 commercially available soybean paste products involved in our study, different samples were distinguished from each other not only by the dramatic differences in the contents of BAs but also by the variance in BA types. Of these BAs, tyramine (Tyr) and agmatine (Agm) were found to be the highest contents in soybean paste products; the contents of histamine (His), 2-phenylethylamine (Phe), serotonin (Ser), spermidine (Spd), and putrescine (Put) were lower, followed by cadaverine (Cad), tryptamine (Try), and spermine (Spm). This method proved to be highly sensitive and effective in determining the contents of BAs in soybean paste products; there was a dramatic difference in the contents of ten BAs among different soybean paste products.
Introduction
Fermented soybean products, as a class of seasoning and table delicacy, including soybean paste, soy sauce, fermented bean curd, and fermented soya beans, have won warm praise from broad masses. Soybean paste is a widely popular product that features reddish-brown color, distinct soy sauce aroma, and delicious taste. Owing to rich nutrition, unique color and flavor, and contribution to appetite, Chinese soybean paste is a traditional Chinese salty flavoring that combines seasoning, nutritive, and medical functions. With the components of soybean polypeptides, melanoidins, soy isoflavone, soyasaponin, and other physiological active substances, soybean paste has medical functions like antioxidation,[Citation1,Citation2] anti-microbial,[Citation3] anticoagulation,[Citation4] antihypertension,[Citation5,Citation6] gastric secretion stimulation,[Citation7] appetite improvement,[Citation7] digestion promotion,[Citation8] and immune enhancement.[Citation8]
Despite of the multiple nutrients and medical functions, soybean paste also contains some substances that may potentially threaten human health, such as biogenic amines (BAs).[Citation9–Citation11] BAs are a kind of bioactive organics with low molecular weight, and their presence in food may lead to public health concerns due to their physiological and toxicological effects. Considering that China is both a big producer and consumer of soybean paste, it is very necessary to investigate the types and contents of BAs in commercially available Chinese soybean paste products.
Materials and methods
Samples and reagents
The samples were purchased from local supermarkets. BAs standards, such as Put, Cad, Spd, Spm, Try, Phe, His, Ser, Hep, Tyr, Agm, and dansyl chloride (Dns-Cl) were from Sigma Chemical Co.; acetonitrile and acetone for high-performance liquid chromatography (HPLC) from Merck; the other reagents used in this study were of HPLC grade.
Instrumentation
The Waters 1525 HPLC system connected to a Waters 2487 Dual Wavelength Absorbance UV Detector was used for separation and quantitation of ten target BAs. A XE-3-WH-2 basic vortex mixer (Zhengzhou Nanbei Instrument and Equipment Co., Ltd, China) was adopted for the vortexing of sample solution. Centrifugation of sample solution was performed on the LD5-10 centrifuge (Beijing Medical Centrifuge Factory, China). A Milli-Q Gradient system (A10, Millipore Corp., Bedford, USA) was used to make deionized water.
Experimental methods
Preparation of standard solution
All standards were prepared from 1 mg/mL stock solution according to their effective content using 0.1 mol/L HCl solution. The same volume of stock solution of ten BAs (Put, Cad, Spm, Spd, Try, Phe, His, Ser, Tyr, and Agm) was taken respectively and then mixed to gain a 100 μg/mL working solution of mixed standards. The 1 mg/mL stock solution of internal standard Hep was diluted to 100 μg/mL internal standard working solution with 0.1 mol/L HCl solution as per the same procedure. All solutions were stored in a dark refrigerator at 4°C.
Standard curve drawing
The 100 μg/mL working solution of mixed BA standards was diluted with 0.1 mol/L HCl solution to 80, 50, 20, 10, 5, 1, 0.5, and 0.1 μg/mL, respectively. The experiment was conducted in the following steps: 2 mL of diluted solutions of different concentrations were taken, respectively, followed by addition of 100 μL of internal standard working solution (100 μg/mL), 1 mL of NaOH (2 mol/L), and 10 μL of benzoyl chloride; after 30 s of vortex oscillation, the solutions were placed in the water bath at 30°C for 40 min of reaction and then taken out; after another 30 s of vortex oscillation, 2 mL of saturated NaCl solution was added to terminate the reaction; then 3 mL of anhydrous ether was added twice separately for extraction, followed by centrifugation at 1000 rpm for 5 min. The ether layer was removed to a 10 mL centrifuge tube and then dried under nitrogen, followed by dissolution with 1 mL of acetonitrile (chromatographic grade) and filtration with 0.45 μm ultrafiltration membrane for subsequent determination. Three parallels were established for each sample. All samples were stored in a dark refrigerator at 4°C and detected within 24 h.
Sample pretreatment
Twenty milliliter of perchloric acid solution and 100 μL of Hep solution were added to 2 g of soybean paste sample (which needed to be ground evenly if any granular bean flaps), followed by oscillation for 5 min and still standing for 5 min. After that, 10 µL of 2 M sodium hydroxide solution and 10 µL of benzoyl chloride were added to 1 mL of the mixture, followed by two cycles of oscillation for 10 s plus reaction in water bath at 30°C for 20 min. Two microliter of saturated sodium chloride solution was then added (to terminate the reaction), followed by oscillation for 10 s. Three mililiter of anhydrous ether was added for extraction, followed by rolling-over shaking for 5 s and centrifugation at 1000 rpm for 5 min. The above described ether extraction steps were repeated three times and the upper ether layers gained in three cycles were removed and mixed to a 10 mL centrifuge tube. After nitrogen gas flushing, 1 mL of acetonitrile was added, followed by vortex oscillation for 15 s and filtration with 0.45 μm ultrafiltration membrane for subsequent liquid phase determination. Three parallels were established for each sample.
Liquid chromatography[Citation9,Citation10]
Column: 250, 4.6, and 5 are in ODS-3 column; column temperature: 30°C; mobile phase A: 0.005 moL/L ammonium acetate solution; mobile phase B: acetonitrile; see for gradient elution methods of mobile phases; sample size: 20 μL; UV detection: 254 nm; chromatography workstation was the Breeze system provided by Waters Corporation, US.
Table 1. Gradient elution program for biogenic amines analysis.
Detection limits and quantitation limits
The derivatives of mixed standards with the concentration of 0.1 μg/mL was appropriately diluted with acetonitrile; signal-to-noise ratio (S/N) of greater than 3 was defined as the criterion for detection limits, and S/N of greater than 10 as the criterion for quantitation limits.
Detection of BAs
Specific methodology referred to the report by Guan et al.[Citation11], Liu et al.,[Citation12] and Zhong et al.[Citation13] The detected BAs included Put, Cad, Spm, Spd, Tyr, Phe, His, Ser, Try, and Agm, where each sample was detected three times.
Data processing
All data were processed using Duncan’s multiple comparison provided by statistical software SPSS, the results were represented by mean ± standard deviation.
Results and analysis
Chromatograms of the standards and actual samples
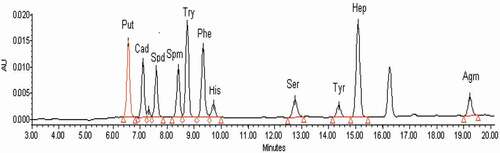
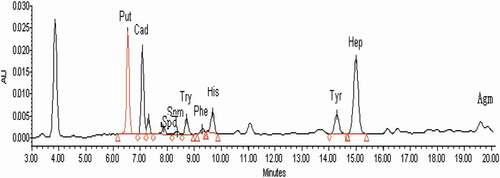
See for the chromatogram of standards of ten BAs. It can be found that both different BAs (5 μg/mL) and the internal standard derivatives could be effectively separated; the samples could be quickly eluted, and all tested derivatives were successfully eluted within 20 min. See for the chromatogram of derivatives of soybean paste sample (soybean paste sample 1) extract. The peak shape of different BAs was symmetrical without impurity interference, suggesting that the mobile phase elution gradient met the requirement of simultaneously determined ten BAs.
Method validation
The 0.1, 0.5, 1, 5, 10, 20, 50, 80, and 100 μg/mL solutions of mixed standards were injected successively. The ratio of the peak area to the internal standard peak area was calculated to develop the standard curve for standard solutions with corresponding concentrations. The obtained standard curve regression equations and correlation coefficients have been summarized in . The linearly dependent coefficients of all standards were greater than 0.99 with the detection limits of 0.01–0.03 μg/g and the quantitation limits of 0.03–0.10 μg/g, which indicated the high sensitivity of our method and its competence for simultaneous determination.
Table 2. Linear ranges, calibration curves, correlation coefficient (R), method limits of detection, and quantitation (MLODs and MLOQs) of ten biogenic amines.
Contents of ten BAs in the samples of commercially available soybean paste products
shows the determination results of BA contents in different brands of soybean paste sold in Hangzhou. Samples as in contained multiple BAs more or less, where Put, Cad, His, Ser, Try, and Agm existed in all samples, while Spm, Spd, Phe, and Tyr were not detected in individual products.
Table 3. Contents of biogenic amines in soybean paste samples.
The data in revealed dramatic differences in the contents of BAs, as well as great variance in BA types among different samples. For example, the content of Tyr in sp4 was 411.04 mg/kg, while that in sp14 turned to be only 15.50 mg/kg; the content of Phe in sp17 was 82.97 mg/kg, but it was not detected in sp10. There were also significant differences among the contents of different BAs for the same brand. In sp1, for example, the content of Tyr was 37.77 mg/kg, the content of His was 28.29 mg/kg, while the content of Spm was only 0.54 mg/kg; in sp3, the content of Put was 34.49 mg/kg, the content of Try was 11.07 mg/kg, while the content of Ser was only 4.16 mg/kg. The BAs category and content differed in different soybean paste, which was probably due to the difference in manufacturing technique, processing environment, etc. for different kinds of soybean paste.[Citation14–Citation17]
Conclusion
With benzoyl chloride as the derivatization reagent and Hep as the internal standard, our experiment established a HPLC method for the detection of ten BAs in soybean paste, by which the derivatives of involved BAs could be effectively separated. The methodological validation suggested good linearity in the derivatives of different BAs, where the linear correlation coefficients of all standards were greater than 0.99 with the linear range of 0.1–80 μg/g, detection limits of 0.01–0.03 μg/g, and quantitation limits of 0.03–0.10 μg/g. Determination of BAs in commercially available soybean paste products indicates great differences in the contents and types of BAs between different samples. Therefore, it is necessary to further explore the impact of different fermentation processes, storage conditions, and storage time on BA synthesis in soybean paste products, thus providing a theoretical basis for the edible safety of soybean paste products.
Additional information
Funding
References
- Kuligowski, M.; Pawłowska, K.; Jasińska-Kuligowska, I.; Nowak, J. Isoflavone Composition, Polyphenols Content and Antioxidative Activity of Soybean Seeds during Tempeh Fermentation. CYTA-Journal of Food. 2017, 15(1), 27–33.
- Ma, H. R.; Liu, R.; Zhao, Z. Y.; Zhang, Z. X.; Cao, Y.; Ma, Y. D.; Guo, Y.; Xu, L. A Novel Peptide from Soybean Protein Isolate Significantly Enhances Resistance of the Organism under Oxidative Stress. Plos One. 2016. DOI: 10.1371/journal.pone.0159938.
- Moon, H. R.; Rhee, M. S. Synergism between Carvacrol or Thymol Increases the Antimicrobial Efficacy of Soy Sauce with No Sensory Impact. International Journal of Food Microbiology 2016, 217, 35–41. DOI: 10.1016/j.ijfoodmicro.2015.10.009.
- Kim, K. Y.; Lim, K. M.; Kim, C. W.; Shin, H. J.; Seo, D. B.; Lee, S. J.; Noh, J. Y.; Bae, O. N.; Shin, S.; Chung, J. H. Black Soybean Extract Can Attenuate Thrombosis through Inhibition of Collagen-Induced Platelet Activation. Journal of Nutritional Biochemistry 2011, 22(10), 964–970. DOI: 10.1016/j.jnutbio.2010.08.008.
- Rudolph, S.; Lunow, D.; Kaiser, S.; Henle, T. Identification and Quantification of ACE-inhibiting Peptides in Enzymatic Hydrolysates of Plant Proteins. Food Chemistry 2017, 224, 19–25. DOI: 10.1016/j.foodchem.2016.12.039.
- Yan, Z. L.; Zhang, X. Y.; Li, C. L.; Jiao, S. C.; Dong, W. Y. Association between Consumption of Soy and Risk of Cardiovascular Disease: A Meta-Analysis of Observational Studies. European Journal of Prevention Cardiology 2017, 24(7), 735–747. DOI: 10.1177/2047487316686441.
- Xia, X. D.; Wang, Y.; Liu, X. L.; Li, Y.; Zhou, J. Z. Soymilk Residue (Okara) as a Natural Immobilization Carrier for Lactobacillus Plantarum Cells Enhances Soymilk Fermentation, Glucosidic Isoflavone Bioconversion, and Cell Survival under Simulated Gastric and Intestinal Conditions. Peer Journal 2016. DOI: 10.7717/peerj.2701.
- Angelis, E. D.; Pilolli, R.; Bavaro, S. L.; Monaci, L. Insight into the Gastro-Duodenal Digestion Resistance of Soybean Proteins and Potential Implications for Residual Immunogenicity. Food & Function 2017. DOI: 10.1039/c6fo01788f.
- Kim, J. H.; Ahn, H. J.; Kim, D. H.; Jo, C.; Yook, H. S.; Park, H. J.; Byun, M. W. Irradiation Effects on Biogenic Amines in Korean Fermented Soybean Paste during Fermentation. Journal of Food Science 2003, 68(1), 80–84. DOI: 10.1111/j.1365-2621.2003.tb14118.x.
- Kim, J. H.; Kim, D. H.; Ahn, H. J.; Park, H. J.; Byun, M. W. Reduction of the Biogenic Amine Contents in Low Salt-Fermented Soybean Paste by Gamma Irradiation. Food Control. 2005, 16(1), 43–49. DOI: 10.1016/j.foodcont.2003.11.004.
- Guan, R. F.; Liu, Z. F.; Zhang, J. J.; Wei, Y. X.; Wahab, S.; Liu, D. H.; Ye, X. Q. Investigation of Biogenic Amines in Sufu (Furu): A Chinese Traditional Fermented Soybean Food Product. Food Control. 2013, 31(31), 345–352. DOI: 10.1016/j.foodcont.2012.10.033.
- Liu, Z. F.; Wei, Y. X.; Zhang, J. J.; Liu, D. H.; Hu, Y. Q.; Ye, X. Q. Changes in Biogenic Amines during the Conventional Production of Stinky Tofu. International Journal of Food Science and Technology 2011, 46, 687–694. DOI: 10.1111/j.1365-2621.2011.02545.x.
- Zhong, J. J.; Ye, X. Q.; Fang, Z. X.; Xie, G. F.; Liao, N. B.; Shu, J.; Liu, D. H. Determination of Biogenic Amines in Semi-Dry and Semi-Sweet Chinese Rice Wines from the Shaoxing Region. Food Control. 2012, 28(1), 151–156. DOI: 10.1016/j.foodcont.2012.05.011.
- Suzzi, G.; Gardini, F. Formation and Destruction of Biogenic Amines in Chunjang (A Black Soybean Paste) and Jajang (A Black Soybean Sauce). Food Chemistry 2013, 141, 1026–1031. DOI: 10.1016/j.foodchem.2013.03.054.
- Guo, Y. Y.; Yang, Y. P.; Peng, Q.; Han, Y. Biogenic Amines in Wine: A Review. International Journal of Food Science and Technology 2015, 50, 1523–1532. DOI: 10.1111/ijfs.12833.
- Jairath, G.; Singh, P. K.; Dabur, R. S.; Rani, M.; Chaudhari, M. Biogenic Amines in Meat and Meat Products and Its Public Health Significance: A Review. Journal of Food Science and Technology 2015, 52(11), 6835–6846. DOI: 10.1007/s13197-015-1860-x.
- Karovičová, J.; Kohajdová, Z. Biogenic Amines in Food. Cheminform. 2005, 59(1), 70–79.