?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The fat content and fatty acid profile of commercially important scallops Flexopecten glaber, Mimachlamys varia, and Pecten jacobaeus were investigated in samples of adductor muscle, gonad, mantle, and viscera. The viscera showed the highest lipid content in all species examined. Significant differences were found in the fatty acid composition among tissues and among scallops. All pectinids exhibited high levels of eicosapentaenoic and docosahexaenoic acids in the adductor muscle, with a maximum value of 211 mg/100 g tissue and 252 mg/100 g tissue in the viscera of F. glaber. Highest n3/n6 ratios were recorded in F. glaber gonad and viscera, in P. jacobaeus muscle, and in the gonad of M. varia. M. varia adductor muscle had the lowest values of atherogenicity and thrombogenicity indices used as indicators of beneficial health effects. These data contribute to the overall evaluation of the nutritional quality of scallops and suggest that their consumption may provide health benefits.
Introduction
The consumption of food from the aquatic environment has long been recognized as healthy for human consumption. Their benefits are related to high biological value proteins, essential vitamins, minerals, and mainly to the presence of bioactive long-chain omega-3 polyunsaturated fatty acids (n-3 PUFAs). In recent years, increasing attention has been focused on significance of n-3 PUFAs in human nutrition, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These fatty acids (FA) play vital roles in disease prevention and health promotion.[Citation1–Citation3]
Contemporary literature abounds with arguments about their potential role in the prevention of cardiovascular diseases (CVDs) that are the leading cause of death around the world. Moreover, they may be beneficial in preventing asthma in children, as well as neurological disorders (retinitis pigmentosa, depression, schizophrenia, and Alzheimer’s disease), infertility, inflammatory diseases (rheumatoid arthritis, Crohn’s disease), and certain types of cancers.[Citation1,Citation4–Citation6] Long-chain n-3 PUFAs cannot be synthesized by humans and must be obtained through the diet.[Citation7]
Nowadays, the Western diet tends to be too low in n-3 PUFAs, mainly due too low consumption of seafoods and a lack of adequate information for consumers regarding the nutritional value of these foodstuffs.[Citation2] Scallops belonging to Pectinidae family are one of the most demanding bivalve species for the customers all over the world. Their European market share has increased significantly in recent years[Citation8] representing an important part of the global seafood market and supporting both commercial fisheries and aquaculture all around the world.[Citation9] Pecten jacobaeus, Mimachlamys varia, and Flexopecten glaber are the most commercially important scallops from the Ionian Sea (central Mediterranean Sea), for the peculiar organoleptic characteristics of their meat, their high market value, and high growth rates in the wild and in cultured.[Citation10] The knowledge of their biochemical composition is extremely important because it reflects their nutritional value. This information can help consumers to make healthy food choices and can be for producers a powerful marketing tool.
Extensive research exists which describes lipids and fatty acid composition of many species of bivalves.[Citation3,Citation11–Citation14] However, the lipid content and the fatty acid composition of bivalves, besides varying for several parameters, such as fishing season, geographical location, size, sex, and reproductive cycle period,[Citation15] are also species-specific.[Citation16] Moreover, almost all the data included in the molluscan lipid studies concern the entire organism and only a few report the distribution of fatty acids in separate tissues.[Citation17,Citation18]
The aim of this study was to evaluate the nutritional quality of four different tissues (adductor muscle, gonad, mantle, and viscera) of three scallops’ species (F. glaber, M. varia, and P. jacobaeus), with emphasis on fatty acids profile. These data may contribute to a better understanding and interpretation of the health benefits given by the lipid fraction of some of the most economically important scallops from the central Mediterranean Sea.
Materials and methods
Sampling area, animal collection, and sample preparation
Adult specimens of P. jacobaeus (shell length: 100.0 ± 5.5 mm; edible weight: 8.6 ± 1.7 g), F. glaber (shell length: 44.2 ± 1.5 mm; edible weight: 5.36 ± 1.7 g), and M. varia (shell length: 41.1 ± 2.1 mm; edible weight: 4.51 ± 0.9 g) were collected from suspended cages of a pilot plant in the Ionian Sea (central Mediterranean, southern Italy: 40° 25ʹ 54ʹʹ N, 17° 14ʹ 22ʹʹ E) during autumn months (October–December 2014). Taxonomic determination of species has been made by use of classification keys available in literature.[Citation19,Citation20] On arrival in the laboratory, samples of the adductor muscles, viscera, gonads, and mantles were taken from 10 specimens for each species and stored at −30°C, until analysed. Biochemical analysis, performed on pooled tissues originating from 10 specimens, was done in triplicate (three for species for each organ).
Total lipid and fatty acids analysis
All used reagents and solvents (analytical grade) were purchased from Sigma (Sigma–Aldrich GmbH, Steinheim, Germany). Total lipid (TL) content was determined gravimetrically after chloroform–methanol extraction according to Folch et al.[Citation21] FAs of TLs were transesterified to methyl esters (FAMEs) in a boron trifluoride-catalyzed methanol:benzene solution (1:2, v:v). The mixture was shaken, and then heated in boiling water for 45 min.[Citation22] Samples were allowed to cool, and 1 ml of distilled water was added followed by vigorous shaking. FAMEs were recovered in the upper benzene phase and concentrated under nitrogen and kept at −20°C until further analysis. Triplicate samples were analysed. Analysis of FAMEs was performed by gas chromatography (GC) using an HP 6890 series GC (Hewlett Packard, Wilmington, DE, USA) equipped with flame ionization detector. FAMEs were separated with an Omegawax 250 capillary column (Supelco, Bellafonte, PA, USA) (30 m long, 0.25-mm internal diameter, and 0.25-mm film thickness). Helium was used as carrier gas at a flow rate of 1 ml/min. The column temperature program was as follows: 150–250°C at 4°C/min and then held at 250°C. FAMEs were identified by comparing retention times with a standard (Supelco 37 Component FAME Mix). The area under each FA peak, relative to the total area of all FA peaks, was used to quantify the FAs identified. The results obtained are reported as percentage of FA. All samples were analysed in triplicate. Relative quantities were expressed as weight % of total fatty acids. Per cent of total fatty acids data were converted to amounts per 100 g wet fillet according to Greenfield and Southgate.[Citation23]
Lipids nutritional quality indexes (NQI)
Atherogenicity index (AI)[Citation24]: AI shows the inhibition of the aggregation of plaque and diminishing the levels of esterified FA, cholesterol, and phospholipids, thereby preventing the appearance of micro- and macro-coronary diseases) where:
AI = (C12:0 + 4×C14:0 + C16:0)/(Sum MUFAs+Sum PUFAs)
Thrombogenicity index (TI)[Citation24]: TI shows the tendency to form clots in the blood vessels):
Fatty acids hypocholesterolemic/hypercholesterolemic ratios (HH): HH[Citation25] shows the hypocholesterolemic effect of PUFA:
Statistical analysis
Results are reported as means ± standard deviations (n = 30). The normality of distributions and homogeneity of variances were assessed by means of the Kolmogorov–Smirnov test of goodness of fit and Levene’s test, respectively. Data with homogeneous variances were analysed using ANOVA and post hoc Tukey’s test to determine differences among species and tissues. A principal component analysis (PCA), based on the Pearson’s correlation matrix, was used to simplify the data interpretation showing a visual representation of the main variations of the fatty acids content in the four tissues (adductor muscle A, gonad G, mantle M, and viscera V) of the three scallops. Analysis was performed with the statistical package software XLSTAT (Version 2008.4.01).
Results and discussion
TL content
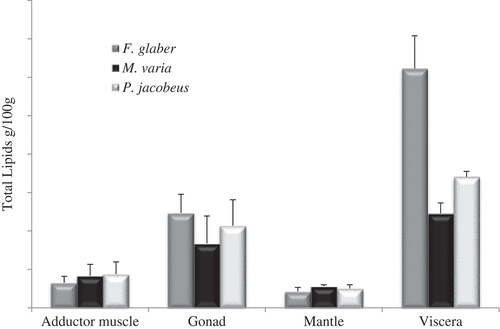
The TL contents of different tissues and pectinid species, expressed on a wet weight basis (ww), are shown in . Of all tissues examined, viscera had the highest lipid content in all species, and particularly in F. glaber with a value of 3.11 ± 0.42 g/100 g ww (p < 0.05) (). No significant differences among species (p > 0.05) were registered in the mantle (range 0.21–0.25 g/100 g ww) and the adductor muscle (range 0.32–0.44 g/100 g ww) that showed the lowest lipid content (p < 0.05). These data are in agreement with values reported by Caers et al.[Citation17] for Argopecten purpuratus from Chile.
Fatty acids
Fatty acid profiles of lipids are listed in . In all species and tissues a relative pattern with saturated fatty acid (SAFA)>polyunsaturated Fatty acids (PUFA)>monounsaturated fatty acid (MUFA) was observed, except for the adductor muscle of M. varia and viscera of F. glaber in which the PUFA was the most represented group.
Table 1. Fatty acid composition (mg/100 g tissue wet weight) and nutritional quality indexes of adductor muscle and gonad of F. glaber, M. varia, and P. jacobaeus (mean value ± SD; n = 30).
Palmitic acid was the major SAFA in all samples, with the lowest contents in the mantle of all species (between 35.76 and 46.71 mg/100 g ww tissue) and the highest 390 mg/100 g ww tissue in the viscera of F. glaber, followed by stearic (C18:0) and myristic (C14:0) acids. Relatively to adductor muscle and gonad, in general the values, expressed as % of total FAs, are consistent with the findings reported by Telahigue et al.[Citation9] in a study on the same species from Tunisia water, while Caers et al.[Citation17,Citation26] for A. purpuratus found values slightly lower than pectinidae of this study.
Diets high in SAFA increase the risk for developing heart disease[Citation27] given that it is known to increase blood total cholesterol and Low Density Lipoprotein (LDL); recent studies have, however, shown that only three SAFAs, lauric (12:0), myristic (14:0), and palmitic acids (16:0), significantly influence total and LDL-cholesterol.[Citation28] These fatty acids reduce the activity of the LDL receptors and thereby decrease the cellular LDL uptake.[Citation29] In this study, the proportion of C14:0 acid was highest in the viscera of the three scallops, registering the maximum content in F. glaber (390.7 mg/100 g ww tissue, representing 17.9% of total FAs). On the contrary, stearic acid (18:0) does not cause a significant increase of cholesterol levels,[Citation28] but rather a High Density Lipoprotein (HDL)-cholesterol-level-lowering effect.[Citation30]
MUFAs represented the lowest proportion of extracted FAs. F. glaber showed in the gonad and viscera higher values than the other species examined, while P. jacobaeus and M. varia in the adductor muscle and mantle (p < 0.05) (). The adductor muscle had values % of MUFA comparable results to those reported by Telahigue et al.[Citation9] in the same species. Oleic acid (C18:1n-9) was the most abundant in all species, with highest content in viscera and gonad (64.24–177.92 mg/100 g and 47.03–73.82 mg/100 g, respectively). Though the mantle had a lower content of oleic acid, in terms of percentage of total fatty acids it was the tissue with the highest value % (12.37–16.46% of total FAs).
Table 2. Summary of ANOVA (F-ratio and p value) for fatty acid content (mg/100 g tissue wet weight) and ratios in four tissues [factor: F. glaber (F), M. varia (M), P. jacobaeus (P), and samples means ranked in order of magnitude according to Tukey post hoc test].
Palmitoleic acid (C16:1n-7) was the other predominant MUFA, with higher values in viscera and gonads of all scallops species (). The same value % of C16:1n-7 was found by Telahigue et al.[Citation9] in the gonad of P. jacobaeus (6.4%).
Caers et al.[Citation17] found lower values % of C18:1n-9 in all studied tissues of A. purpuratus than those of this study. The percentage values of C16:1n-7 in the gonad of P. jacobaeus and viscera of M. varia (6.4% and 8.3%, respectively), were equivalent to those found by Caers et al.[Citation17] in the female gonad (6.6%) and viscera of A. purpuratus (8.2%). Both these MUFAs may have exogenous origins, from the diets, or endogenous by desaturation of C16:0 and C18:0 acids.
From a nutrition point of view, MUFAs have received increasing attention because of their mixed effects on human health. Recent evidence tends to indicate more beneficial effects and, in particular, on reducing risk of CVDs and other inflammation-related diseases.[Citation31,Citation32] When MUFAs (primarily, oleic acid: 18:1, n-9) are supplied instead of SAFA in metabolic studies, they lower total and LDL-cholesterol significantly.[Citation33]
PUFAs showed values ranging from 27% of total FAs in the mantle of P. jacobaeus to 52% of total FAs in the adductor muscle of M. varia. In general, PUFAs are considered as hypocholesterolemic and hypotriglyceridemic compared to SAFAs,[Citation34] but different PUFAs have different effects. PUFAs content proved to be predominant in the viscera and gonad of all species, reaching the maximum value in the viscera of F. glaber (903 mg/100 g tissue). DHA was the predominant PUFA in all studied species and in all tissues and mean % ranged from 6.8 ± 0.5% of total FAs (viscera of M. varia) to 24.9 ± 2.6% of total FAs (adductor muscle of M. varia). Caers et al.[Citation17,Citation26] report similar results for A. purpuratus. However, the highest DHA content was found in the viscera with its value ranging from 58.6 mg/100 g tissue (M. varia) to 252 mg/100 g tissue (F. glaber).
EPA was the second most abundant PUFA, ranging from 5.5 ± 1.5% of total FAs (mantle of P. jacobaeus) to 13.1 ± 0.9% of total FAs (adductor muscle of M. varia) (). Although the content of EPA is significantly low in the adductor muscle of all species (23.75–36.8 mg/100 g tissue), in terms of percentage values of EPA on the total of fatty acids it shows to be more represented in the muscle compared to the other tissues (–)
Table 3. Summary of ANOVA (F-ratio and p value) for fatty acid content (mg/100 g tissue wet weight) and ratios in F. glaber, M. varia, and P. jacobaeus [factor: adductor muscle (A), gonad (G), mantle (M), viscera (V)] and samples means ranked in order of magnitude according to Tukey post hoc test.
Highest proportions (%) of EPA and DHA were observed in adductor muscle of all species (p < 0.05), and this is consistent with available literature data, which report a low lipid content in the adductor muscle compared to the other tissues and a fatty acid profile characterized by a high n-3 PUFA level.[Citation17,Citation35] Telahigue et al.[Citation9] reported, for the same species, lower % values of DHA and EPA than those found in this study, mainly in the adductor muscle of F. glaber (3.64% and 3.29% of total FAs for DHA and EPA vs. 10.4 and 13.8 in this study, respectively).
Humans can synthesize SAFAs and MUFAs but cannot synthesize PUFA n-3 and n-6 fatty acids de novo. This is because humans, like other animals, lack the desaturase enzymes required to produce the simplest members of these families (α-linolenic acid and linoleic acid, respectively).[Citation36] However, DHA and EPA are considered to be among the major beneficial nutrients obtained from seafood consumption. They inhibit inflammatory processes by influencing the eicosanoid metabolism. Furthermore, they influence blood coagulation by reducing platelet adhesion and aggregation and have blood pressure-reducing properties and CVDs.[Citation37] EPA and DHA have also been shown to contribute to the reduction of certain types of cancers, diabetes, mental health disorders, and asthma.[Citation7] DHA, also, is highly abundant in brain and retina where it plays important structural and functional roles. Consequently, DHA status is important to ensure optimum neural and visual functions.[Citation38]
There were also noteworthy amounts of stearidonic acid (18:4n3) in the gonad (39.2–71.6 mg/100 g tissue; 6.7–8.9% of total FAs) and in the viscera (57.8–218.6 mg/100 g tissue; 6.7–10% of total FAs) of the studied species, as also noted by Telahigue et al.[Citation9] Furthermore, small contents of linoleic acid (18:2n-6), α-linolenic acid (18:3n-3), arachidonic acid (ARA; 20:4n-6), and docosapentaenoic acid (22:5n-3) were also detected (). In all species and tissues, more than 80% of PUFA (except for mantle of P. jacobaeus and F. glaber) are n-3 fatty acids. Statistical analysis showed that the sum of n-3 PUFAs was significantly higher in the viscera of all species (217–800 mg/100 g tissue) than in the other tissues (–). High levels of PUFA n-3 are important for the human health and seafood products are the only significant source in the diet. Epidemiological findings of a negative correlation between the intake of n-3 fatty acids and mortality from coronary heart disease (CHD) provide further evidence for the protective effects of n-3 PUFA.[Citation39]
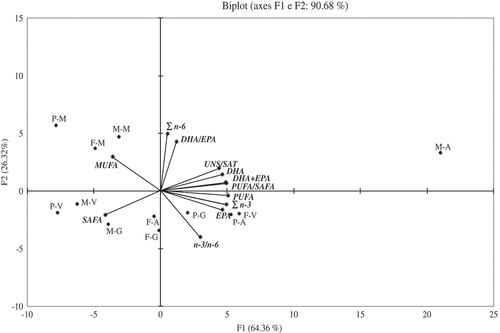
The level of the n-6 series was detected as lower than n-3, with % values ranging from 3.8% in the gonad of M. varia to 7% in the mantle of P. jacobaeus. Within tissues no significant differences were recorded among species (p > 0.05). P. jacobeus exhibited in the gonad a content of n-6 higher than the other species (p < 0.05) (–). All species had the highest n-6 percentage in the mantle (p < 0.05) (). Linoleic (C18: 2n‐6) and ARAs (20: 4n-6) dominated n‐6 PUFA, even though ARA was reported as present in low amount in the gonads of all pectinids species by Caers et al.[Citation17,Citation26] and Telhaigue et al.[Citation9] ARA is associated with membrane phospholipids and can be oxidized to a variety of eicosanoid compounds important in cell–cell signalling.[Citation40] A biplot summarized the PCA results of the most significant fatty acids, expressed as % of total FAs, along the two first principal components and the groupings and/or differences among organs of the three pectinids examined (). The PCA clearly discriminated among the fatty acid composition of tissues and species. The values for fatty acid profiles were determined as 64.36% and 26.32% for PC1 and PC2, respectively. Therefore, the two-axis ordination diagram described 90.68% of the variation. The scatter plot of scores on the first two principal components PC1 and PC2 shows a separation among species and tissues (). The samples were clustered in four zones of the plot. A cluster formed by the viscera of P. jacobaeus, viscera, and gonad of M. varia and adductor muscle of F. glaber grouped for the high SAFAs content. Another distinct cluster from the other species was represented by M. varia, characterized by highest levels of PUFAs, PUFA/SAFA, DHA, DHA + EPA UNS/SAT, n-3, DHA, and EPA in the adductor muscle. A third group located on the left of the top was characterized by the higher values of MUFA in the mantle of the three scallop species. The last group was formed by the gonad and adductor muscle of P. jacobaeus and viscera of F. glaber with a relatively high amount of EPA and n-3/n-6 ratio.
Lipid NQI
In order to evaluate and compare the nutritional quality of the investigated scallops, the amount of the main bioactive fatty acids, DHA + EPA, DHA/EPA, n-3/n-6, and PUFA/SFA ratios were investigated in the TL matter of the scallops’ edible part. DHA + EPA and DHA/EPA are important for nutritional qualities of seafood. In this study, the sum of DHA + EPA significantly varied among the scallops species and among tissues examined (p < 0.05), while, as regards DHA/EPA ratio, significant differences were observed only for the gonad, with highest value (1.80) found in P. jacobaeus (p < 0.05) (). Gogus and Smith[Citation41] reported that high ratio of DHA/EPA has an advantageous impact on consumer health and that DHA is more efficient than EPA in reducing the risk of CHDs. In this study, DHA/EPA ratio was always ≥1 and the DHA + EPA sum was found to be higher in the adductor muscle and mantle than in any other tissue in all species.
In the last 50 years, in developed countries diet patterns have changed, resulting in a higher intake of fat, specifically SAFA and n‐6 PUFA and relatively low in n-3 PUFA. It is widely accepted that a high n-3/n-6 fatty acid ratio is healthful, particularly with regard to reducing the risk of CVD[Citation42] and it can be used as an index for comparing the nutritional values of shellfish. The U.K. Department of Health recommends an ideal relationship of n3/n6 of 4.0 at maximum.[Citation43] In this study, all tissues of all species showed a high n-3/n-6 ratio, always >5, except in the mantle. Gonad and viscera of F. glaber (n-3/n-6 ratio 8 and 7.9, respectively) showed the highest values (p < 0.05), followed by adductor muscle of P. jacobaeus (n-3/n-6 ratio 7.4) and M. varia (n-3/n-6 ratio 7.2 for adductor and 7.1 for gonad) (). These aspects contribute to a positive evaluation of the lipid quality in the scallops examined.
The n-3/n-6 ratio of gonads in F. glaber and P. jacobaeus was higher than the ratio reported by Telhaigue et al.[Citation9] from the Tunisian coast (n-3/n-6 ratio of 8 and 6.8 in this study; c.f. 4.76 and 5.27, respectively), but comparable to that of M. varia (7.72). Caers et al.[Citation17] reported values of 12.1 for adductor muscle of A. purpuratus and male and female gonads (11.3 and 11.9, respectively). In bivalve species such as Chamelea gallina from the central Adriatic Sea (Italy), this ratio varies between 4.28 and 10.8[Citation11], referred to the whole organism.
Another useful factor for assessing the nutritional quality of the lipid fraction of foods is the PUFA/SAFA ratio, considered as a measure of the propensity of the diet to influence the incidence of CHD. A recommended minimum value of this ratio is 0.45 or not less than 0.1.[Citation43] In this study, the PUFA/SAFA ratio in all tissues and in all species was found to be high, with the highest values found in the adductor muscle of M. varia (1.47) and in the viscera of F. glaber (1.08) ( and –).
Ulbricht and Southgate[Citation24] used the AI to measure of the ability of a diet to reduce blood lipid content, a TI as a measure of the ability to reduce platelet activity, and H/H to describe the functional effects of different PUFAs of cholesterol metabolism. Diets with low AI and TI values could reduce the potential risk of CHD. AI values from 0.33 to 2.37 and TI values from 0.01 to 1.18 are reported in the literature for different seafoods.[Citation28] In this study, AI and TI fall within the above-mentioned range, with the lowest values of AI and TI in the adductor muscle of M. varia (0.48 and 0.19, respectively; p < 0.05), while the ‘worst’ in health terms (the highest) AI and TI values were recorded in the viscera of P. jacobaeus (1.91 and 0.52) (p < 0.05) (–). Therefore, the presented AI and TI values () provide beneficial information for human nutrition. As regards the HH fatty acid ratio, higher ratio amounts are desirable. In this study, the highest values were shown in adductor of M. varia (2.58) while lower values in the viscera of P. jacobaeus (0.67). However, all species showed values within those reported in the literature for some other species (0.25–3.23).[Citation44]
Conclusion
This work investigated for the first time the composition and distribution of fatty acids in the different edible portions of three scallop species from Ionian Sea (southern Italy coast) during the autumn months. The results obtained reveal that F. glaber, M. varia, and P. jacobaeus are beneficial nutritionally due to their low levels of lipid and high percentage of healthy PUFAs. Indeed, the pectinids’ tissues contained high levels of n-3 PUFA, especially bioactive fatty acids, such as EPA and DHA. In addition, all three species have low amounts of n-6 PUFA; consequently, the n3/n6 PUFA ratio is high. While analyses of selected tissues showed the three scallop species have different characteristic fatty acids profiles, and all the scallops examined provide the consumers with a satisfying level of n-3 PUFA. M. varia appeared to be the species with highest nutritional quality as evidenced by the lipid quality indices. In general, adductor muscle presented a healthier lipid profile compared to the other tissues and showed good anti-thrombogenic, anti-atherogenic, and hypo-cholesterolemic properties of scallop lipids.
Additional information
Funding
References
- Simopoulos, A. P.;. N-3 Fatty Acids and Human Health: Defining Strategies for Public Policy. Lipids 2001, 36, S83–S89.
- Prato, E.; Biandolino, F. Total Lipid Content and Fatty Acid Composition of Commercially Important Fish Species from the Mediterranean, Mar Grande Sea. Food Chemistry 2012, 131, 1233–1239.
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive Compounds from Marine Mussels and Their Effects on Human Health. Food Chemistry 2014, 142, 48–60.
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 Fatty Acids and Antioxidants in Neurological and Psychiatric Diseases: An Overview. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2007, 31, 12–26.
- Calder, P. C.; Yagoo, P. Marine Omega-3 Fatty Acids and Coronary Heart Disease. Current Opinion in Cardiology 2013, 27, 412–419.
- Gerber, M.;. Omega-3 Fatty Acids and Cancers: A Systematic Update Review of Epidemiological Studies. British Journal of Nutrition 2012, 107, S228–239.
- Alasalvar, C.; Shahidi, F.; Quantick, P. Food and Health Applications of Marine Nutraceucitals: A Review. In Seafoods Quality, Technology and Nutraceutical Applications, Springer, Berlin Heidelberg;; Alasalvar, C., Taylor, T., Eds.; Springer: New York, NY, 2002; pp. 175–204.
- Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Rehbein, H.; Schröder, U. Meat Composition and Quality Assessment of King Scallops (Pecten Maximus) and Frozen Atlantic Sea Scallops (Placopecten Magellanicus) on a Retail Level. Foods 2015, 4, 524–546.
- Telahigue, K.; Chetoui, I.; Rabeh, I.; Romdhane, M. S.; El Cafsi, M. Comparative Fatty Acid Profiles in Edible Parts of Wild Scallops from the Tunisian Coast. Food Chemistry 2010, 122, 744–746.
- Prato, E.; Parlapiano, I.; Biandolino, F.; Gianguzza, P.; Fanelli, G. The Recruitment of Scallop (And Beyond) by Two Different Artificial Collectors (Gulf of Taranto, Mediterranean Sea). Aquaculture Research 2016, 47, 3319–3331.
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Caproni, R.; Santoroni, G.; Giulini, G. Nutritional and Commercial Quality of the Striped Venus Clam, Chamelea Gallina, from the Adriatic Sea. Food Chemistry 2006, 101, 1063–1070.
- Biandolino, F.; Prato, E.; Caroppo, C. Preliminary Investigation on the Phytoplankton Contribution to the Mussel Diet on the Basis of Fatty Acids Analysis. Journal of the Marine Biological Association of the UK 2008, 28(7), 1009–1017.
- Prato, E.; Danieli, A.; Maffia, M.; Biandolino, F. Lipid and Fatty Acid Compositions of Mytilus Galloprovincialis Cultured in the Mar Grande of Taranto (Southern Italy): Feeding Strategies and Trophic Relationships. Zoological Studies 2010, 49(2), 211–219.
- Karnjanapratum, S.; Benjakul, S.; Kishimura, H.; Tsai, Y. Chemical Compositions and Nutritional Value of Asian Hard Clam (Meretrix Lusoria) from the Coast of Andaman Sea. Food Chemistry 2013, 141, 4138–4145.
- Luzia, L. A.; Sampaio, G. R.; Castellucci, C. M. N.; Torres, E. A. F. S. The Influence of Season on the Lipid Profiles of Five Commercially Important Species of Brazilian Fish. Food Chemistry 2003, 83, 93–97.
- Nogueira, N.; Fernandes, I.; Fernandes, T.; Cordeiro, N. A Comparative Analysis of Lipid Content and Fatty Acid Composition in Muscle, Liver and Gonads of Seriola Fasciata Bloch 1793 Based on Gender and Maturation Stage. Journal of Food Composition and Analysis 2017, 59, 68–73.
- Caers, M.; Coutteau, K. C.; Cure, K.; Morales, V.; Gajardo, G.; Sorgeloos, P. The Chilien Scallop Argopecten Pupuratus (Lamarck, 1819): I. Fatty Acid Composition and Lipid Content of Six Organs. Comparative Biochemistry and Physiology 1999, 123(B), 89–96.
- Najdek, M.; Blažina, M.; Ezgeta-Balic, D.; Peharda, M. Diets of Fan Shells (Pinna Nobilis) of Different Sizes: Fatty Acid Profiling of Digestive Gland and Adductor Muscle. Marine Biology 2013, 160, 921–930.
- Turolla, E.;. Atlante Dei Bivalve Dei Mercati Italiani. Adriatica, G., Ed.; Taglio di Po (Ro), Taglio di Po: Italy, 2007; p. 95.
- Dijkstra, H. H.;. Annotations to the Described and Figured Scallops (Mollusca, Bivalvia, Pectinidae) in the German, French and Dutch Editions of Georg Wolfgang Knorr’s “Vergnügen” (1757-1775). Basteria 2010, 74, 3–20.
- Folch, J.; Lees, M.; Stanley, G. H. S. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. Journal of. Biological Chemistry 1957, 226, 497–509.
- Allinger, N. L.; Cava, M. P.; De Jough, D. C.; Johnson, C. R.; Lebel, N. A.; Stevens, C. L. Chimica Organica. Zanichelli: Bologna, 1986; pp. 976pp.
- Greenfield, H.; Southgate, D. A. T. Food Composition Data. Production, Management and Use. FAO: Rome, 2003; pp. 289pp.
- Ulbricht, T. L.; Southgate, D. A. T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992.
- Fernández, M.; Ordóñez, J. A.; Cambero, I.; Santos, C.; Pin, C.; De La Hoz, L. Fatty Acid Compositions of Selected Varieties of Spanish Ham Related to Their Nutritional Implications. Food Chemistry 2007, 101, 107–112.
- Caers, M.; Couteau, P.; Sorgellos, P.; Gajardo, G. Impact of Algal Diets and Emulsions on the Fatty Acid Composition and Content of Selected Tissues of Adult Broodstock of the Chilean Scallop Argopecten Purpuratus (Lamarck, 1819). Aquaculture 2003, 217, 437–452.
- Hu, F. B.; Manson, J. E.; Willett, W. C.Types of Dietary Fat and Risk of Coronary Heart Disease: A Critical Review. Journal of the American College of Nutrition 2001, 2001(20), 5–19.
- Kris-Etherton, P. M.; Yu, S. Individual Fatty Acid Effects on Plasma Lipids and Lipoproteins: Human Studies. American Journal of Clinical Nutrition 1997, 65(5), 1628S–1644S.
- Dietschy, J. M.;. Dietary Fatty Acids and the Regulation of Plasma Low Density Lipoprotein Cholesterol Concentrations. Journal of Nutrition 1998, 128, 444S–448S.
- Yu, S.; Derr, J.; Etherton, T. D.; Kris-Etherton, P. M. Plasma Cholesterol-Predictive Equations Demonstrate that Stearic Acid Is Neutral and Monounsaturated Fatty Acids Are Hypocholesterolemic . American Journal of Clinical Nutrition 1995, 61, 1129–1139.
- Kalogeropoulos, N.; Andrikopoulos, N.; Hassapidou, M. Dietary Evaluation of Mediterranean Fish and Molluscs Pan-Fried in Virgin Olive Oil. Journal of the Science of Food and Agriculture 2004, 84, 1750–1758.
- Mashek, D. G.; Wu, C. MUFA’s. Advavances in Nutrition 2015, 6, 276–277.
- Gardner, C. D.; Kraemer, H. C. Monounsaturated versus Polyunsaturated Dietary Fat and Serum Lipids. A Meta-Analysis. Arteriosclerosis. Thrombosis, and Vascular Biology 1995, 15, 1917–1927.
- Mensink, R. P.; Zock, P. L.; Kester, A. D.; Katan, M. B. Effects of Dietary Fatty Acids and Carbohydrates on the Ratio of Serum Total to HDL Cholesterol and on Serum Lipids and Apolipoproteins: A Meta-Analysis of 60 Controlled Trials. American Journal of Clinical Nutrition 2003, 77(5), 1146–1155.
- Napolitano, G. E.; Ackman, R. G. Fatty Acid Dynamics in Sea Scallops Placopecten Magellanicus (Gmelin, 1791) from Georges Bank, Nova Scotia. Journal of Shellfish Research 1993, 12, 267–277.
- Calder, P. C.;. Omega‐3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? British Journal of Clinical Pharmacology 2013, 75(3), 645–662.
- Candela, C. G.; Lopez, L. M. B.; Kohen, V. L. Importance of a Balanced Omega 6/Omega3 Ratio for the Maintenance of Health, Nutritional Recommendations. Nutricion hospitalaria 2011, 26(2), 323–329.
- Eilander, A.; Hundscheid, D. C.; Osendarp, S. J.; Transler, C.; Zock, P. L. Effects of N-3 Long Chain Polyunsaturated Fatty Acid Supplementation on Visual and Cognitive Development Throughout Childhood: A Review of Human Studies. Prostaglandins. Leukotrienes and Essential Fatty Acids 2007, 76, 189–203.
- De Lorgeril, M.; Salen, P.; Martin, J. L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications after Myocardial Infarction: Final Report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785.
- Wheelan, J.;. Antagonistic Effects of Dietary Arachidonic Acid and N-3 Polyunsaturated Fatty Acids. Journal of Nutrition 1996, 12, 1086S–10.
- Gogus, U.; Smith, C. N-3 Omega Fatty Acids: A Review of Current Knowledge. International Journal Food Science and Technology 2010, 45, 417–436.
- Simopoulos, A. P.;. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Experimental Biology and Medicine 2008, 233, 674–688.
- HMSO, UK. Nutritional Aspects of Cardiovascular Disease (report on health and social subjects). London: HMSO 1994, No. 46
- Testi, S.; Bonaldo, A.; Gatta, P. P.; Badiani, A. Nutritional Traits of Dorsal and Ventral Fillets from Three Farmed Fish Species. Food Chemistry 2006, 98, 104–111.