ABSTRACT
In the present study, crude polysaccharides from Ziziphus Jujuba cv. Muzao were isolated and purified using DEAE cellulose-52 and Sephadex G-100 size-exclusion chromatography; four fractions were collected, namely GZMP-1, GZMP-2, GZMP-3, and GZMP-4. The molecular weights of these four fractions were measured to be 111.2, 95.1, 84.2, and 571.4 kDa, respectively, using high-performance gel permeation chromatography. Gas chromatography analysis of the monosaccharide composition confirmed that GZMP-1 was composed of rhamnose, arabinose, glucose, and galactose. Rhamnose, arabinose, and galactose were the main components present in GZMP-2 and GZMP-3, whereas GZMP-4 was composed of only rhamnose and arabinose. Scanning electron microscopy showed relatively smooth surfaces for GZMP-1 and GZMP-4, whereas GZMP-2 and GZMP-3 had more folds on their surfaces. Fourier transform infrared spectroscopy analyses indicated that GZMP-1 and ZMP mainly had α-type glycosidic linkages. The in vitro antioxidant activities of the polysaccharides revealed that jujube polysaccharides exhibit remarkable antioxidant activity, and can scavenge DPPH radical and OH radical in a concentration-dependent manner. The results of this work suggest that polysaccharides from Z. Jujuba cv. Muzao have potential to be used as functional food and in the development of natural antioxidant drug carriers.
Introduction
Ziziphus Jujuba cv. Muzao is a key member of Chinese jujube, and has long been used in diet therapy for thousands of years.[Citation1] It is not only a good source of nutrients, but also contains a variety of bioactive substances, such as polysaccharides, phenolics, flavonoids, triterpenoids, and saponins.[Citation2,Citation3] Jujube fruits have been used as a traditional Chinese medicine to prevent and treat diseases because of its immunological activities, sedative and hypnotic effects, anti-cancer properties, antioxidative activities, and anti-inflammatory effects.[Citation4–Citation8]
Polysaccharides with different properties and structures of constituent monosaccharides widely exist in plants, microorganisms, animals, and algae.[Citation9] Previous studies have demonstrated that polysaccharides isolated from jujube fruits show various biological activities, including antioxidative, immunomodulatory, anti-viral, anti-tumor, and anti-cancer effects.[Citation10–Citation13] All the acidic polysaccharide fractions were more effective in chelating radical scavenging activity than the neutral one among jujube polysaccharides.[Citation11] However, a few studies have described the activity and structural characterization of polysaccharides extracted from Z. Jujuba cv. Muzao.[Citation14,Citation15] Little information is available in the literature regarding the isolation, purification, characterization, and antioxidant activity of polysaccharides extracted from Z. Jujuba cv. Muzao.
In this study, the polysaccharides from Z. Jujuba cv. Muzao were subjected to ultrasonic-assisted extraction with acid buffer and further purified by DEAE cellulose-52 and Sephadex G-100 size-exclusion chromatography, resulting in four fractions: GZMP-1, GZMP-2, GZMP-3, and GZMP-4. Moreover, the purified fractions were characterized by chemical analysis, high-performance gel permeation chromatography (HPGPC), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and gas chromatography (GC). Further, the antioxidant activities of the four fractions on DPPH radical and OH radical were investigated.
Materials and methods
Materials
Z. Jujuba cv. Muzao fruits without diseases or visible blemishes were picked at the red, ripened stage in the Loess Plateau of China in Northern Shaanxi Province. Removal of the seed was followed by drying for 48 h at 50°C. The dried jujube was ground to pass through a 60-mesh sieve and stored at room temperature in desiccators until further use.
2,2-diphenyl-1-picrylhydrazyl (DPPH) and dextran standards of analytical grade, including rhamnose, arabinose, xylose, galactose, glucose, and mannose, were purchased and from Sigma Chemical Co. (St. Louis, MO, USA). DEAE cellulose-52 and Sephadex G-100 were purchased from Pharmacia Co (Kalamazoo, MI, USA). All other chemical reagents used were of analytical grade.
Preparation of crude polysaccharides
The jujube polysaccharides were extracted as previously described.[Citation16] Briefly, the dried jujube powder was first refluxed with 80% ethanol twice in a water bath at 85°C for 3 h in order to remove any soluble pigments and lipids. Then, the dried residue was extracted twice with distilled water under optimized conditions. The supernatants were collected, combined, and concentrated, and then mixed with four volumes of ethanol to precipitate the crude polysaccharides. The collected pellets were resuspended in distilled water, deproteinated with Sevag reagent, decolored with hydrogen peroxide solution, dialyzed (molecular weight cut-off: 3500 Da), and concentrated, and then underwent secondary sedimentation by ethanol, yielding the yellowish colored crude polysaccharides (ZMP).[Citation17]
Separation and purification of ZMP
ZMP were subjected to a DEAE-52 cellulose column (2.6 cm × 40 cm) for purification with stepwise elution of water and 0.05, 0.1, and 0.3 M aqueous solutions of sodium chloride (NaCl) at a flow rate of 1.0 mL/min. Different fractions (8 mL/tube), namely ZMP-1, ZMP-2, ZMP-3, and ZMP-4, were collected according to the phenol-sulfuric acid method. After dialysis, concentration, and lyophilization, ZMP-1, ZMP-2, ZMP-3, and ZMP-4 were further purified with a Sephadex G-100 column (1.6 cm × 60 cm) and eluted with distilled water at a flow rate of 0.3 mL/min to yield the four final purified fractions of GZMP-1, GZMP-2, GZMP-3, and GZMP-4. Finally, the four fractions were lyophilized (LGJ-25C, −50°C, 30 Pa, 24 h) before further investigation.
Analytical methods
Chemical composition analysis: The total carbohydrate content was determined by the phenol-sulfuric acid method,[Citation18] and the protein content was measured by the Bradford method.[Citation19,Citation20] Finally, the galacturonic acid content was determined via the modified m-hydroxydiphenyl method.[Citation16] Two milliliter of galacturonic acid standard and polysaccharide fractions were poured into a screw-capped tube separately and inserted in an bath for 10 min. Next, 5 mL of 0.125 M sodium tetraborate in sulfuric acid was added and mixed thoroughly, followed by heating in boiling water for 10 min, cooling in an ice-bath, adding 100 μL of 1.5 mg/mL m-hydroxybiphenyl in sodium hydroxide, shaking for 20 min, sonicating to remove air bubble and measuring the absorbance at 520 nm.[Citation16]
Homogeneity and molecular weight determination: The homogeneity and molecular weights of the fractions were determined by gel permeation chromatography on a Waters HPLC system (Waters Co. Ltd., Milford, MA, USA) equipped with three Ultrahydrogel linear columns (7.8 mm × 300 mm) in series and a model 2414 refractive index detector. Homogeneity and molecular weight analysis were estimated using the standard curve, which was obtained with T-series dextran standards.[Citation21]
Monosaccharide composition analysis: The monosaccharide composition analysis of GZMP-1, GZMP-2, GZMP-3, and GZMP-4 was detected by GC analysis as described previously.[Citation22,Citation23] GC (Shimadzu 2014 C; Shimadzu Corp., Kyoto, Japan) with a high-performance capillary column (DB-17; 30 mL × 0.25 mm ID, 0.25-μm film thickness; Agilent Technologies, Santa Clara, CA, USA) was used to determine the neutral monosaccharides’ derivatized products.
FTIR spectroscopy assay: The ZMP purified fractions were characterized using an FTIR; MP. 0331.04 (Bruker Corp., Billerica, MA, USA). The dried samples were ground with KBr powder and then pressed into pellets for FTIR measurement over the frequency range of 4000–400 cm–1.[Citation24]
SEM analysis: The shape and surface characteristics were observed and recorded using a field emission scanning electron microscope (FESEM; S-4800; Hitachi, Tokyo, Japan). The samples were examined at an acceleration voltage of 10 kV under high vacuum conditions, with image magnification of 20,000 ×.[Citation25]
In vitro antioxidant properties
DPPH free radical scavenging activity: The DPPH radical scavenging capacity was estimated with some modification of a previous method.[Citation26] Briefly, various concentrations (0.5–2.5 mg/mL) of the ZMP purified fractions (2 mL) were mixed with 4 mL of DPPH radical solution (0.1 mM, in ethanol). The mixtures were shaken vigorously and left to stand in the dark for 30 min, and the absorbance of the mixtures was then determined at 517 nm. The DPPH radical scavenging effect was estimated by the following equation: DPPH radical scavenging activity (%) = [1 – (A1 – A2)/A0]×100%; where A0 was the absorbance of the mixture with sample replaced by deionized water, and A1, and A2 were the absorbances of DPPH solution with and without jujube polysaccharides, respectively.
Hydroxyl radical scavenging activity: The hydroxyl radical scavenging capacity was measured using an improved Fenton-type reaction.[Citation12] Various concentrations (0.5–2.5 mg/mL) of the ZMP purified fractions (2.0 mL) were mixed with 2.0 mL FeSO4, 2.0 mL salicylic acid-ethanol, and 2.0 mL H2O2, and then incubated in a water bath at 37°C for 60 min, followed by determination of the absorbance at 510 nm. The scavenging activity of the jujube polysaccharide fractions toward hydroxyl radicals was calculated using the equation: Scavenging rate (%) = [1 – (Ai – Aj)/Ao]×100%; where Ai is the absorbance of ZMP fractions, Aj is the absorbance of background solution, and Ao is the absorbance of the control (deionized water instead of samples).
Statistical analyses
Data were expressed as means ± standard deviation (SD). Statistical analysis was performed using SPSS software (IBM Corp., Armonk, NY, USA), and one-way analysis of variance was used to evaluate differences between treatments. P-values less than 0.05 were recognized as statistically significant.
Results and discussion
Isolation and purification of ZMP
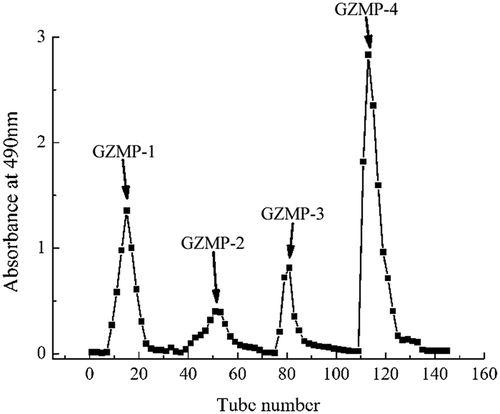
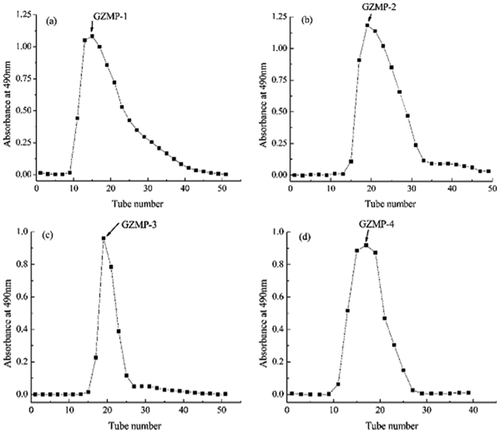
The crude polysaccharides, named ZMP, were extracted from Z. Jujuba cv. Muzao fruit by ethanol precipitation, lyophilization, and then deproteination by Sevag reagent. The total yield rate of ZMP in the isolation procedure was 4.31% (), which is similar to that reported in the previous research.[Citation27] In order to increase the purity and obtain homogeneous polysaccharide products, ZMP were purified using an anion-exchange chromatography of DEAE cellulose-52 column (2.6 cm × 40 cm). The four fractions, eluted with deionized water, 0.05, 0.10, and 0.30 M NaCl, were named ZMP-1, ZMP-2, ZMP-3, and ZMP-4, respectively (). ZMP-1, which was eluted with deionized water, may be a neutral polysaccharide, whereas ZMP-2, ZMP-3, and ZMP-4, which were eluted with the 0.05, 0.10, and 0.30 mol/L NaCl solutions, respectively, are acidic polysaccharides. Each of the four fractions were further purified by gel filtration chromatography of Sephadex G-100 using distilled water as an eluent at a flow rate of 1.0 mL/min. The eluate (8 mL/tube) was collected automatically, resulting in GZMP-1, GZMP-2, GZMP-3, and GZMP-4 were obtained (). The four fractions were recovered at rates of 0.209%, 0.093%, 0.418%, and 0.255%, respectively, based on the original amount of ZMP. All four of these elution curves had a single, symmetrical peak, which indicates that all of the purified products were homogeneous polysaccharides.[Citation28]
Chemical compositions of the ZMP fractions
The monosaccharide compositions of the ZMP purified fractions are presented in . Both GZMP-2 and GZMP-3 were shown to be composed of rhamnose, arabinose, and galactose, and arabinose was found to be the main monosaccharide in these fractions. Conversely, GZMP-1 was shown to be composed of four monosaccharides, namely rhamnose, arabinose, glucose, and galactose, with a molar ratio of 0.59:72.80:0.26:7.48, respectively, whereas only rhamnose and arabinose were detected in GZMP-4. The results indicated that the four fractions were mainly composed of rhamnose and arabinose, which is similar to the results by Wang et al.[Citation14] The difference in monosaccharide composition may be caused by various in the species, the regions of cultivation and extraction methods.
Table 1. Molecular weight, monosaccharides composition, protein and galacturonic acid contents of four ZMP purified fractions.
Notably, the galacturonic acid content was comparatively higher in GZMP-3 and GZMP-4 than in GZMP-1 and GZMP-2. The molecular weights of GZMP-1, GZMP-2, GZMP-3, and GZMP-4 were 111.2, 95.1, 84.2, and 571.4 kDa, respectively, which are higher than previously reported.[Citation16] Different molecular weights in the range of 104–106 Da have been observed in various Z. jujuba fruits and under different experimental conditions. The lower molecular weight and higher negatively charges polysaccharides yield the extended conformations and higher hydrodynamic volume in the aqueous solution, which might exhibit more significant biological activity.[Citation15] Their protein contents were measured to be around 2%, and these ZMP purified fractions are most likely protein-bound polysaccharides because the Sevag method is an effective method for removing free proteins.
FTIR spectroscopy of the ZMP fractions
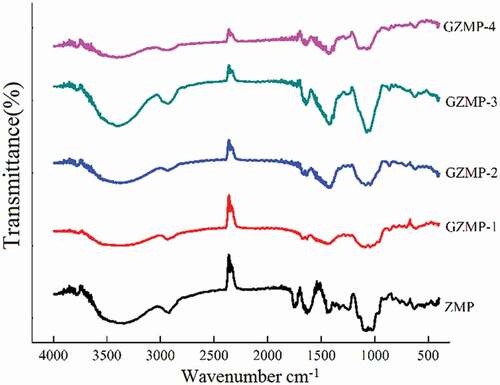
FTIR spectroscopy is a powerful tool for identifying organic group characteristics in polysaccharides.[Citation29] As shown in , a strong and broad absorption peak at 3500–3000 cm−1 attributed to O–H stretching vibrations and a peak at 3000–2800 cm−1 for C–H stretching vibrations were observed in all of the measured polysaccharides, indicating that ZMP, GZMP-1, GZMP-2, GZMP-3, and GZMP-4 were all polysaccharides.[Citation30] Furthermore, the band at 1641 cm−1 was attributed to the bound water, and the presence of a band in the 1200–1000 cm−1 region suggests that the monosaccharides have pyranose rings.[Citation31] The two peaks at 1631 and 1426 cm−1, representing a carboxylic group, suggest the presence of uronic acids in GZMP-1, GZMP-2, GZMP-3, and GZMP-4. The absorption peak at around 860 cm−1 in the FTIR spectrum of GZMP-1 and ZMP indicates the presence of α-type glycosidic linkages.[Citation32] Another obvious absorption peak at 612–631 cm−1 indicates C–H out-of-plane flexural vibration in all the samples. All the FTIR characterization results are in agreement with the results in .
SEM analysis
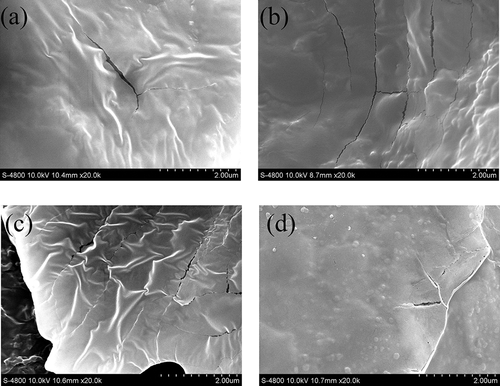
The SEM micrographs of the ZMP purified fractions are shown in , and these allow the surface morphology of the polymers to be determined. The micrographs revealed that the surface of the ZMP purified fractions is smooth and dense, which indicates strong intermolecular forces. GZMP-3 presented more folds than the other polysaccharide fractions. The SEM micrographs also showed the formation of cracks on the surface of the samples caused by the high power of the electron beam during scanning. These findings differ from those of Cui et al. (2014) for polysaccharide fractions from Fructus Jujubae because different methods of extraction, purification, and preparation were used.[Citation33]
In vitro antioxidant activity of the ZMP fractions
The antioxidative effects of polysaccharides closely related to their chemical properties and structural characteristics.[Citation34] It is well-known that specific functional groups such as sulfate, amino, hydroxyl, and carboxyl can be related to the antioxidative effects of polysaccharides, as well as their molecular weights.
Scavenging effect on DPPH radicals: DPPH, a stable free radical with a nitrogen center, is one of the capture-agents for living radicals. DPPH radical bleaching is a rapid method for determining the antioxidant activity of herbal extracts.[Citation35] The DPPH radical scavenging activity of the ZMP fractions is shown in ) and is related to the concentration of the samples. All the polysaccharide fractions showed a low scavenging effect at 0.5 mg/mL, ranging from 1.56% for GZSP-1 to 18.18% for ZMP. Notably, ZMP exhibited the strongest scavenging activity, however, GZMP-1 had the weakest scavenging activity. This sequence was in accordance with the galacturonic acid content, which suggests that higher galacturonic acid content is an obvious indicator of the antioxidant ability of jujube polysaccharides.[Citation10] In addition, except for GZMP-1 and GZMP-2, the scavenging activity of the ZMP fractions increased significantly with increasing sample concentration, which ranged from 1.5 to 2.0 mg/mL. This is similar to the result of Chang et al. (2010), who examined the water-soluble polysaccharides from Zizyphus jujuba purchased from a local farm in Gong-Guan with different monosaccharide.[Citation16] Monosaccharides with active hydroxyl groups can supply hydrogen or an electron to free radicals, thus forming stable radicals that can terminate the radical chain reaction.[Citation36]
Figure 6. Scavenging effects of ZMP, GZMP-1, GZMP-2, GZMP-3, and GZMP-4 on (a) the DPPH radical and (b) the hydroxyl radical.
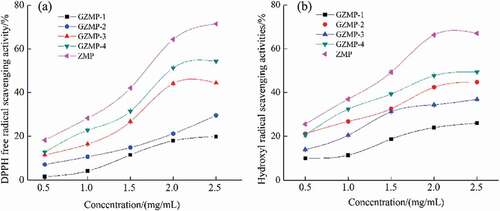
Scavenging effect on hydroxyl radicals: Hydroxyl radicals can react with almost all biomacromolecules, and induce severe oxidative injury in adjacent biomolecules.[Citation36,Citation37] Therefore, the elimination of hydroxyl radicals key to health protection and disease prevention. Especially, the scavenging activity of the ZMP fractions against OH increased of concentration up to 2.5 mg/mL. At 2.5 mg/mL, the hydroxyl radical scavenging activity of ZMP, GZMP-4, GZMP-3, GZMP-2, and GZMP-1 was 67.05%, 49.38%, 36.94%, 44.77%, and 26.03%, respectively. These results show that the antioxidant capacity of the ZMP purified fractions decreased dramatically relative to that of ZMP, which could be attributed to the absence of other phytochemicals, such as phenolics, tocopherol, or pigments.[Citation38] Higher galacturonic acid content and smaller molecular weight of the jujube polysaccharides may have more significant effects on the scavenging of the hydroxyl radical.[Citation34,Citation38] The hydroxyl radical scavenging activity is also related to anomeric hydrogen in the polysaccharides.
Conclusion
In this work, crude polysaccharides (ZMP) were obtained from Ziziphus Jujuba cv. Muzao fruit through ultrasonic-assisted extraction with acid buffer. After purified by DEAE cellulose-52 and Sephadex G-100, four fractions, namely GZMP-1, GZMP-2, GZMP-3, and GZMP-4, with molecular weights of 111.2, 95.1, 84.2, and 571.4 kDa, respectively, were obtained. Monosaccharides analysis revealed that rhamnose, arabinose, glucose, galactose, and galacturonic acid were the major components present in the polysaccharide fractions. The surface structure of GZMP-4 and GZMP-1 was smooth, whereas GZMP-2 and GZMP-3 had pore openings with folds on their surfaces. Further, the FTIR spectra showed that GZMP-1 and ZMP had mainly α-type glycosidic linkages. The assay for the in vitro antioxidant activities demonstrated that ZMP and GZMP-4 exhibited significant antioxidant activity, whereas GZMP-1, GZMP-2, and GZMP-3 had moderate DPPH free radical scavenging and ·OH scavenging activity, which could be related to their structural characteristics, including molecular weight, monosaccharide composition, and galacturonic acid content. These results suggested that the jujube polysaccharides obtained from Z. Jujuba cv. Muzao may be suitable for use in novel, natural functional food. However, more studies are required to ascertain the complete structural characteristics of polysaccharides and the structure-antioxidant activity relationships.
Acknowledgment
The authors are grateful to Xin Wang for providing technical support.
Additional information
Funding
References
- Du, L. J.; Gao, Q. H.; Ji, X. L.; Ma, Y. J.; Xu, F. Y.; Wang, M. Comparison of Flavonoids, Phenolic Acids, and Antioxidant Activity of Explosion-Puffed and Sun-Dried Jujubes (Ziziphus Jujuba Mill.). Journal of Agricultural and Food Chemistry 2013, 61, 11840–11847. DOI: 10.1021/jf401744c.
- Gao, Q. H.; Wu, P. T.; Liu, J. R.; Wu, C. S.; Parry, W. J.; Wang, M. Physico-Chemical Properties and Antioxidant Capacity of Different Jujube (Ziziphus Jujuba Mill.) Cultivars Grown in Loess Plateau of China. Scientia Horticulturae 2011, 130, 67–72. DOI: 10.1016/j.scienta.2011.06.005.
- Gao, Q. H.; Wu, C. S.; Wang, M. The Jujube (Ziziphus Jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. Journal of Agricultural and Food Chemistry 2013, 61, 3351–3363. DOI: 10.1021/jf4007032.
- Cao, J. X.; Zhang, Q. Y.; Cui, S. Y.; Cui, X. Y.; Zhang, J.; Zhang, Y. H; et al. Hypnotic Effect of Jujubosides from Semen Ziziphi Spinosae. Journal of Ethnopharmacology 2010, 130, 163–166. DOI: 10.1016/j.jep.2010.03.023.
- Chen, J. P.; Du, C. Y. Q.; Lam, K. Y. C.; Zhang, W. L.; Lam, C. T. W; Yan, A. L; et al. The Standardized Extract of Ziziphus Jujuba Fruit (Jujube) Regulates Pro-Inflammatory Cytokine Expression in Cultured Murine Macrophages: Suppression of Lipopolysaccharide-Stimulated NF-kappa B Activity. Phytotherapy Research 2014, 28, 1527–1532. DOI: 10.1002/ptr.5160.
- Choi, S. H.; Ahn, J. B.; Kim, H. J.; Im, N. K.; Kozukue, N.; Levin, C. E.; et al. Changes in Free Amino Acid, Protein, and Flavonoid Content in Jujube (Ziziphus Jujuba) Fruit during Eight Stages of Growth and Antioxidative and Cancer Cell Inhibitory Effects by Extracts. Journal of Agricultural and Food Chemistry 2012, 60, 10245–10255. DOI: 10.1021/jf302848u.
- Li, J. W.; Shan, L.; Liu, Y. F.; Fan, L. P.; Ai, L. Z. Screening of a Functional Polysaccharide from Zizyphus Jujuba Cv. Jinsixiaozao and Its Property. International Journal of Biological Macromolecules 2011, 49, 255–259. DOI: 10.1016/j.ijbiomac.2011.04.006.
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y. B.; Ren, F. Z. Systematic Evaluation of Antioxidant Capacities of the Ethanolic Extract of Different Tissues of Jujube (Ziziphus Jujuba Mill.) From China. Food Chemical and Toxicology 2010, 48, 1461–1465. DOI: 10.1016/j.fct.2010.03.011.
- Chen, S. H.; Chen, H. X.; Tian, J. G.; Wang, J.; Wang, Y. W.; Xing, L. S. Enzymolysis-Ultrasonic Assisted Extraction, Chemical Characteristics and Bioactivities of Polysaccharides from Corn Silk. Carbohydrate Polymers 2014, 101, 332–341. DOI: 10.1016/j.carbpol.2013.09.046.
- Li, J. W.; Liu, Y. F.; Fan, L. P.; Ai, L. Z.; Shan, L. Z. Antioxidant Activities of Polysaccharides from the Fruiting Bodies of Zizyphus Jujuba Cv. Carbohydrate Polymers 2011, 84, 390–394. DOI: 10.1016/j.carbpol.2010.11.051.
- Ji, X. L.; Peng, Q.; Yuan, Y. P.; Shen, J.; Xie, X. Y.; Wang, M. Isolation, Structures and Bioactivities of the Polysaccharides from Jujube Fruit (Ziziphus Jujuba Mill.): A Review. Food Chemistry 2017, 227, 349–357. DOI: 10.1016/j.foodchem.2017.01.074.
- Wang, D. Y.; Zhao, Y.; Jiao, Y. D.; Yu, L. H.; Yang, S.; Yang, X. B. Antioxidative and Hepatoprotective Effects of the Polysaccharides from Zizyphus Jujuba Cv. Shaanbeitanzao. Carbohydrate Polymers 2012, 88, 1453–1459. DOI: 10.1016/j.carbpol.2012.02.046.
- Xie, J. H.; Tang, W.; Jin, M. L.; Li, J. E.; Xie, M. Y. Recent Advances in Bioactive Polysaccharides from Lycium Barbarum L., Zizyphus Jujuba Mill, Plantago Spp., And Morus Spp.: Structures and Functionalities. Food Hydrocolloids 2016, 60, 148–160. DOI: 10.1016/j.foodhyd.2016.03.030.
- Wang, Y. J.; Liu, X. Q.; Zhang, J. Z.; Liu, G. P.; Liu, Y.; Wang, K. M; et al. Structural Characterization and in Vitro Antitumor Activity of Polysaccharides from Zizyphus Jujuba Cv. Muzao. RSC Advances 2015, 5, 7860–7867. DOI: 10.1039/C4RA13350A.
- Zhang, L.; Liu, X. Q.; Wang, Y. J.; Liu, G. P.; Zhang, Z.; Zhao, Z. X; et al. In Vitro Antioxidative and Immunological Activities of Polysaccharides from Zizyphus Jujuba Cv. Muzao. International Journal of Biological Macromolecules 2017, 95, 1119–1125. DOI: 10.1016/j.ijbiomac.2016.10.102.
- Chang, S. C.; Hsu, B. Y.; Chen, B. H. Structural Characterization of Polysaccharides from Zizyphus Jujuba and Evaluation of Antioxidant Activity. International Journal of Biological Macromolecules 2010, 47, 445–453. DOI: 10.1016/j.ijbiomac.2010.06.010.
- Qu, C. L.; Yu, S. C.; Jin, H. L.; Wang, J. S.; Luo, L. The Pretreatment Effects on the Antioxidant Activity of Jujuba Polysaccharides. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2013, 114, 339–343. DOI: 10.1016/j.saa.2013.05.084.
- Zhang, L.; Zhang, W. X.; Wang, Q. J.; Wang, D. D.; Dong, D. Q.; Mu, H. B.; et al. Purification, Antioxidant and Immunological Activities of Polysaccharides from Actinidia Chinensis Roots. International Journal of Biological Macromolecules 2015, 72, 975–983. DOI: 10.1016/j.ijbiomac.2014.09.056.
- Bradford, M. M.; A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein Binding. Analytical Biochemistry 1976, 72, 248–254. DOI: 10.1016/0003-2697(76)90527-3.
- Wang, X.; Chen, Q. R.; Lü, X. Pectin Extracted from Apple Pomace and Citrus Peel by Subcritical Water. Food Hydrocolloids 2014, 38, 129–137. DOI: 10.1016/j.foodhyd.2013.12.003.
- Yang, H. H.; Wu, Y. J.; Gan, C. J.; Yue, T. L.; Yuan, Y. H. Characterization and Antioxidant Activity of a Novel Polysaccharide from Pholidota Chinensis Lindl. Carbohydrate Polymers 2015, 38, 327–334
- Wang, X.; Lü, X. Characterization of Pectic Polysaccharides Extracted from Apple Pomace by Hot-Compressed Water. Carbohydrate Polymers. 2014, 102, 174–184. DOI: 10.1016/j.carbpol.2013.11.012.
- Wang, X.; Zhang, L. H.; Wu, J. L.; Wang, X. Q.; Lü, X. Improvement of Simultaneous Determination of Neutral Monosaccharides and Uronic Acids by Gas Chromatography. Food Chemistry 2017, 220, 198–207. DOI: 10.1016/j.foodchem.2016.10.008.
- Sun, L. Q.; Wang, L.; Li, J.; Liu, H. H. Characterization and Antioxidant Activities of Degraded Polysaccharides from Two Marine Chrysophyta. Food Chemistry 2014, 160, 1–7. DOI: 10.1016/j.foodchem.2014.03.067.
- Romdhane, M. B.; Haddar, A.; Ghazala, I.; Jeddou, K. B.; Helbert, C. B.; Ellouz-Chaabouni, S. Optimization of Polysaccharides Extraction from Watermelon Rinds: Structure, Functional and Biological Activities. Food Chemistry 2017, 216, 355–364. DOI: 10.1016/j.foodchem.2016.08.056.
- Lin, C. L.; Wang, C. C.; Chang, S. C.; Inbaraj, B. S.; Chen, B. H. Antioxidative Activity of Polysaccharide Fractions Isolated from Lycium Barbarum Linnaeus. International Journal of Biological Macromolecules 2009, 45, 146–151. DOI: 10.1016/j.ijbiomac.2009.04.014.
- Li, J. W.; Ai, L. Z.; Hang, F.; Ding, S. D.; Liu, Y. F. Composition and Antioxidant Activity of Polysaccharides from Jujuba by Classical and Ultrasound Extraction. International Journal of Biological Macromolecules 2014, 63, 150–153. DOI: 10.1016/j.ijbiomac.2013.10.043.
- Tang, X. H.; Yu, F.; Liu, J.; Gao, J.; Yan, L. F.; Dong, M. M. Isolation and Identification of Anti-Tumor Polysaccharide LSP21 from Limonium Sinense (Girard) Kuntze. International Journal of Biological Macromolecules 2014, 70, 138–142. DOI: 10.1016/j.ijbiomac.2014.06.042.
- Tang, H. L.; Chen, C.; Wang, S. K.; Sun, G. J. Biochemical Analysis and Hypoglycemic Activity of a Polysaccharide Isolated from the Fruit of Lycium Barbarum L. International Journal of Biological Macromolecules 2015, 77, 235–242. DOI: 10.1016/j.ijbiomac.2015.03.026.
- Liu, C. H.; Li, X. D.; Li, Y. H.; Feng, Y.; Zhou, S.; Wang, F. S. Structural Characterisation and Antimutagenic Activity of a Novel Polysaccharide Isolated from Sepiella Maindroni Ink. Food Chemistry 2008, 110, 807–813. DOI: 10.1016/j.foodchem.2008.02.026.
- Li, X.; Zhao, L.; Zhang, Q.; Xiong, Q. P.; Jiang, C. X. Purification, Characterization and Bioactivity of Polysaccharides from Glossaulax Didyma. Carbohydrate Polymers 2014, 102, 912–919. DOI: 10.1016/j.carbpol.2013.10.057.
- Manrique, G. D.; Lajolo, F. M. FT-IR Spectroscopy as a Tool for Measuring Degree of Methyl Esterification in Pectins Isolated from Ripening Papaya Fruit. Postharvest Biology and Technology. 2002, 25, 99–107. DOI: 10.1016/S0925-5214(01)00160-0.
- Cui, G. T.; Zhang, W. X.; Wang, Q. J.; Zhang, A. M.; Mu, H. B.; Bai, H. J.; et al. Extraction Optimization, Characterization and Immunity Activity of Polysaccharides from Fructus Jujubae. Carbohydrate Polymers 2014, 111, 245–255. DOI: 10.1016/j.carbpol.2014.04.041.
- Chen, H. X.; Zhang, M.; Qu, Z. S.; Xie, B. J. Antioxidant Activities of Different Fractions of Polysaccharide Conjugates from Green Tea (Camellia Sinensis). Food Chemistry 2008, 106, 559–563. DOI: 10.1016/j.foodchem.2007.06.040.
- Elboutachfaiti, R.; Delattre, C.; Petit, E.; Michaud, P. Polyglucuronic Acids: Structures, Functions and Degrading Enzymes. Carbohydrate Polymers 2011, 84, 1–13. DOI: 10.1016/j.carbpol.2010.10.063.
- Xie, J. H.; Shen, M. Y.; Xie, M. Y.; Nie, S. P.; Chen, Y.; Li, C.; et al. Ultrasonic-Assisted Extraction, Antimicrobial and Antioxidant Activities of Cyclocarya Paliurus (Batal.) Iljinskaja Polysaccharides. Carbohydrate Polymers 2011, 89, 177–184. DOI: 10.1016/j.carbpol.2012.02.068.
- Gao, J.; Zhang, T.; Jin, Z. Y.; Xu, X. M.; Wang, J. H.; Zha, X. Q.; et al. Structural Characterisation, Physicochemical Properties and Antioxidant Activity of Polysaccharide from Lilium Lancifolium Thunb. Food Chemistry 2015, 169, 430–438. DOI: 10.1016/j.foodchem.2014.08.016.
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H. E.; Browder, I. W.; Williams, D. L. Glucans Exhibit Weak Antioxidant Activity, but Stimulate Macrophage Free Radical Activity. Free Radical Biology and Medicine 2001, 30, 393–402. DOI: 10.1016/S0891-5849(00)00485-8.