 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bioactive compounds and antioxidant activity from 44 fruit were evaluated. The data were statistically evaluated by analysis of common components and specific weights (CCSWA). Panã, acerola, açaí, and jabuticaba showed higher values of total phenolic compounds (TPCs) and antioxidant activity. The analysis of CCSWA was able to explain almost 100% of the variance of the data and established the correlation between TPC and antioxidant capacity, being the most influential variables in the classification of samples. This statistical method is ideal for quickly analyzing a large amount of data, as obtained in this research, which facilitates routines of industrial analysis.
Introduction
Brazil has a large variety of native and exotic underexploited fruit species of great potential and interest for the agroindustry.[Citation1] These fruits are differentiated from the conventional ones because they are produced in specific regions of the Brazilian biomes, such as Cerrado and Mata Atlântica, and that only a small part of the population has access, besides having peculiar characteristics like flavor and texture. Consumption of these fruits is no longer exclusively a result of personal taste and preference, but has become a concern for health due to recognition of their nutritive and therapeutic value.[Citation2] However, there are several exotic fruits that have not yet been studied with regard to their bioactive compounds as well as their antioxidant potential.
Because of this recognition, industries are transforming fresh fruits into pulps marketed in frozen form, which can be used as raw material for the production of other products, in addition to providing practicality for the consumer, and avoiding the seasonality of fruits.[Citation3]
Fruit pulps are a source of antioxidant compounds, such as phenolic compounds, vitamins, and flavonoids, which can protect cellular constituents against oxidative damage and therefore limit the risk of various degenerative diseases associated with oxidative stress. The content of bioactive compounds in fruits depends directly on natural factors such as cultivar, growing region, and maturity[Citation4], and in addition, the industrialization process itself can affect these characteristics. Experimental studies strongly support the role of phenolic compounds in the prevention of cancer, diabetes, and neurodegenerative disease.[Citation5–Citation7]
Bioactive compounds from different matrixes are receiving more attention, especially in the last two decades, due to the potential of these substances to replace the synthetic antioxidants widely used.[Citation8,Citation9] Studies where different fruits species are analyzed are of great interest for the industry of food processing.[Citation10] Therefore, in this context, the use of chemometric tools for the characterization, origin determination, authentication, adulteration, and quality control of fruits has been increasingly employed in food science and technology.[Citation11] Thus, there are many applications of multivariate statistical techniques in order to explore and classify fruit bioactivity and functionality.[Citation12,Citation13]
The analysis of common components and specific weights (CCSWA) was developed by Qannari et al.[Citation14], and it is used in the treatment of sensory test data. This research is the first study to use CCSWA as a method of classifying bioactive compounds in fruits. The analysis of CCSWA was used in this research as a precursor tool in the classification of samples of fruit pulps, from results of phenolic compounds and antioxidant activity. This study aimed to evaluate the bioactive compounds of 44 Brazilian fruit pulps using in vitro colorimetric tests and chromatographic analysis, making use of the analysis of CCSWA as a statistical method of classification of samples by their most relevant characteristics.
Materials and methods
Solvents and chemicals
Folin–Ciocalteu reagent, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), DPPH (1,1-diphenyl-2-picrylhydrazyl), ABTS (2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), and chemical HPLC-grade standards (purity ≥ 95%) of caffeic acid, p-coumaric acid, ferulic acid, chlorogenic acid, trans-cinnamic acid, gallic acid, syringic acid, vanillic acid, quercetin, catechin, rutin, and resveratrol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol and acetic acid were of HPLC grade, while the other reagents used in the experiments were of analytical grade. The aqueous solutions were prepared using ultrapure water (Milli-Q, Millipore, São Paulo, SP, Brazil).
Raw material
Forty-four fruit pulps were used in this study: açaí (açai) (Euterpe oleracea), acerola (acer) (Malpighia emarginata), apple (appl) (Malus sp.), araça (araç) (Psidium guineenses), blackberry (blac) (Rubus fruticosus), blueberry (blue) (Vaccinium myrtillum), buriti (buri) (Mauritia vinifera), cocoa (coca) (Theobroma cacao), cagaita (caga) (Eugenia dysenterica), cajá (caja) (Spondias mombin), cambuci (camb) (Paivaea langsdorfii), cashew (cash) (Anacardium occidentale), coconut (coco) (Cocos nucifera), cupuassu (cupu) (Theobroma grandiflorum), garcinia (garc) (Garcinia sp.), guava (guav) (Psidium guajava), grape (grap) (Vitis sp.), graviola (grav) (Annona muricata), grumixama (grum) (Eugenia brasiliensis), jabuticaba (jabu) (Myrciaria cauliflora), jackfruit (jack) (Artocarpus heterophyllus), jatobá (jato) (Hymenaea courbaril), kiwi (kiwi) (Actinidia deliciosa), lemon (lemo) (Citrus limon), orange (oran) (Citrus sinensis), mango (mang) (Mangifera indica), melon (melo) (Cucumis melo), murici (muri) (Byrsonima verbascifolia), papaya (papa) (Carica papaya), panã (pana) (Annona crassiflora), passion fruit (pass) (Passiflora edulis), peach (peac) (Prunus persica), pineapple (pine) (Ananas comosus), pitanga (pita) (Eugenia uniflora), pitomba (pito) (Talisia esculenta), raspberry (rasp) (Rubus idaeus), seriguela (seri) (Spondias purpurea), star fruit (star) (Averrhoa carambola), strawberry (stra) (Fragaria sp.), tamarind (tama) (Tamarindus indica), tangerine (tang) (Citrus reticulata), umbu (umbu) (Spondias tuberosa), uvaia (uvai) (Eugenia uvalha), and watermelon (wate) (Citrullus lanatus). The pulps were purchased from the companies Polpa Norte (Campo Mourão, PR, Brazil) and Sítio Bello (Paraibuna, SP, Brazil). All samples were stored in an ultrafreezer at −80°C until analysis.
Bioactive compound extraction process
The extraction was carried out in the proportion 1:10 (m/v), where 2 g of pulp was transferred to Erlenmeyer flasks and 20 mL of a 40% hydroethanolic solution was added. Then the Erlenmeyer flasks were placed in a shaking incubator (Shaker SL 222, Solab, Piracicaba, SP, Brazil) at 130 rpm for 120 min at 25°C. After extraction, the solution was transferred to centrifuge tubes and centrifuged at 5000 rpm for 10 min. The supernatant fraction (extract) was transferred to amber flasks and stored under 5°C refrigeration until analysis.
Quantification of total phenolic compounds (TPCs)
Quantification of the phenolic compounds in the extracts was performed according to the colorimetric method of Folin–Ciocalteu described by Singleton & Rossi[Citation15] with some modifications. Into 10-mL volumetric flasks were pipetted 7 mL of ultrapure water, 0.1 mL of fruit extract, and 0.5 mL of Folin–Ciocalteu reagent. After 3 min, 1.5 mL of 20% sodium carbonate was added and the volume completed to 10 mL in a volumetric flask with ultrapure water. Readings were taken with a spectrophotometer (UV M51, BEL Engineering, Monza, MB, Italy) at 765 nm. Gallic acid was used at different concentrations (0–500 mg/L) to construct the calibration curve. The results were expressed in GAE/100 g of fresh fruit.
Quantification of total flavonoids
The concentration of total flavonoids content (TFC) in the extracts was determined according to the methodology of Chang et al.[Citation16]; 250 μL of the fruit extract and 1250 μL ultrapure water were used. Subsequently 75 μL NaNO2 (5%) was added and after 6 min 150 μL AlCl3.6H2O (10%) was added. After 5 min, 0.5 mL of 1 M NaOH and 275 μL of ultrapure water were added. Readings were taken with a spectrophotometer (UV M51, BEL Engineering, Monza, MB, Italy) at 510 nm. Catechin was used as the standard and the calibration curve was constructed with concentrations of 0–65 mg/L. All values were expressed in milligrams equivalent of catechin per 100 grams fresh weight (mg CTE/100 g fw).
Monomeric anthocyanin content (MAC)
Quantification of the monomeric anthocyanins in the extracts was determined by the differential pH method proposed by Giusti & Wrolsted.[Citation17] The appropriate dilution factor was determined for each type of sample so that its absorbance was within the linearity range of the spectrophotometer at 510 nm (cyanidin-3-glycoside + extraction system). Dilutions were performed using potassium chloride buffer solution pH 1.0 and sodium acetate buffer pH 4.5. After dilution, the extracts were rested for 15 min. A reading at 510 nm and then at 700 nm was performed for each diluted sample. The absorbance (A) was calculated by Equation 1:
where A510nm pH1.0 and A700nm pH1.0 are the absorbances of the samples at the dilution at pH 1.0 at 510 and 700 nm, respectively, and A510nm pH4.5 and A700nm pH4.5 are the absorbances of the samples at the dilution at pH 4.5 at 510 and 700 nm, respectively. The concentration of anthocyanin content was calculated initially in milligrams of cyanidin-3-glycoside per liter of extract according to Eq. (2). The results were expressed in milligrams of cyanidin-3-glycoside per 100 g of fresh weight.
where A is the absorbance calculated in Eq. (1); M is cyanidin-3-glycoside molar mass (449.2 g/mol), DF is the dilution factor, Ε is the molar extinction coefficient (26 900 L/mol cm), and λ is the optical path length (1 cm).
Antioxidant activity evaluation
Antioxidant activity – DPPH•: Determination of the antioxidant activity in the extracts by the DPPH• method was performed according to the methodology described by Brand-Williams et al.[Citation18] with some modifications. In test tubes, 2.75 mL of ethanol, 1.75 mL of DPPH solution and 0.5 mL of extract were added, and maintained in the dark for 30 min. Readings were taken with a spectrophotometer (UV M51, BEL Engineering, Monza, MB, Italy) at 517 nm absorbance. Ethanol was used as blank. The Trolox calibration curve was constructed with concentrations varying from 0 to 250 μmol/L. The results were expressed in μmol of Trolox equivalent per 100 g of fresh weight (μmol Trolox/100 g fw).
Antioxidant activity – ABTS•+: Determination of the ABTS•+ radical inhibition activity in the extracts was performed according to Re et al.[Citation19] with some modifications. The antioxidant values of the extracts were presented as μmol Trolox/100 g of fw and calculated from a calibration curve with a concentration ranging from 0 to 1000 μmol/L of Trolox.
Phenolic profile by RP-HPLC-DAD
The phenolic profile analysis was performed according to the methodology described by Haminiuk et al.[Citation20], with injection of 10 μL of hydroethanol extracts previously filtered in a 0.45 μm syringe filter. A Shimadzu Prominence model high-performance liquid chromatograph (HPLC), coupled to a diode array detector (DAD) and auto sampler, was used. Data were collected by the LCSolution software provided by Shimadzu. The separation was performed on an Acclaim® 120 C18 column, with dimensions of 4.6 mm × 250 mm, 5 μm (Dionex, Salt Lake City, UT, USA) at 40°C. The mobile phase was composed of two solvents: water acidified with acetic acid (solvent A) and methanol (solvent B). The following gradient was performed: 0–10% B for 2 min; 10–20% B for 3 min; 20–30% B for 5 min; 30–35% B for 5 min; 35–50% for 10 min; 50–60% B for 5 min; 60–80% B for 5 min; and 80–100% B for 10 min, followed by washing and column reconditioning with 100% B for 7 min; 100–5% B for 5 min. Quantification of the phenolic compounds was carried out using calibration curves for caffeic acid, p-coumaric acid, ferulic acid, chlorogenic acid, trans-cinnamic acid, gallic acid, syringic acid, vanillic acid, quercetin, catechin, rutin, and resveratrol, from Sigma-Aldrich (St. Louis, MO, EUA).
Data analysis
Each parameter was analyzed in triplicate. Results were presented as mean ± standard deviation (SD). The Pearson correlation coefficient was used to evaluate the correlation strength between the experimental values obtained by the colorimetric analysis. A chemometric approach consisting of the analysis of CCSWA was applied in order to merge the data of bioactive compounds obtained by colorimetric assays and compounds isolated by RP-HPLC-DAD/UV-Vis. This statistical analysis allows evaluation of the variables of greater relevance in the separation of the different samples. CCSWA was performed with Matlab R2008b following an algorithm proposed by Qannari et al.[Citation14] and detailed by Bouveresse et al.[Citation21]
Results and discussion
Determination of bioactive compounds by spectrophotometry
Bioactive compounds from the fruits were extracted with a mixture of solvents, ethyl alcohol and water at a 40 : 60 (v/v) ratio. According to studies by Haminiuk et al.,[Citation22] this proportion of solution is the best extractive solution of these compounds. According to Chaicouski et al.,[Citation23] a hydroalcoholic extractive solution can carry both polar and nonpolar compounds, presenting advantages in extraction. The concentration of TPC in the extracts ranged from 66.09 to 902.18 mg GAE/100 g fw (). Vasco et al.[Citation24] studied the concentration of TPC in 17 fruits from Ecuador and the fruit were classified into three concentration categories of TPC: low (< 100 mg GAE/100 g), medium (100–500 mg GAE/100 g), and high (> 500 mg GAE/100 g) for the samples based on fresh weight.
Table 1. Concentrations of the bioactive compounds.
Extracts of the fruits with high concentrations of TPC were panã (902.18 ± 10.68 mg GAE/100 g), açaí (708.22 ± 10.21 mg GAE/100 g), jabuticaba (626.57 ± 4.39 mg GAE/100 g), and acerola (593.77 ± 10.16 mg GAE/100 g). On the other hand, extracts classified with an average concentration of TPC presented concentrations ranging from 344.09 ± 5.74 mg GAE/100 g for araça to 100.77 ± 0.97 mg GAE/100 g for blueberry. Finally, fruit extracts classified as having a low concentration of TPC showed variation from 99.40 mg GAE/100 g for jatobá to 55.66 mg GAE/100 g for watermelon.
According to Souza et al.,[Citation25] which quantified the bioactive compounds of five fruit pulps of the Brazilian Cerrado, the marolo fruit (panã), was also classified as having a high concentration of TPC, with a value of 739.37 ± 7.92 mg GAE/100 g, indicating that the fruit is an excellent source of phenolic compounds. The structure of these compounds present in fruits and plants may have beneficial properties, mainly due to the vicinal hydroxyls attached to the aromatic ring.[Citation26] In addition, phenolic compounds can interrupt chain oxidation reactions by donating hydrogen atom or by chelating metals.[Citation27] Therefore, they act as antioxidant and reducing agents.
Fruits are the main sources of flavonoids, and their health benefits are associated with hydrogen donors and also their reducing properties, which contribute to cell regulation.[Citation28] Total flavonoid concentrations ranged from 2.45 to 449.18 mg CTE/100 g. The extracts of panã and açaí were also the samples with the highest concentrations of total flavonoids, whereas, 449.18 mg and 197.249 mg CTE/100 g, respectively. The samples that presented the highest content of monomeric anthocyanins (MAC) were açaí with 46.12 mg/100 g fw and blackberry with 54.65 mg/100 g fw.
The antioxidant activity values evaluated by free radical scavenging assays ranged from 176.85 ± 16.22 to 7433.37 ± 26.26 μmol Trolox/100 g fw (DPPH•) and from 335.43 ± 3.82 to 8511.84 ± 61.44 μmol Trolox/100 g fw (ABTS•+). The extracts with the highest values of antioxidant activity were acerola (DPPH• = 7433.37 ± 26.26 μmol Trolox/100 g fw and ABTS•+ = 8511.84 ± 61.44 μmol Trolox/100 g fw) and panã pulp (DPPH• = 4486.11 ± 30.40 μmol Trolox/100 g fw and ABTS•+ = 4778.32 ± 19.87 μmol Trolox/100 g fw). The DPPH and ABTS methods are based on different mechanisms. The ABTS method includes a wider range of compounds, which can be hydrophilic or lipophilic. In contrast, the DPPH method is widely used to analyze antioxidants that are soluble in organic solvents.[Citation29] Although the DPPH radical is more stable compared to ABTS, the ABTS has been more widely used because of the range of compounds with which it reacts.[Citation30] The antioxidant capacity of fruits may vary according to their concentration of TPC, flavonoids, carotenoids, and vitamins C and E.[Citation25]
The results of this study showed a positive and significant Pearson correlation between the antioxidant capacity measured by the DPPH• and ABTS•+ assays, with TPC (r = 0.863 and 0.839, respectively; p < 0.001) and total flavonoids (r = 0.4701 and 0.4758, respectively; p < 0.001). The DPPH• and ABTS•+ assays showed a significant correlation (r = 0.969; p < 0.001). The highest correlation between the DPPH• assay and the Folin–Ciocalteu test occurs due to the fact that both are based on similar mechanisms of action, that is, electron transfer.[Citation28] The results suggest that phenolic compounds, such as phenolic acids, tannic acid, and proanthocyanidins, may be responsible for presenting the most important contributions to the antioxidant activity of the fruits studied.[Citation25] Almeida et al.[Citation31] evaluated 11 fruits and verified that the antioxidant activity measured in ABTS•+ and DPPH• assays was highly correlated (r = 0.94 and r = 0.88, respectively; p < 0.001) with TPC. Other studies have also reported a high correlation between phenolic compound concentration and antioxidant activity.[Citation11,Citation24] Lin et al.[Citation32] reported that the antioxidant capacity of flavonoids can occur due to the presence of double bonds in C-rings, which increases nucleophilic power.
Evaluation of phenolic compounds by liquid chromatography
Five hydroxycinnamic acids, three hydroxybenzoic acids, four flavonoids, and one stilbene were identified in fruit pulp extracts by RP-HPLC-DAD/UV-Vis (caffeic acid with a retention time (RT) of 14.36 min, p-coumaric acid (RT 18.3 min), ferulic acid (RT 19.6 min), chlorogenic acid (RT 12.71 min), trans-cinnamic acid (RT 29.95), gallic acid (RT 6.55 min), syringic acid (RT 15.25), vanillic acid (RT 14.30), quercetin (RT 30.53 min), catechin (RT 11.30 min), rutin (RT 23.55 min), and resveratrol (RT 24.18 min)). The results are shown in .
Table 2. Concentration of individual phenolic by HPLC.
Flavonoids were the most abundant compounds in the extracts of panã (4.60 ± 0.10 mg/100 g), araça (4.39 ± 0.14 mg/100 g), and pitanga (4.21 ± 0.05 mg/100 g) fruits. Among the flavonoids, catechin is the main one responsible for the concentration of flavonoids in the fruits studied. Lage et al.[Citation33] reported the presence of flavonoids in panã pulp, with catechin being one of the main flavan-3-ols found in the fruit. Although the presence of galloyl groups and the number and position of hydroxyl groups (based on the redox potential) are responsible for increasing the antioxidant activity, methoxylation and glycosylation of the 3-position apparently inhibit the reduction capacity.[Citation34]
The extract of murici presented a higher concentration with respect to the stilbene resveratrol in fresh weight (0.03 ± 0.01 mg/100 g). Stilbenes are considered a group that acts as potential chemopreventive and antiaging agents.[Citation35] In addition, resveratrol inhibits the formation of free radicals and has antimutagenic activity.[Citation36] Studies by Malta et al.[Citation36] reported the presence of resveratrol in murici fruit at a concentration of 0.31 ± 0.04 mg/100 g of dry weight.
Hydroxybenzoic acids were found at higher concentrations in açaí (0.81 ± 0.04 mg/100 g), with vanillic acid showing the highest concentration in the fruit. Hydroxybenzoic acids are generally present in the attached form and are typically a component of a complex structure such as hydrolyzable lignins and tannins. They can also be found linked to sugar derivatives and organic acids in foods of plant origin.[Citation37] Pacheco-Palencia et al.[Citation38] found a predominance of vanillic (0.505 ± 0.027 mg/100 g) and syringic acids (0.402 ± 0.036 mg/100 g) in açaí. Vanillic and syringic acids have antimicrobial, anticarcinogenic, and antioxidation properties.[Citation39]
In relation to hydroxycinnamic acids, higher concentrations in the extracts of apple (0.89 ± 0.01 mg/100 g) and peach (0.80 ± 0.01 mg/100 g) were found, highlighting the chlorogenic acid. Chlorogenic acid is widely distributed in fruits, having several beneficial biological properties, including antibacterial, antiphlogistic, antiviral, and inhibitory effects on carcinogenesis in the large intestine and liver.[Citation40,Citation41] Studies by Pingret et al.[Citation42] and Hao et al.[Citation41] have shown that apple contains considerable amounts of hydroxycinnamic acid derivatives which are represented mainly by chlorogenic acid. Hydroxycinnamic acids are generally found primarily esterified with small molecules, as well as bound to cell wall structural components, such as cellulose, lignin and proteins, through ester bonds.[Citation37]
Multivariate statistical analysis
The distribution of fruit extracts in the CCSWA was obtained from colorimetric data from TPC, TFC, MAC, DPPH•, and ABTS•+ and chromatographic analysis. In this analysis it is possible to demonstrate grouping tendencies according to the relevant characteristics of each sample, finding a common structure between the tables, analyzed iteratively, giving them the same weight, generating a consensus space for sample representation.[Citation43] This analysis makes it possible to evaluate a large number of samples, facilitating the elucidation of possible relationships between the data set, which is essential in industrial analysis routines that evaluate the quality of the product before being commercialized.
It was observed that only two common components (CC1 and CC2) were sufficient to explain almost 100% of the data variance (). A main group containing most of the samples can be observed (), which represent the samples containing similar values as verified by the analyses. This is because, since they are fruits, many have similar characteristics, having high amounts of phenolic compounds and, consequently, antioxidants, when compared to other food sources. Some samples were distanced from the others, characterized mainly by the antioxidant capacity of samples such as acerola, which has a very superior antioxidant potential compared to the others. Acerola is known as a source rich in ascorbic acid (vitamin C), a fundamental nutrient for human health and its biological functions are centered on its antioxidant properties in biological systems, helping to prevent common degenerative processes.[Citation4] In addition to this component, research has shown that acerola is also a source of carotenoids, highlighting β-carotene, β-52 cryptoxanthin, lutein, and violaxanthin.[Citation44] These components are mainly responsible for the antioxidant capacity of the fruit and their high concentration may have been the factor responsible for the separation of this pulp from the other samples. Samples of panã, açaí, and jabuticaba also distanced themselves from the main group of samples by the same characteristic (antioxidant capacity), as well as by the high content of phenolic compounds, especially the panã sample.
Figure 1. Distribution of samples according to common components and specific weights across CC1 and CC2.
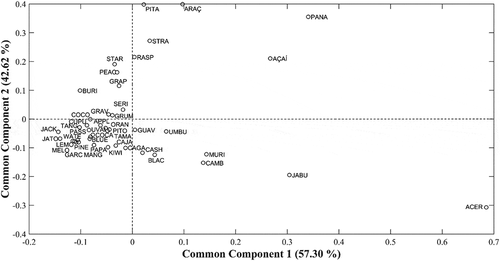
The saliences of the RP-HPLC-DAD and UV-Vis result tables correspond to the participation of each table in the separation. In CC1, the saliences/specific weights for RP-HPLC-DAD and UV-Vis analysis were 0.08 and 0.98, respectively. In CC2, there was only the representation of the saliences for the analysis of RP-HPLC-DAD, with a value of 0.85. Although the first two common components were sufficient to explain the variance, the data were evaluated up to the fifth common component (CC5), demonstrating that the saliences obtained from the third common component were not relevant in explaining the data (specific weight < 0.01).
From the saliences, it was possible to observe that in CC1 the data presented variance predominantly as a function of the results found in the spectrophotometric analyses, whereas in CC2, a predominance of the variance of the results found by the liquid chromatography was obtained. From this, the relevance of each UV-Vis analysis and each compound identified by RP-HPLC-DAD in different correlations was evaluated separately, as shown in and . Regarding spectrophotometric analyses, the variance of the samples was explained mainly by CC1, as can be observed in , which shows the correlation of the most influential variables in the separation of spectrophotometric data in CC1 and CC2. In CC2, the most influential variables were TPC and TFC.
Figure 2. (A) Correlation between spectrophotometric (UV-Vis) variables and common components (CC1 and CC2); (B) correlation between chromatographic (RP-HPLC-DAD) variables and common components (CC1 and CC2). (Legend: black bars—CC1, gray bars—CC2) .
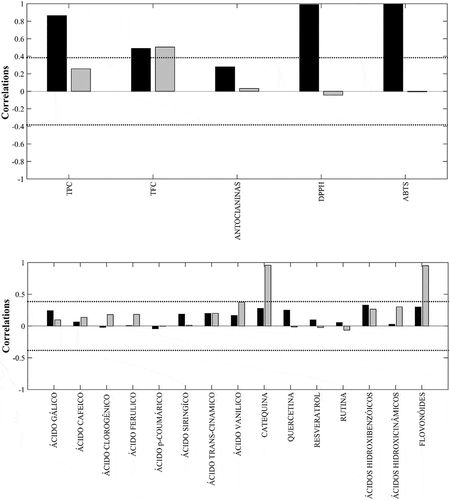
DPPH• and ABTS•+ variables highly influenced in the separation of the samples, followed by the TPC analysis. This may have occurred because it is proven that phenolic compounds may have antioxidant activity,[Citation27] and as previously confirmed by the Pearson test, the samples showed a high correlation between phenolic compounds and antioxidants.
MAC showed a greater influence on CC1; however, when related to the other variables, little influence on the separation of the samples was observed, due to the lower concentration in the analyzed fruits when compared to the other parameters. Although the anthocyanin concentration was very small or even absent in some of the samples, this phytochemicals has great importance for the antioxidant capacity of the samples, such as blackberry, açaí, and blueberry, due to its effect against free radicals.[Citation45]
shows the correlation of the most influential variables in the separation of the chromatographic data in CC1 and CC2. In this correlation, both common components had an influence on the separation, highlighting CC2, which presented higher correlations for catechin and vanillic acid compounds. The group of flavonoids was the main group responsible for the distribution of the samples in CC2, due to catechin, which is a flavonoid group present in a larger quantity in the samples, especially panã, araça, and pitanga fruits. These samples were distanced from the others along CC2 due to their high catechin content. Catechin is an important phenolic compound, belonging to the flavonoid group and has gained considerable attention in recent years due to its health-beneficial properties such as antidiabetic, anti-inflammatory, antimutagenic, anticarcinogenic, and antimicrobial activities.[Citation46]
Conclusion
In this study, through the colorimetric analyses, it was possible to demonstrate a positive correlation between the content of total phenolic compounds and antioxidant activity, in the same way, it was possible to verify that the main quantified phenolic compounds are part of the flavonoid group. Among the major phenolic compounds identified by liquid chromatography, catechin was found in the majority of the fruit pulps. Other important compounds that make up the groups of hydroxycinnamic acids, hydroxybenzoic acids, and stilbene were also identified. It was possible to visualize the relationship between the data using the analysis of CCSWA, highlighting the most important characteristics of each fruit pulp with respect to the composition of bioactive compounds. The relationship between TPC content and antioxidant activity was confirmed by means of this analysis, and the highest amount of compound in the samples (catechin) was one of the most relevant factors influencing the sample distribution.
In this way, this research has achieved the objective of presents important characteristics of a great diversity of fruits, especially exotic fruits, such as those of the Cerrado and Mata Atlântica, which results in greater recognition of their beneficial biological activity and to increase their value and sale territory. These data reinforce the importance of a regular intake of fruits to provide antioxidant compounds in the human diet. However, more studies are needed to identify and quantify the major bioactive compounds present in fruits, especially their antioxidant activity in in vivo tests.
Additional information
Funding
References
- Schiassi, M. C. E. V.; Souza, V. R.; Lago, A. M. T.; Campos, L. G.; Queiroz, F. Fruits from the Brazilian Cerrado Region: Physico-Chemical Characterization, Bioactive Compounds, Antioxidant Activities, and Sensory Evaluation. Food Chemistry 2018, 245, 305–311. DOI: 10.1016/j.foodchem.2017.10.104.
- Siriamornpun, S.; Kaewseejan, N. Quality, Bioactive Compounds and Antioxidant Capacity of Selected Climacteric Fruits with Relation to Their Maturity. Scientia Horticulturae 2017, 221, 33–42. DOI: 10.1016/j.scienta.2017.04.020.
- Dutra, R. L. T.; Dantas, A. M.; Marques, D. A.; Batista, J. D. F.; Meireles, B. R. L. A.; Cordeiro, A. M. T. M.; Magnani, M.; Borges, G. S. C. Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Frozen Pulps of Brazilian Exotic Fruits Exposed to Simulated Gastrointestinal Conditions. Food Research International 2017, 100, 650–657. DOI: 10.1016/j.foodres.2017.07.047.
- Hanamura, T.; Uchida, E.; Aoki, H. Changes of the Composition in Acerola (Malpighia Emarginata DC.) Fruit in Relation to Cultivar, Growing Region and Maturity. Journal of the Science of Food and Agriculture 2008, 88, 1813–1820. DOI: 10.1002/jsfa.v88:10.
- Cho, N.; Choi, J. H.; Yang, H.; Jeong, E. J.; Lee, K. Y.; Kim, Y. C.; Sung, S. H. Neuroprotective and Anti-Inflammatory Effects of Flavonoids Isolated from Rhus Verniciflua in Neuronal HT22 and Microglial BV2 Cell Lines. Food and Chemical Toxicology 2012, 50, 1940–1945. DOI: 10.1016/j.fct.2012.03.052.
- Lin, S.-T.; Tu, S.-H.; Yang, P.-S.; Hsu, S.-P.; Lee, W.-H.; Ho, C.-T.; Wu, C.-H.; Lai, Y.-H.; Chen, M.-Y.; Chen, L.-C. Apple Polyphenol Phloretin Inhibits Colorectal Cancer Cell Growth through Inhibition of Type 2 Glucose Transporter and Activation of P53-Mediated Signaling Proteins. Journal of Agricultural and Food Chemistry 2016, 64, 6826–6837. DOI: 10.1021/acs.jafc.6b02861.
- Saleem, F.; Sarkar, D.; Ankolekar, C.; Shetty, K. Phenolic Bioactives and Associated Antioxidant and Anti-Hyperglycemic Functions of Select Species of Apiaceae Family Targeting for Type 2 Diabetes Relevant Nutraceuticals. Industrial Crops and Products 2017, 107, 518–525. DOI: 10.1016/j.indcrop.2017.06.023.
- Delfanian, M.; Kenari, R. E.; Sahari, M. A. Influence of Extraction Techniques on Antioxidant Properties and Bioactive Compounds of Loquat Fruit (Eriobotrya Japonica Lindl.) Skin and Pulp Extracts. Food Science & Nutrition 2015, 3, 179–187. DOI: 10.1002/fsn3.201.
- Caleja, C.; Barros, L.; Antonio, A. L.; Oliveira, M. B. P. P.; Ferreira, I. C. F. R. A Comparative Study between Natural and Synthetic Antioxidants: Evaluation of Their Performance after Incorporation into Biscuits. Food Chemistry 2017, 216, 342–346. DOI: 10.1016/j.foodchem.2016.08.075.
- Berto, A.; Ribeiro, A. B.; Souza, N. E.; Fernandes, E.; Chisté, R. C. Bioactive Compounds and Scavenging Capacity of Pulp, Peel and Seed Extracts of the Amazonian Fruit Quararibea Cordata against ROS and RNS. Food Research International 2015, 77, 236–243. DOI: 10.1016/j.foodres.2015.06.018.
- Zielinski, A. A. F.; Ávila, S.;Ito, V.; Nogueira, A.; Wosiacki, G.; Haminiuk, C. W. I. The Association between Chromaticity, Phenolics, Carotenoids, and in Vitro Antioxidant Activity of Frozen Fruit Pulp in Brazil: An Application of Chemometrics. Journal of Food Science 2014, 79, 510–516. DOI: 10.1111/1750-3841.12389.
- Meléndez, E.; Ortiz, M. C.; Sarabia, L. A.; Íniguez, M.; Puras, P. Modelling Phenolic and Technological Maturities of Grapes by Means of the Multivariate Relation between Organoleptic and Physicochemical Properties. Analytica Chimica Acta 2013, 761, 53–61. DOI: 10.1016/j.aca.2012.11.021.
- Dorta, E.; González, M.; Lobo, M. G.; Sánchez-Moreno, C.; Ancos, B. Screening of Phenolic Compounds in By-Product Extracts from Mangoes (Mangifera Indica L.) By HPLC-ESI-QTOF-MS and Multivariate Analysis for Use as a Food Ingredient. Food Research International 2014, 57, 51–60. DOI: 10.1016/j.foodres.2014.01.012.
- Qannari, E. M.; Wakeling, I.; Courcoux, P.; MacFie, H. J. H. Defining the Underlying Sensory Dimensions. Food Quality and Preference 2000, 11, 151–154. DOI: 10.1016/S0950-3293(99)00069-5.
- Singleton, V. L.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Chang, C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. Journal of Food and Drug Analysis 2002, 10, 178–182.
- Giusti, M. M.; Wrolstad, R. E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. In Current Protocols in Food Analytical Chemistry, Wrolstad, R. E., Editor, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Science and Technology 1995, 28, 25–30.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying and Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237. DOI: 10.1016/S0891-5849(98)00315-3.
- Haminiuk, C.; Plata-Oviedo, M. S.; Mattos, G.; Carpes, S. T.; Branco, I. G. Extraction and Quantification of Phenolic Acids and Flavonols from Eugenia Pyriformis Using Different Solvents. Journal of Food Science and Technology 2014, 51, 2862–2866. DOI: 10.1007/s13197-012-0759-z.
- Bouveresse, D.; Pinto, R. C.; Schimidtke, L. M.; Locquet, N.; Rutledge, D. N. Identification of Significant Factors by an Extension of ANOVA-PCA Based on Multi-Block Analysis. Chemometrics and Intelligent Laboratory Systems 2011, 106, 173–182. DOI: 10.1016/j.chemolab.2010.05.005.
- Haminiuk, C. W. I.; Plata-Oviedo, M. S. V.; Guedes, A. R.; Stafussa, A. P.; Bona, E.; Carpes, S. T. Chemical, Antioxidant and Antibacterial Study of Brazilian Fruits. International Journal Food Science Technology 2011, 46, 1529–1537. DOI: 10.1111/j.1365-2621.2011.02653.x.
- Chaicouski, A.; Silva, J. E.; Trindade, J. L. F.; Canteri, M. H. G. Determination of the Amount of Phenolic Compounds Present in Liquid and Dry Extracts of Yerba Mate (Ilex Paraguariensis). Brazilian Journal of Agro-industrial Products 2014, 16, 33–41.
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total Phenolic Compounds and Antioxidant Capacities of Major Fruits from Ecuador. Food Chemistry 2008, 111, 816–823. DOI: 10.1016/j.foodchem.2008.04.054.
- Souza, V. R.; Pereira, P. A. P.; Queiroz, F.; Borges, S. V.; Carneiro, J. D. S. Determination of Bioactive Compounds, Antioxidant Activity and Chemical Composition of Cerrado Brazilian Fruits. Food Chemistry 2012, 134, 381–386. DOI: 10.1016/j.foodchem.2012.02.191.
- Halliwell, B.; Aeschbach, R.; Loliger, J.; Aruoma, O. I. The Characterization on Antioxidants. Food and Chemical Toxicology 1995, 33, 601–617. DOI: 10.1016/0278-6915(95)00024-V.
- Bursal, E.; Köksal, E.; Gülçin, I.; Bilsel, G.; Gören, A. C. Antioxidant Activity and Polyphenol Content of Cherry Stem (Cerasus Avium L.) Determined by LC– MS/MS. Food Research International 2013, 51, 66–74. DOI: 10.1016/j.foodres.2012.11.022.
- Gonçalves, A. E. S. S.; Lajolo, F. M.; Genovese, M. I. Chemical Composition and Antioxidant/Antidiabetic Potential of Brazilian Native Fruits and Commercial Frozen Pulps. Journal of Agricultural and Food Chemistry 2010, 58, 4666–4674. DOI: 10.1021/jf903875u.
- Prior, R. L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53, 4290–4302. DOI: 10.1021/jf0502698.
- Marecek, V.; Mikyska, A.; Hampel, D.; Cejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH Methods as a Tool for Studying Antioxidant Capacity of Spring Barley and Malt. Journal of Cereal Science 2017, 73, 40–45. DOI: 10.1016/j.jcs.2016.11.004.
- Almeida, M. M. B.; Sousa, P. H. M.; Arriaga, A. M. C.; Prado, G. M.; Magalhães, C. E. C.; Maia, G. A.; Lemos, T. L. F. Bioactive Compounds and Antioxidant Activity of Fresh Exotic Fruits from Northeastern Brazil. Food Research International 2011, 44, 2155–2159. DOI: 10.1016/j.foodres.2011.03.051.
- Lin, S. D.; Liu, E. H.; Mau, J. L. Effect of Different Brewing Methods on Antioxidant Properties of Steaming Green Tea. LWT- Food Science and Technology 2008, 41, 1616–1623. DOI: 10.1016/j.lwt.2007.10.009.
- Lage, G. A.; Medeiros, F. S.; Furtado, W. L.; Takahashi, J. A.; Filho, J. D. S.; Pimenta, L. P. S. The First Report on Flavonoid Isolation from Annona Crassiflora Mart. Natural Product Research 2014, 28, 808–811. DOI: 10.1080/14786419.2014.885518.
- Aron, P. M.; Kennedy, J. A. Flavan-3-Ols: Nature, Occurrence and Biological Activity. Molecular Nutrition and Food Research 2008, 52, 79–104. DOI: 10.1002/mnfr.200700137.
- Rimando, A.; Kalt, W.; Magee, J.; Dewey, J.; Ballington, J. Resveratrol, Pterostilbene, and Piceatannol in Vaccinium Berries. Journal of Agricultural and Food Chemistry 2004, 52, 4713–4719. DOI: 10.1021/jf040095e.
- Malta, L. G.; Ghiraldini, F. G.; Reis, R.; Oliveira, M. V.; Silva, L. B.; Pastore, G. M. In Vivo Analysis of Antigenotoxic and Antimutagenic Properties of Two Brazilian Cerrado Fruits and the Identification of Phenolic Phytochemicals. Food Research International 2012, 49, 604–611. DOI: 10.1016/j.foodres.2012.07.055.
- Barros, L.; Dueñas, M.; Ferreira, I. C. F. R.; Baptista, P.; Santos-Buelga, C. Phenolic Acids Determination by HPLC–DAD–ESI/MS in Sixteen Different Portuguese Wild Mushrooms Species. Food and Chemical Toxicology 2009, 47, 1076–1079. DOI: 10.1016/j.fct.2009.01.039.
- Pacheco-Palencia, L. A.; Duncan, C. E.; Talcott, S. T. Phytochemical Composition and Thermal Stability of Two Commercial Açai Species, Euterpe Oleracea and Euterpe Precatória. Food Chemistry 2009, 115, 1199–1205. DOI: 10.1016/j.foodchem.2009.01.034.
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on CCl4-induced Liver Injury. Biological and Pharmaceutical Bulletin 2010, 33, 983–987. DOI: 10.1248/bpb.33.983.
- Wang, H. Q.; Li, D. L.; Du, Z. Y.; Huang, M. T.; Cui, X. X.; Lu, Y. J.; Zhang, K. Antioxidant and Anti-Inflammatory Properties of Chinese Ilicifolius Vegetable (Acanthopanax Trifoliatus (L) Merr) and Its Reference Compounds. Food Science and Biotechnology 2015, 24, 1131–1138. DOI: 10.1007/s10068-015-0144-6.
- Hao, Y.; Gao, R.; Liu, D.; He, G.; Tang, Y.; Guo, Z. Selective Extraction and Determination of Chlorogenic Acid in Fruit Juices Using Hydrophilic Magnetic Imprinted Nanoparticles. Food Chemistry 2016, 200, 215–222. DOI: 10.1016/j.foodchem.2016.01.004.
- Pingret, D.; Fabiano-Tixier, A.-S.; Le Bourvellec, C.; Renard, C. M. G. C.; Chemat, F. Lab and Pilot-Scale Ultrasound-Assisted Water Extraction of Polyphenols from Apple Pomace. Journal of Food Engineering 2012, 111, 73–81. DOI: 10.1016/j.jfoodeng.2012.01.026.
- Mazerolles, G.; Preys, S.; Bouchut, C.; Meudec, E.; Fulcrand, H.; Souquet, J. M.; Cheynier, V. Combination of Several Mass Spectrometry Ionization Modes : A Multiblock Analysis for A Rapid Characterization of the Red Wine Polyphenolic Composition. Analytica Chimica Acta 2010, 678, 195–202. DOI: 10.1016/j.aca.2010.07.034.
- Mezadri, T.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoid Pigments in Acerola Fruits (Malpighia Emarginata DC.) And Derived Products. European Food Research and Technology 2005, 220, 63–69. DOI: 10.1007/s00217-004-1042-y.
- Rufino, M. S.; Alves, R. E.; Brito, E. S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Nontraditional Tropical Fruits from Brazil. Food Chemistry 2010, 121, 996–1002. DOI: 10.1016/j.foodchem.2010.01.037.
- Jullian, C.; Miranda, S.; Zapata-Torres, G.; Mendizábal, F.; Oleoa-Azar, C. Studies of Inclusion Complexes of Natural and Modified Cyclodextrin with (+) Catequin by NMR and Molecular Modeling. Bioorganic & Medicinal Chemistry 2007, 15, 3217–3224. DOI: 10.1016/j.bmc.2007.02.035.
