ABSTRACT
The phytochemical composition and antioxidant activity of fruits are associated with their health benefits. Longan is a traditional Chinese edible and medicinal fruit, but its varietal differences in phytochemical profiles and antioxidant activity were unknown. Twenty-four longan cultivars from southern China were investigated in this study. Their total phenolic and flavonoid contents in pulp were 22.09–132.47 mg gallic acid equivalents/100 g and 2.48–14.26 mg catechin equivalents/100 g, respectively. The mean contributions of free phenolics and flavonoids to the total were 82.5% and 94.0%, respectively. Among 10 individual phenolics detected by HPLC, the contents of epicatechin, 4-methylcatechol, chlorogenic, and vanillic acids were relatively higher. The cellular antioxidant activity (CAA) of longan pulp showed significant varietal discrepancies and positive correlation with phenolic and flavonoid contents. Phenolics in longan pulp of 24 cultivars showed lower CAA potential than many other fruits with the CAA qualities from 0.35 to 1.21 µmol quercetin equivalent/100 µmol total phenolics. Longan cultivars were classified into six groups by hierarchical clustering analysis. Six cultivars in group 4 and 6 had higher phytochemical contents and CAA activity.
Introduction
Regular consumption of fruits and vegetables plays an important role in maintaining good health due to their possible effects on reducing the risk of many chronic diseases. These health benefits are partly attributed to their unique phytochemical constituents. Polyphenols are the most extensively investigated compounds that constitute the major portion of antioxidants in the human diet. The antioxidant and anti-inflammatory activity of dietary polyphenols accounted for many health benefits of fresh fruits and vegetables.[Citation1]
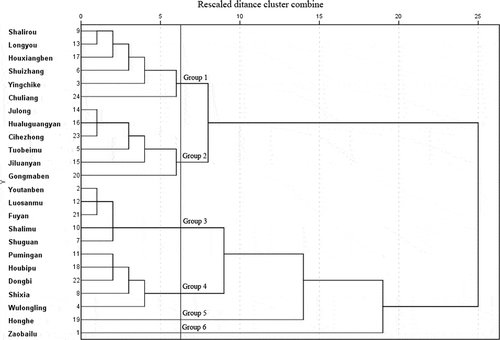
Figure 1. Hierarchical clustering analysis dendrogram of 24 longan cultivars based on their phenolic profiles and cellular antioxidant activity.
Longan (Dimocarpus longan Lour.) is a subtropical fruit, grown commercially in the southern part of China and some southeastern Asian countries. China contributes 60% to the total production of longan in the world.[Citation2] Longan has gained popularity worldwide due to its delicious flavor and health effects.[Citation3] Longan pulp has been traditionally employed in Chinese medicine to cure insomnia, neural pain, and swelling, promoting blood metabolism.[Citation4] Extracts from longan fruits, pericarps, and seeds have shown excellent antioxidant and anticancer activities.[Citation5–Citation7]
Previous research has shown that the pericarp and seeds of longan are abundant in phenolics and have excellent antioxidant activity, whereas the longan pulp has been rarely investigated.[Citation6,Citation8] Three compounds, gallic acid, corilagin, and ellagic acid, were identified in fresh longan pulp, but showed very low content in nine commercially cultivated cultivars of Thailand.[Citation9] Being a largest longan producing country, China has the richest longan germplasm resources in the world. However, little is known about the varietal differences in phytochemical profiles and biological activity of Chinese longan. He et al.[Citation6] analyzed the phenolic content and antioxidant activity of 12 Chinese longan cultivars based on solvent extractable phenolics, but individual phenolic profiles in each longan cultivar were still unknown. Furthermore, bound phenolics, which are covalently conjugated to cell wall macromolecules through ester bounds, were also not involved. Bound phenolics were reported to averagely contribute approximately 24% to the total phenolics in commonly consumed fruits.[Citation10] Therefore, the phenolic content and antioxidant capacity of fruits would be underestimated if bound fractions were not included.
Furthermore, to our knowledge, the antioxidant activity of longan pulp was investigated in very few studies and only chemical assays were used in the analysis, including ferric reducing antioxidant power (FRAP)[Citation6] and trolox equivalent antioxidant capacity (TEAC).[Citation11] Recently, the “test-tube” chemical methods for antioxidant assay have been questioned due to their inability to predict the in vivo activity.[Citation12] In contrast, the cellular antioxidant activity (CAA) based on cell culture represents biologically more relevant estimates of antioxidant activity,[Citation13] and hence could enhance our understanding regarding the biological activity of longan fruits.
Therefore, to get a complete overview of the phenolic profiles and antioxidant activity of Chinese longan germplasm resources, 24 representative longan cultivars distributed in several provinces of China were collected and analyzed in the present study. The objectives of this study is (1) to assess the free and bound phenolic profiles and cellular antioxidant activity of longan pulp of different Chinese longan cultivars; (2) to investigate the correlation among phenolic contents and antioxidant activity in longan pulp.
Materials and methods
Chemicals and reagents
Trolox, 2′,7′-dichlorofluorescin diacetate (DCFH-DA), and 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPH), gallic acid, chlorogenic acid, catechin, caffeic acid, epicatechin, rutin, and Folin–Ciocalteu’s phenol reagent were purchased from Sigma (Sigma Chemical Co., Saint Louis, MO, USA). Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum were from HyClone (Logan, UT, USA), Hank’s Buffered Salt Solution (HBSS), HPLC-grade acetic acid, and acetonitrile were obtained from Fisher Scientific (Waltham, MA, USA). All other chemicals used were of analytical grade.
Extraction of free phenolics
Longan fruits of different cultivars were obtained in commercial maturation season from the Institute of Fruit Tree Research, Guangdong Academy of Agricultural Sciences from July to September 2013. Mature longan fruits, free from visible blemish or disease, were selected. Free phenolics were extracted as stated previously[Citation14] with slight modification. Briefly, 100 g of fresh longan pulp was homogenized with pre-cooled 80% aqueous acetone (1:2, w/v) in a Philips blender for 5 min and then further homogenized for additional 5 min in an ice bath using an XHF-D homogenizer (Ningbo Xin-zhi-Bio Technology Co., Ltd. Ningbo, China). The homogenates were centrifuged at 3000 g for 10 min at 4 °C. The residue was extracted again in the same manner. The combined supernatants were concentrated under vacuum at 45 °C until approximately 90% of the supernatants had been evaporated. The concentrates were then recovered with distilled water to 50 mL and stored at −80 °C until analysis.
Extraction of bound phenolics
Bound phenolics were extracted as reported earlier.[Citation15] Briefly, the residue from free phenolic extraction was hydrolyzed with 20 mL of 4 mol/L NaOH at room temperature for 1 h with continuous shaking under nitrogen gas. The mixture was then neutralized with concentrated hydrochloric acid and extracted six times with ethyl acetate. The ethyl acetate fractions were pooled together and concentrated under vacuum till dryness at 45 °C. The bound phenolics obtained were reconstituted with 10 mL distilled water and stored at −80 °C for analysis.
Determination of the total phenolic contents
The contents of the total phenolics were determined by the Folin–Ciocalteu (FC) method.[Citation15] Briefly, 125 μL of appropriately diluted extract was mixed with 0.5 mL of distilled water and subsequently with 125 μL of FC reagent. After 6 min, 1.25 mL of 7% aqueous sodium carbonate solution was added into the mixture. The total volume was adjusted to 3 mL with distilled water. After 90 min in the dark, the absorbance was read at 760 nm using a Shimadzu UV-1800 spectrometer (Shimadzu Inc., Tokyo, Japan). Gallic acid was used as standard, and the total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per 100 g of fresh weight (FW).
Determination of the total flavonoid contents
The total flavonoid contents of the longan extracts were determined using a modified colorimetric method.[Citation13] A 250 μL aliquot of appropriately diluted extract was mixed with 1.25 mL of distilled water and subsequently 75 μL of 5% NaNO2 solution. After 6 min, 150 μL of 10% AlCl3 · 6H2O solution was added and allowed to stand for 5 min before the addition of 0.5 mL of 1 mol/L NaOH solution. The final volume was marked to 2.5 mL with distilled water and absorbance was measured immediately at 510 nm using a Shimadzu UV-1800 spectrometer. The total flavonoid contents were expressed as milligrams of catechin equivalents (CE) per 100 g of FW.
Determination of phenolic compositions
Phenolic compositions were determined using an Agilent 1260 HPLC system with a DAD detector. The chromatographic conditions were set according to the previous procedure.[Citation16] The samples were eluted using 0.4% aqueous solution of acetic acid (solution A) and acetonitrile (solution B) as the mobile phase with a gradient flow as follows: 0–40 min, solution B 5–25%; 40–45 min, solution B 25–35%; and 45–50 min, solution B 35–50%. The flow rate was kept at 1.0 mL/min and the injection volume was 20 μL. All the phenolic compounds were detected at 280 nm except quercetin at 350 nm. All the samples were filtered through a 0.22 μm membrane filter (Millipore, MA, USA) before sampling. Identification of each peak was primarily based on comparison of their retention times with the known authentic standards. The percent recovery of all the phenolics was 92–112%.
Determination of CAA
The CAA assay was conducted as suggested by Wolfe & Liu.[Citation17] Briefly, 6 × 104 HepG2 cells suspended in a 100 μL DMEM medium supplemented with 10% fetal bovine serum were seeded in each well of a black 96-well microplate (Corning) and maintained at 37 °C for 24 h. After being rinsed with PBS once, cells in each well were treated with 100 μL DMEM containing different concentrations of quercetin or phenolic extracts together with DCFH-DA at a final concentration of 25 μmol/L for 1 h. For the blank and positive control wells, only 25 μmol/L of DCFH-DA was added. After removing the treatment medium from each well, 100 μL of AAPH (600 μmol/L) dissolved in HBSS was immediately added to each well except blank wells, where 100 μL of HBSS without AAPH was added. The fluorescence in each well was recorded with excitation at 485 nm and emission at 538 nm using an Infinite® M200 PRO plate reader at 37 °C every 5 min for 1 h. The results were expressed as micromoles of quercetin equivalents (µmol QE) per 100 g FW of longan pulp. Each treatment in the same plate was made in triplicates and the CAA value of each sample was obtained from three separate experiments.
Statistical analyses
Data were presented as mean ± standard deviation (SD) from three replicate determinations of each sample. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by the SNK-q test for different longan cultivars using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). Significant differences were considered at p < 0.05. A correlation between variables was determined by conducting the Pearson correlation test. Hierarchical clustering analysis was performed using Ward’s linkage analysis to classify the 24 longan cultivars based on their phenolic profiles and CAA activity.
Results and discussion
Total phenolic and flavonoid content
The free and bound phenolic contents of longan pulp and the percentage contribution of each fraction to the total of different cultivars are given in . Their free and bound phenolic contents ranged from 16.89 (Shuizhang) to 109.17 (Zaobailu) mg GAE/100 g, and from 3.67 (Yingchike) to 25.14 (Dongbi) mg GAE/100 g, respectively. The contribution of the free phenolic fraction to the total was from 76.45% (Shuizhang) to 86.93% (Yingchike). The total phenolic contents were in the range of 22.09–132.47 mg GAE/100 g with the average value of 75.5 mg GAE/100g. The coefficient of variation (CV) was 43.7% for the total phenolic contents in longan varieties. Zaobailu had the highest total phenolic content followed by Wulongling and Houbipu, while Shuizhang and Hualuguangyan had the lowest total phenolic content.
Table 1. Total phenolic contents of 24 longan cultivars and percentage contribution of free and bound fractions to the total.
The free and bound flavonoid contents of longan pulp and the percentage contribution of each fraction to the total in different cultivars are presented in . Free flavonoid contents of 24 longan cultivars ranged from 2.26 (Yingchike) to 13.40 (Zaobailu) mg CE/100 g. The percentage contribution of free flavonoids to the total ranged from 89.86% (Honghe) to 96.26% (Jiluanyan). Bound flavonoid contents ranged from 0.14 (Jiluanyan) to 0.86 (Zaobailu) mg CE/100 g. Zaobailu (14.26 mg CE/100 g) and Yingchike (2.48 mg CE/100 g) had the highest and lowest total flavonoid contents, respectively.
Table 2. Total flavonoid contents of 24 longan cultivars and percentage contribution of free and bound fractions to the total.
Fu et al.[Citation11] previously reported that longan contained 80.32 mg GAE/100 g FW of the total soluble phenolics, which is equivalent to the average total phenolics of 24 longan cultivars in the present study (75.53 mg GAE/100g). The total phenolic and flavonoid contents of selected tropical fruits in Malaysia were from 24.4 to 191.0 mg GAE/100g and from 1.24 to 44.6 mg CE/100g, respectively.[Citation18] Some tropical fruits in French island including pineapple, papaya, mango, banana, litchi, and passion fruit were reported to contain 33–286.6 mg GAE/100 g of phenolics and 1.5–70.1 mg CE/100 g of flavonoids.[Citation19] The longan cultivars Zaobailu, Youtanben, Wulongling, Luosanmu, Houbipu, and Gongmaben had higher total phenolic and flavonoid contents than the aforementioned fruits except banana, litchi, and passion fruits. These results indicate that longan is a tropical fruit with a moderate level of phenolic contents.
Our results showed that phenolics in longan pulp mainly exist in free form. The average percentage contribution of bound to total phenolics was 17.5%, which is comparable to previous reports on litchi (21.2%)[Citation16] and other commonly consumed fruits (24%),[Citation10] but quite higher than the reported average contribution of eight strawberry cultivars (5%).[Citation20] However, only 5.9% of flavonoids were bound form in determined longan cultivars on average, which is much lower than that of litchi (26.9%).[Citation16]
Phenolic compositions
Ten phenolic compounds were detected in the free and bound fractions of longan pulp, which were gallic acid, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, epicatechin, 4-methylcatechol, ferulic acid, coumarin, and quercetin. The content of these 10 compounds in 24 longan cultivars and percentage contribution of free and bound form to the total are summarized in . Gallic and ferulic acids were found only in the free form, while chlorogenic acid and coumarin existed only in the bound form. The other six compounds were detected in free and bound forms.
Table 3. Phenolic compositions of 24 longan cultivars (μg/100 g).
The content of epicatechin ranged from 146.41 to 1819.57 µg/100 g and 17.36 to 166.58 µg/100 g in free and bound fractions, respectively. Zaobailu contained the most total epicatechin followed by Wulongling, Shixia, and Houbipu. Shuguan contained the least total epicatechin. The contribution of the free fraction to the total epicatechin was from 80.0 to 97.6%. Epicatechin was the most abundant phenolic compound detected in longan pulp with an average content of 577.51 µg/100g. Shi et al.[Citation21] previously found it in the longan pericarp of cultivar Shixia (0.15–0. 26 mg/g FW) and Chuliang (0.09–0.56 mg/g FW). Epicatechin is present in high concentrations in many plant foods, especially in fruits. Many healthy effects of epicatechin have been reported previously, including improving insulin sensitivity,[Citation22] anti-allergy,[Citation23] retarding Alzheimer’s disease,[Citation24] etc. Epicatechin was detected in all 15 determined Bulgarian fruits,[Citation25] except melon. The content of epicatechin in these fruits was from less than 0.3 mg/kg in fig to 87 mg/kg in black grape. In this study, Houbipu, Shixia, Wulongling, and Zaobailu even contained more epicatechin than black grape, and could be considered good sources of epicatechin.
The second abundant phenolic compound in longan pulp was 4-methylcatechol followed by chlorogenic acid, vanillic acid, and gallic acid with an average content of 160.10, 149.19, 143.38, and 131.63 µg/100 g, respectively. Nineteen out of 24 longan cultivars contained 4-methylcatechol with the total content ranging from 14.35 (Honghe) to 561.12 µg/100 g (Gongmaben). The contents of gallic acid ranged from 27.90 µg/100 g in Honghe to 230.30 µg/100 g in Zaobailu. Gongmaben, Luosanmu, Shuguan, and chuliang had more gallic acid than other cultivars except Zaobailu. Chlorogenic acid contents were from 27.99 to 492.79 µg/100 g. Chuliang had the highest chlorogenic acid content followed by Shixia, Shalirou, and Tuobeimu, and the latter two cultivars were similar (p > 0.05). The total contents of vanillic acid ranged from 28.98 to 411.97 µg/100 g, with the contribution of the free fraction to the total from 16.4 to 94.6%. Zaobailu contained the most vanillic acid followed by Dongbi and Gongmaben. Besides epicatechin, quercetin was the only another flavonoid compound detected from longan pulp in this study. It was found in 17 longan cultivars. The free and bound contents of quercetin ranged from 2.93 to 219.40 µg/100 g and 6.24 to 46.11 µg/100 g, respectively. Zaobailu had the most total quercetin (219.40 µg/100 g) followed by Youtanben (76.34 µg/100 g).
Cellular antioxidant activity
The CAA activity and antioxidant quality of the pulp of 24 longan cultivars are presented in . The CAA values ranged from 0.49 to 6.71 µmol QE/100 g with a mean value of 2.76 µmol QE/100 g. Wulongling and Zaobailu showed the first and second highest CAA values, respectively, followed by Shixia, Houbipu, Dongbi, and Honghe. The CAA values of the last four cultivars were not significantly different (p > 0.05). Shuizhang, Yingchike, and Cihezhong had lower CAA values than any other cultivars. The CAA assay has now been widely used in a great number of studies to estimate the antioxidants of fruits,[Citation26] vegetables,[Citation27] grains,[Citation28] and medicinal plants.[Citation29] However, to our knowledge, this study firstly reported the CAA activity of longan pulp. The CAA activity of longan is much lower than that of many previously reported fruits including berries (47.9–292 µmol QE/100g), apple (34.4 µmol QE/100g), pineapple (49.8 µmol QE/100g), etc. (Wolfe et al. 2008). Wulongling, Zaobailu, Shixia, and Houbipu showed the highest CAA values, which are equivalent to those of nectarine, honeydew, avocado, cantaloupe, and banana (3.15–6.91 µmol QE/100g).[Citation30]
Table 4. Cellular antioxidant activity (CAA) and antioxidant quality of 24 longan cultivars.
The CAA values showed a significant correlation with the free phenolics (r = 0.828, p < 0.001) and flavonoid (r = 0.693, p < 0.001) contents of longan cultivars, suggesting that phenolics and flavonoids are the main contributors to the antioxidant capacity of longan fruits. We also analyzed the correlation between the individual phenolic compound content and CAA activity. Out of 10 phenolic compounds, only epicatechin showed a positive correlation with the CAA activity (r = 0.703, p < 0.001), indicating that epicatechin may be the main component contributing to the CAA activity of longan pulp.
To compare the antioxidant potential of phytochemicals in various longan cultivars, the CAA values per 100 μmol of phenolics present in 24 cultivars were calculated as CAA qualities. The CAA qualities were in the range of 0.35–1.21 µmol QE/100 µmol total phenolics. The cultivars Houxiangben, Tuobeimu, Wulongling, and Longyou showed the highest antioxidant qualities while Shuguan, Youtanben, Yingchike, and Cihezhong presented the lowest antioxidant qualities.
The CAA qualities of longan cultivars in the present study are much lower than that in pomegranate (12.6 µmol QE/100 µmol total phenolics), berries (7.7–11.6 µmol QE/100 µmol total phenolics), and many other commonly consumed fruits.[Citation30] Only Longyou, Wulongling, Tuobeimu, and Houxiangben had antioxidant qualities higher than 1.0 µmol QE/100 µmol total phenolics, which are equivalent to those of pear and banana.[Citation30] The CAA activity of different phenolic compounds exhibited big differences.[Citation17] The most abundant phenolic compound detected from longan pulp is epicatechin in the present study, which was reported to show much lower CAA activity in previous research.[Citation17] The lower cellular antioxidant potential of phenolics in longan cultivars may be the main reason for their weak CAA activity.
Hierarchical clustering analysis of longan cultivars
The dendrogram obtained from Ward’s minimum-variance method based on the phenolic profiles and CAA activity of longan cultivars is shown in Fig. 1. The 24 Chinese longan cultivars were clustered into six groups. Group 1 and 2 both grouped six cultivars. These two groups were similar to each other but had significantly lower total phytochemical contents and CAA activity than other groups. Groups 3 and 4 both contained five cultivars. Youtanben, Luosanmu Fuyan, Shalimu, and Shuguan constituted group 3 and Pumingan, Houbipu, Dongbi, Shixia, and Wulongling constituted group 4. These two groups had equivalent total phenolic contents, but group 4 showed much higher CAA activity than group 3 (4.61 vs 2.45 µmol QE/100 g). Furthermore, they had different phenolic profiles, for example epicatechin (315.48 vs. 865.43 µg/100 g in groups 3 and 4, respectively). Zaobailu was solely grouped into group 6 due to its higher phenolic content and antioxidant activity than any other cultivars.
These results may provide helpful information on fresh consumption and deep processing of longan. Since phenolics are the main components resulting in a browning reaction of longan products, the cultivars with a lower phenolic content would be more suitable for deep processing. In contrast, consumers prefer to choose longan cultivars having more phytochemicals and higher antioxidant activity for fresh consumption considering their health benefits.
In summary, the phytochemical content and antioxidant activity of pulp from 24 longan cultivars had significant varietal differences. Phenolics and flavonoids of longan pulp existed mainly in free form. HPLC analysis revealed 10 individual phenolic compounds in longan pulp with a relatively higher content of epicatechin, 4-methylcatechol, chlorogenic acid, and vanillic acid. Longan pulp contained higher phenolic and flavonoid contents than some of the commonly consumed fruits, which were highly correlated with their antioxidant activities. As a dietary ingredient, longan may not provide high CAA activity due to the lower CAA potential of their main phenolic components.
Additional information
Funding
References
- Siriwardhana, N.; Kalupahana, N. S.; Cekanova, M.; Lemieux, M.; Greer, B.; Moustaidmoussa, N. Modulation of Adipose Tissue Inflammation by Bioactive Food Compounds. Journal of Nutritional Biochemistry 2013, 24, 613–623. DOI: 10.1016/j.jnutbio.2012.12.013.
- Wu, Y.-L.; Yi, G.-J.; Zhou, B.-R.; Zeng, J.; Huang, Y.-H. The Advancement of Research on Litchi and Longan Germplasm Resources in China. Scientia Horticulturae 2007, 114, 143–150. DOI: 10.1016/j.scienta.2007.07.016.
- Yang, B.; Jiang, Y.; Shi, J.; Chen, F.; Ashraf, M. Extraction and Pharmacological Properties of Bioactive Compounds from Longan (Dimocarpus Longan Lour.) Fruit - A Review. Food Research International 2011, 44, 1837–1842.
- Li, L.; Xu, J.; Mu, Y.; Han, L.; Liu, R.; Cai, Y.; Huang, X. Chemical Characterization and Anti-Hyperglycaemic Effects of Polyphenol Enriched Longan (Dimocarpus Longan Lour.) Pericarp Extracts. Journal of Functional Foods 2015, 13, 314–322. DOI: 10.1016/j.jff.2015.01.006.
- Chung, Y.-C.; Lin, -C.-C.; Chou, -C.-C.; Hsu, C.-P. The Effect of Longan Seed Polyphenols on Colorectal Carcinoma Cells. European Journal of Clinical Investigation 2010, 40, 713–721. DOI: 10.1111/eci.2010.40.issue-8.
- He, N.; Wang, Z.; Yang, C.; Lu, Y.; Sun, D.; Wang, Y.; Shao, W.; Li, Q. Isolation and Identification of Polyphenolic Compounds in Longan Pericarp. Separation And Purification Technology 2009, 70, 219–224. DOI: 10.1016/j.seppur.2009.09.019.
- Park, S.-J.; Dong, H.-P.; Dong, H.-K.; Lee, S.; Yoon, B.-H.; Jung, W.-Y.; Lee, K.-T.; Cheong, J.-H.; Ryu, J.-H. The Memory-Enhancing Effects of Euphoria Longan Fruit Extract in Mice. Journal of Ethnopharmacology 2010, 128, 160–165. DOI: 10.1016/j.jep.2010.01.001.
- Chen, J.-Y.; Xu, Y.-J.; Ge, -Z.-Z.; Zhu, W.; Xu, Z.; Li, C.-M. Structural Elucidation and Antioxidant Activity Evaluation of Key Phenolic Compounds Isolated from Longan (Dimocarpus Longan Lour.) Seeds. Journal of Functional Foods 2015, 17, 872–880. DOI: 10.1016/j.jff.2015.06.028.
- Rangkadilok, N.; Worasuttayangkurn, L.; Bennett, R. N.; Satayavivad, J. Identification and Quantification of Polyphenolic Compounds in Longan (Euphoria Longana Lam.) Fruit. Journal of Agricultural and Food Chemistry 2005, 53, 1387–1392. DOI: 10.1021/jf0403484.
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.-H. Antioxidant and Antiproliferative Activities of Common Fruits. Journal of Agricultural and Food Chemistry 2002, 50, 7449–7454. DOI: 10.1021/jf0207530.
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chemistry 2011, 129, 345–350. DOI: 10.1016/j.foodchem.2011.04.079.
- Schaich, K.-M.; Tian, X.; Xie, J. Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays. Journal of Functional Foods 2015, 14, 111–125. DOI: 10.1016/j.jff.2015.01.043.
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the Free and Bound Phenolic Profiles and Cellular Antioxidant Activities of Litchi Pulp Extracts from Different Solvents. BMC Complementary and Alternative Medicine 2014, 14, 1–10. DOI: 10.1186/1472-6882-14-9.
- Wolfe, K.; Wu, X.; Liu, R. H. Antioxidant Activity of Apple Peels. Journal of Agricultural and Food Chemistry 2003, 51, 609–614. DOI: 10.1021/jf020782a.
- Dewanto, V.; Wu, X.; Liu, R.-H. Processed Sweet Corn Has Higher Antioxidant Activity. Journal of Agricultural and Food Chemistry 2002, 50, 4959–4964. DOI: 10.1021/jf0255937.
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic Profiles and Antioxidant Activity of Litchi Pulp of Different Cultivars Cultivated in Southern China. Food Chemistry 2013, 136, 1169–1176. DOI: 10.1016/j.foodchem.2012.09.085.
- Wolfe, K.-L.; Liu, R.-H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. Journal of Agricultural and Food Chemistry 2007, 55, 8896–8907. DOI: 10.1021/jf0715166.
- Alothman, M.; Rajeev, B.; Karim, -A.-A. Antioxidant Capacity and Phenolic Content of Selected Tropical Fruits from Malaysia, Extracted with Different Solvents. Food Chemistry 2009, 115, 785–788. DOI: 10.1016/j.foodchem.2008.12.005.
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.-P. Evaluation of Nutritional and Antioxidant Properties of the Tropical Fruits Banana, Litchi, Mango, Papaya, Passion Fruit and Pineapple Cultivated in Réunion French Island. Food Chemistry 2016, 212, 225–233. DOI: 10.1016/j.foodchem.2016.05.147.
- Meyers, K.-J.; Watkins, C.-B.; Pritts, M.-P.; Liu, R.-H. Antioxidant and Antiproliferative Activities of Strawberries. Journal of Agricultural and Food Chemistry 2003, 51, 6887–6892. DOI: 10.1021/jf034506n.
- Shi, J.; Sun, J.; Wei, X.; Shi, J.; Cheng, G.; Zhao, M.; Wang, J.; Yang, B.; Jiang, Y. Identification of (−)-Epicatechin as the Direct Substrate for Polyphenol Oxidase from Longan Fruit Pericarp. LWT - Food Science and Technology 2008, 41, 1742–1747.
- Cremonini, E.; Bettaieb, A.; Haj, F.-G.; Fraga, C.-G.; Oteiza, P.-I. (-)-Epicatechin Improves Insulin Sensitivity in High Fat Diet-Fed Mice. Archives of Biochemistry and Biophysics 2016, 599, 13–21. DOI: 10.1016/j.abb.2016.03.006.
- Singh, A.; Demont, A.; Actisgoretta, L.; Holvoet, S.; Lévêques, A.; Lepage, M.; Nutten, S.; Mercenier, A. Identification of Epicatechin as One of the Key Bioactive Constituents of Polyphenol-Enriched Extracts that Demonstrate an Anti-Allergic Effect in a Murine Model of Food Allergy. British Journal of Nutrition 2014, 112, 358–368. DOI: 10.1017/S0007114514000877.
- Cox C-J, Fahd C, Eleanor P, Perkinton M-S, Richardson J-C, Howlett D-R, Lichtenthaler S-F, Francis P-T, Williams R-J. Dietary (-)-Epicatechin as a Potent Inhibitor of βγ-secretase Amyloid Precursor Protein Processing. Neurobiology of Aging 2015, 36, 178–187. DOI: 10.1016/j.neurobiolaging.2014.07.032.
- Tsanovasavova, S.; Ribarova, F.; Gerova, M. (+)-Catechin and (-)-Epicatechin in Bulgarian Fruits. Journal of Food Composition and Analysis 2005, 18, 691–698. DOI: 10.1016/j.jfca.2004.06.008.
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.-H. Comparison of Phytochemical Profiles, Antioxidant and Cellular Antioxidant Activities of Different Varieties of Blueberry (Vaccinium Spp.). Food Chemistry 2017, 217, 773–781. DOI: 10.1016/j.foodchem.2016.09.002.
- Murador, D.-C.; Mercadante, A.-Z.; Rosso, -V.-V.-D. Cooking Techniques Improve the Levels of Bioactive Compounds and Antioxidant Activity in Kale and Red Cabbage. Food Chemistry 2016, 196, 1101–1107. DOI: 10.1016/j.foodchem.2015.10.037.
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Phenolic Contents, Cellular Antioxidant Activity and Antiproliferative Capacity of Different Varieties of Oats. Food Chemistry 2018, 239, 260–267. DOI: 10.1016/j.foodchem.2017.06.104.
- Mcdowell, A.; Thompson, S.; Stark, M.; Ou, Z.-Q.; Gould, K.-S. Antioxidant Activity of Puha (Sonchus Oleraceus L.) As Assessed by the Cellular Antioxidant Activity (CAA) Assay. Phytotherapy Research 2011, 25, 1876–1882. DOI: 10.1002/ptr.3648.
- Wolfe, K. L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R. H. Cellular Antioxidant Activity of Common Fruits. Journal of Agricultural and Food Chemistry 2008, 56, 8418–8426. DOI: 10.1021/jf801381y.
