?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The findings of this study suggests that chemical composition, essential oil yield, antioxidant and antimicrobial activity of Boswellia serrata oleo gum resin essential oils extracted by hydro distillation, steam distillation and supercritical fluid carbon dioxide methods vary greatly from each other. The optimum essential oil yield was obtained using hydro distillation method (8.18 ± 0.15 %). The essential oils isolated through different extraction methods contained remarkable amounts of total phenolics and total flavonoids. Essential oil isolated through supercritical fluid carbon dioxide extraction exhibited better antioxidant activity with highest free radical scavenging potential (96.16 ± 1.57 %), inhibition of linoleic acid oxidation (94.18 ± 1.47 %) and hydrogen peroxide free radical scavenging potential (68.25 ± 1.02 %). Moreover, the antimicrobial activity of essential oils was performed through well diffusion, resazurin microtiter plate and micro dilution broth assay assays. The essential oil isolated through steam distillation method revealed highest antimicrobial activity with maximum inhibition zone (24.21 ± 0.34 to12.08 ± 0.30 mm) and least MIC values (35.18 ± 0.77 to 281.46 ± 7.03 µg/mL). The comparison of chemical composition of essential oils isolated at different extraction methods have shown that the concentration of α-thujene, camphene, β-pinene, myrcene, limonene, m-cymene and cis-verbenol was higher in steam distilled essential oil as compared to hydro and supercritical fluid carbon dioxide extracted essential oils. These compounds may be responsible for the higher antimicrobial activity of Boswellia serrata oleo gum resin steam distilled essential oil.
Introduction
Boswellia serrata Roxb. commonly known as Indian frankincense tree, belongs to Burseraceae family, distributed in dry regions of India, Nigeria, Yemen, Somalia, Arabia, Oman, and Pakistan.[Citation1–Citation3] Among 43 different species of Boswellia, Boswellia serrata is a commercially important plant due to its highly valuable oleo gum resin essential oil. Boswellia serrata essential oil is used in food, flavor, and perfume industries.[Citation4] Moreover, it has biological activities such as anticarcinogenic, antimicrobial, psychopharmacological, antiulcer, antioxidant, and anticancer.[Citation3,Citation5–Citation7] Reactive nitrogen and oxygen species are produced in human body that is associated with various health problems such as ageing, arteriosclerosis, rheumatoid arthritis, cirrhosis, and cancer.[Citation8,Citation9] Antioxidants are the first line of defense against these reactive nitrogen and oxygen species to maintain the optimum health conditions.[Citation10] Moreover, lipid peroxidation in fat and food products cause chemical spoilage and rancid of the flavor. Lipid peroxidation also decreases fat and food products nutritional and sensory quality.[Citation11] A wide range of synthetic antioxidant compounds have been used as preserver in food products to over lipid peroxidation reactions. Similarly, food-borne diseases are one of most emergent public health concern around the world, caused by consumption of microbial contaminated food products.[Citation12,Citation13] The existence and growth of microbes on food products lead to spoilage and formation of toxins.[Citation14] An increasing concern about use of synthetic antioxidants and food additives has increased researchers interest in finding out new food additives and antioxidants from natural sources.[Citation15]
Essential oils are secondary metabolites of aromatic plants and well known to have antioxidant, antibacterial, antiviral, antifungal, and insecticidal activities.[Citation16,Citation17] Hydro-distillation and steam distillation are the conventional extraction methods used for isolation of essential oil from plant materials that are used in aromatherapy, paints, beauty products, and perfume industries.[Citation4,Citation18] These conventional extraction methods have few drawbacks such as potential degradation of labile compounds, large fuel consumption, and long extraction periods.[Citation19–Citation21] Supercritical fluid extraction is an interesting alternative technique as compared to these conventional extraction methods with advantages like lower working temperatures, environmental friendly, and lower solvent consumptions. Wide ranges of supercritical fluids are available but carbon dioxide is extensively used due to its comparatively lower critical temperature and critical pressure. CO2 as a supercritical fluid give better extraction for nonpolar and moderately polar compounds. Polar organic solvents can be added as modifiers for the polar compounds extraction.[Citation22] It has been previously reported that the extraction methods may affect the essential oil yield, chemical composition, and biological activities of essential oils.[Citation23–Citation25] The extensive literature review confirmed that there was no report published on comparison of extraction methods and biological activity of Boswellia serrata oleo gum resin essential oils. Therefore, the present study was undertaken to evaluate the effect of extraction methods on the essential oil yield, chemical composition, and biological activity of Boswellia serrata oleo gum resin essential oil.
Materials and methods
Collection of plant materials
Boswellia serrata oleo gum resin was collected from wild habitat of Zhob district of Balochistan, Pakistan, in May 2016. The plant sample was identified and authenticated by a Taxonomist, Dr. Mansoor Hameed, Associate Professor, Department of Botany, University of Agriculture, Faisalabad, Pakistan.
Isolation of essential oil
Hydro- and steam distillation methods
The air-dried and finely grounded (80 mesh) oleo gum resin (300 g) was subjected to hydro- and steam distillations for 3 h separately. Distillates of essential oils were dried over anhydrous sodium sulfate, filtered, and stored at +4°C until further analysis. Essential oil extraction process was repeated five times for enhanced reproducibility in results.
Supercritical fluid CO2 extraction
Extraction of essential oil from Boswellia serrata oleo gum resin was carried out at Rose Lab, Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan. Liquid carbon dioxide chilled at −10°C was charged into extraction vessel (already maintained at 45 ± 1°C) using a high-pressure pump. The pressure was controlled to an accuracy of about 1% over the measuring range. The extraction vessel was packed with raw materials and glass beads to facilitate easy effusion of supercritical CO2 through it. The supercritical CO2 with dissolved compounds passed through a heated micrometer valve, and was subsequently expanded to ambient pressure. The extract was precipitated in a collection vessel at ambient pressure and temperature. A calibrated wet-test meter at known temperature and pressure measured the total amount of CO2. For each extraction test, the extractor was charged with about 500 g of oleoresin. CO2 flow rate was 2 L/min. The oil weight was measured by precision analytical balance.[Citation26]
Antioxidant activity
Total phenolic contents
To 1.0 mL of each essential oil isolated at different extraction temperature, 5 mL of Folin–Ciocalteu and 4 mL of sodium carbonate (7%, w/v) were added and samples were shaken to mix the components completely. After keeping all the samples in dark for 30 min, absorbance was measured at 765 nm using a spectrophotometer (model 721D). Same procedure was adopted for gallic acid standard solutions (10, 40, 70, 100, and 130 mg/mL). Total phenolic contents were expressed as gallic acid equivalents (GAE) in milligram per liter on dry weight basis.[Citation27]
Total flavonoid contents
The total flavonoid contents in essential oils isolated at different extraction temperatures were determined using aluminum trichloride colorimetric assay as described in the literature[Citation28] with minor modifications. To 1.0 mL of each essential oil (isolated at different extraction temperature) or catechin standard solution (20, 40, 60, 80, and 100 mg/L) taken in a 10 mL volumetric flask, 4.0 mL of water and 0.3 mL of 5% NaNO2 were added. After waiting for 5 min, 0.3 mL of 10% AlCl3 was added and after further waiting for 1 min more, 2 mL of 1 M NaOH was added and total volume was made up to 10 mL using deionized distilled water. After mixing solution properly, the absorbance was measured at 510 nm using a reagent blank. The amount of total flavonoids was expressed as catechin equivalents in milligram per liter of dry plant materials.
DPPH free radical scavenging activity
The DPPH assay was performed according to method described in the literature[Citation29] with minor modifications. To 2.5 mL of essential oils isolated at different extraction temperatures, 1 mL of 0.09 mM DPPH solution was added and final volume was made up to 4 mL using 95% MeOH. The absorbance of the resulting solutions and the blank were recorded after 1 h at room temperature in dark at 515 nm using a spectrophotometer. Butylated hydroxyl toluene (100 ppm) was used as a positive control. Percentage (%) inhibition of free radicals by DPPH was calculated using following Eq. (1):
where, Ablank is the absorbance of the control reaction mixture and Asample is the absorbance of sample.
Total antioxidant contents (FRAP assay)
Total antioxidant contents were estimated using ferric reducing antioxidant power (FRAP) assay as described previously[Citation30] with minor modifications. One milliliter of each essential oil isolated at different extraction temperature (or gallic acid solutions (20, 40, 60, 80 and 100 mg/L)) was mixed with 2.5 mL of phosphate buffer (0.2 M; pH 6.6) and 2.5 mL of potassium ferricyanide (1%, w/v). The test tubes were incubated in water at 50°C for 25 min. Then 2.5 mL of trichloroacetic acid solution (10%, w/v) was added to each test tube. From each test tube, 2.5 mL of each reaction mixture was taken in a separate test tube and diluted with 2.5 mL of distilled water, followed by addition of 500 µL of ferric chloride (0.1%, w/v) solution. The test tubes were incubated for 30 min at room temperature. The absorbance of each reaction mixture was measured at 700 nm using UV–Vis spectrophotometer. Total antioxidant activity was calculated using gallic acid calibration curve (0–100 mg/mL) and total antioxidant contents were expressed as mg/mL of GAE.
Percentage inhibition in linoleic acid system
The percentage inhibition in linoleic acid peroxide formation of essential oils isolated at different extraction temperature was determined by the method described previously in the literature[Citation31] with minor modifications. Fifty microliters of essential oil dissolved in 1 mL of ethanol was mixed with 1 mL of linoleic acid (2.5%, v/v), 4 mL of 99.5% ethanol, and 4 mL of 0.05 M sodium phosphate buffer (pH 7). The solution was incubated at 40°C for 175 h. The extent of oxidation was measured by peroxide value using the colorimetric method.[Citation32] To 0.2 mL sample solution, 10 mL of ethanol (75%), 0.2 mL of an aqueous solution of ammonium thiocyanate (30%), and 0.2 mL of ferrous chloride solution (20 mM in 3.5% HCl) were added sequentially. After 3 min of stirring, the absorbance was measured at 500 nm, using a spectrophotometer. A control sample (without essential oil) was also run by following similar procedure. Butylated hydroxytoluene (BHT) was used as a positive control. Inhibition of linoleic acid oxidation expressed as percent was calculated by following Eq. (2):
Hydrogen peroxide scavenging activity
The ability of the essential oils to scavenge hydrogen peroxide was determined spectrophotometrically.[Citation17] Briefly, a solution of hydrogen peroxide (2 mM) was prepared in 0.17 M phosphate buffer (pH 7.4). Essential oil (600 µL) isolated at different extraction temperatures was mixed with 600 µL of 2 mM hydrogen peroxide. After 10 min of incubation at room temperature, the absorbance was read against a blank at 230 nm. Similar procedure was adopted for ascorbic acid (100 ppm).The percentage hydrogen peroxide scavenging activity of samples was calculated by following Eq. (3):
where, A0 was the absorbance of the control and A1 was the absorbance of samples.
Antimicrobial activity
Microbial strains
The essential oils isolated at different temperatures were individually tested against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Pasteurella multocida, Fusarium solani, Aspergillus niger, Alternaria alternate, and Aspergillus flavus. The microbial strains were obtained from Biological Division of the Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan.
Agar well diffusion method
The antibacterial activity of Boswellia serrata oleo gum resin essential oils isolated at different temperatures was performed by agar well diffusion method.[Citation33] The overnight cultures of the indicator strains were transferred to flasks containing 25 mL of liquid nutrient agar. The contents of the flasks were transferred to 6-inch petri plates and allowed to solidify at room temperature. Sterilized cork borer was used for well formation. These wells were filled with 10 µL essential oils isolated at different temperatures and standard drug (ampicillin 1 mg/mL) for determination of antibacterial activity. The petri plates were incubated at 37°C for 24 h. After the incubation period, antimicrobial activity was evaluated by measuring the width of the zones of inhibition (mm).
Resazurin microtiter plate assay
For the measurement of minimum inhibitory concentration (MIC) of essential oils isolated at different extraction temperatures,[Citation34] dimethyl sulfoxide (DMSO) was diluted with distilled water (10 mL/100 mL) for preparing the working solutions of essential oils and antibiotic. About 10 µL essential oils, fractions and subfractions solutions (8.4 mg/mL, w/v, in DMSO) was pipetted into the first row of the 96-well plates. Standard antibiotic (10 µL, 1.0 mg/mL in DMSO) was also added to the first row of the 96-well plates. To all other wells, 50 µL of nutrient broth was added. Twofold serial dilutions were performed using a multichannel pipette such that each well had 50 µL of the test material in serially descending concentrations. A volume of 30 µL of 3.3× strength isosensitized broth and 10 µL of resazurin indicator solution (prepared by dissolving 270 mg tablet in 40 mL of sterile distilled water) were added in each well. Finally, 10 µL of bacterial suspension was added to each well to achieve a concentration of ~5 × 105 cfu/mL. Each plate was wrapped loosely with cling film to avoid dehydration of bacteria. Each plate had a set of controls: a column with a positive control, a column with all solutions with the exception of the test compound, a column with all solutions with the exception of the bacterial solution (added 10 µL of nutrient broth instead), and a column with DMSO solution as a negative control. The plates were prepared in triplicate and incubated at 37°C for 24 h. The change in the color of resazurin indicator was then assessed visually. The growth was indicated by color changes from purple to pink or colorless. The lowest concentration at which color change occurred was taken as the MIC value.
Microdilution broth susceptibility assay
For MIC, a microdilution broth susceptibility assay was used.[Citation35] Essential oils were solubilized in 10% DMSO followed by dilution in culture media for use. Dilutions series (0.66–1351.08 µg/mL) of the essential oils, fractions, and subfractions in a 96-well microtiter plate, including one growth control, solvent control, and one sterility control were prepared. About 160 μL of sabouraud dextrose broth was added to microplates and 20 μL of test solution. Then, 20 μL of 5 × 105 cfu/mL (confirmed by viable count) of standard/microorganism suspension were inoculated onto microplates. Plates were incubated at 30ºC for 48 h in case of fungi. Fluconazole (1.0 mg/mL in 10% DMSO) was used as positive control. The growth was indicated by the presence of a white “pellet” on the well bottom. The MIC was calculated as the highest dilution showing complete inhibition of the test strains.
Gas chromatographic–mass spectrometric (GC-MS) analysis
The chemical composition of all essential oils samples was determined by gas chromatography–mass spectrometry (GC–MS) using Agilent-Technologies 6890N Network GC system, equipped with 7890A series inert XL mass selective detector and 5975C series auto injector (Agilent-Technologies) along with DB-5 capillary column (50 m × 0.25 mm, film thickness of 0.25 µm). Column temperature was selected in the range of 60–240°C with a flow rate of 4°C/min. Moreover, lower and upper temperatures were held for 3 and 10 min, respectively. Helium was used as a carrier gas at a flow rate of 2 mL/min. The split mode method was employed for the injection of 2 µL essential oil (split ratio, 1:20). The MS detection was done by using an electron ionization mode with ionization energy of 70 eV. Injector and MS transfer line temperatures were set at 220°C and 290°C, respectively.[Citation36] The identification of compounds present in essential oils was carried out on comparison of retention indices relative to NIST mass spectral library.[Citation37,Citation38]
Statistical analysis
All the experiments were triplicated and statistical analysis of the data were performed by analysis of variance using STATISTICA 5.5 (Stat Soft Inc., Tulsa, OK, USA) software. A probability value at p ≤ 0.05 was considered statistically significant. Data are presented as mean values ± standard deviation calculated from triplicate determinations.
Results and discussion
Essential oil yield
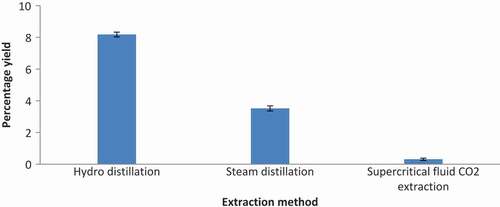
Essential oil yield of Boswellia serrata oleo gum resin isolated through hydro-distillation, steam distillation, and supercritical fluid CO2 extraction method is shown in . It was observed that extraction methods significantly affected the essential oil yield of Boswellia serrata oleo gum resin (). The highest essential oil yield was obtained through hydro-distillation method (8.18 ± 0.15) and lowest essential oil recovery was noted in the supercritical fluid CO2 extraction method (0.31 ± 0.07). The experimental results obtained are similar to the previous studies.[Citation39,Citation40] In these previous studies, it was reported that hydro- and steam distillation methods gave higher essential oil yield than supercritical fluid CO2 extraction method from Piper nigrum L. In another study, it was reported that essential oil yield varied with extraction conditions and extraction methods.[Citation26] The variation in essential oil yield of hydro-distillation, steam distillation, and supercritical fluid CO2 extraction method may be due to the polarity of CO2, as it is nonpolar solvent with different isolating power as compared to water which is a polar solvent. Moreover, it is also possible that some of the volatile compounds escaped with CO2 from the separation vessel. The essential yields of hydro-distilled Boswellia serrata oleo gum resin are comparable to a previous study,[Citation41] which reported that Boswellia serrata oleo gum resin collected from different locations of India, contained 5.0–9.0% essential oil. The comparison of oleo gum resin essential oil yield between Boswellia serrata and other Boswellia species shows that Boswellia serrata oleo gum resin contains higher amount of essential oil (3.30–9.37%) than Boswellia elongate (2.3%), Boswellia socotrana (1.2%), Boswellia sacra (5.5%), and Boswellia ameero (1.8%).[Citation42,Citation43] Such variation in essential oil yield may be due to difference in geological, seasonal, and agro-climatic conditions. Furthermore, it has been reported that yield and chemical composition of oleo gum resin essential oil significantly vary with season, genotype, extraction conditions, depth of the draining (drilling) holes in the tree, plant nutrition, location of plant, parts of plant, environment stress, extraction methods, and storing conditions.[Citation44–Citation46]
Antioxidant activity of essential oils
Extraction methods may affect the biological activity and chemical components of Boswellia serrata oleo gum resin essential oil. Antioxidant activity of Boswellia serrata oleo gum resin essential oils isolated through different extraction methods was determined through various antioxidant assays (). It was found that extraction methods significantly affect the free radical scavenging potential of essential oils. Maximum free radical scavenging potential was observed in essential oil isolated through supercritical fluid CO2 extraction method (96.16 ± 1.57%), while least potential was found in essential oil isolated through hydro-distillation method (53.39 ± 0.43%). The results obtained are comparable to a previous study which stated that Boswellia serrata oleo gum resin essential oil show good free radical scavenging potential.[Citation6] It was observed that all the essential oils revealed good antioxidant activity with least amount of linoleic acid peroxide formation.[Citation47] In linoleic acid assay, greater the number of peroxides formed during the reaction, higher will be the absorbance and the lowest is the antioxidant activity. Supercritical fluid CO2 extracted essential oil showed maximum ability to inhibit the oxidation of linoleic acid (94.18 ± 1.47%). Phenolic compounds are the most important plant secondary metabolites and act as antioxidants due to their hydroxyl groups.[Citation48] Folin–Ciocalteu reagent is frequently used for determination of total phenolics. This reagent is used to find out the reducing capacity of samples.[Citation49] The total phenolic contents in essential oils isolated through different extraction methods were determined from calibration curve (y = 0.0088x + 0.0422, R2 = 0.99) and is represented as mg/L of GAE. Total phenolic contents in essential oils isolated through different extraction methods, varied from 598.24 ± 13.15 to 400.42 ± 10.01 mg/L of GAE. Supercritical fluid CO2 extracted essential oil revealed highest amount of total phenolic compounds (598.24 ± 13.15 mg/L of GAE), whereas essential oil isolated through steam distillation contained least amount of total phenolic compounds (400.42 ± 10.01 mg/L of GAE). Total flavonoid contents of essential isolated through supercritical fluid CO2 extraction method exhibited maximum value (376.18 ± 10.14 mg/L of catechin equivalents), whereas essential oil isolated through steam distillation method contained minimum amount of total flavonoid contents (202.66 ± 5.06 mg/L of catechin equivalents). Essential oil isolated through supercritical fluid CO2 extraction method have shown the highest hydrogen peroxide free radical scavenging (68.25 ± 1.02%) and essential oil isolated through steam distillation technique exhibited the least hydrogen peroxide free radical scavenging (50.74 ± 0.68%). Total antioxidant contents of essential oils isolated through different extraction methods were determined from calibration curve (y = 0.021x − 0.0151, R2 = 0.99) and represented as mg/L of GAE. Total antioxidant contents in essential oils isolated through different extraction methods were ranged from 94.39 ± 1.04 to 56.74 ± 0.79 mg/L GAE. Essential oil isolated through steam distillation technique contained highest amount of total antioxidant contents (94.39 ± 1.04 mg/L GAE), whereas essential oil isolated through supercritical fluid CO2 extraction method contained least amount of total antioxidant contents (56.74 ± 0.79 mg/L GAE). The variation in antioxidant activity of essential oils may be due to difference in chemical components of essential oils isolated through different extraction methods.
Table 1. Total phenolic, total flavonoid contents, DPPH free radical scavenging, hydrogen peroxide scavenging, and total antioxidant/FRAP activities of Boswellia serrata oleo gum resin essential oils isolated through different extraction methods.
Antimicrobial activity of essential oils
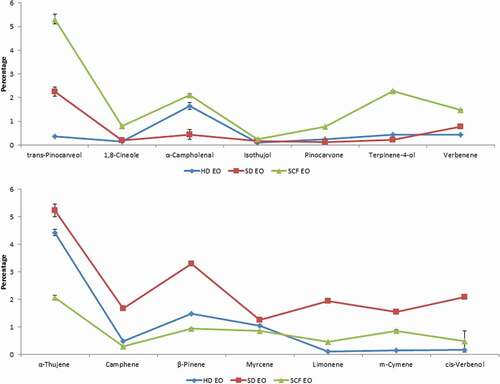
Antimicrobial activity of isolated essential oils against two gram-positive bacteria, two gram-negative bacteria, and four fungal strains was determined by well diffusion, resazurin microtiter plate, and microdilution broth assays (). The values of inhibition zones (mm) and MICs (µg/mL) for the essential oil isolated through different extraction methods were 7.57 ± 0.19 to 24.21 ± 0.34 mm and 35.18 ± 0.77 to 562.94 ± 8.07 µg/mL, respectively. These results show that all essential oils revealed good antimicrobial activity against tested microbial strains. Steam distillated essential oil have shown higher antimicrobial activity with larger inhibition zones (24.21 ± 0.34–12.08 ± 0.30 mm) and smaller MIC values (35.18 ± 0.77–281.46 ± 7.03 µg/mL). Supercritical fluid CO2 extracted essential oil revealed least antimicrobial activity with smaller inhibition zones (7.57 ± 0.19–16.24 ± 0.32 mm) and larger MIC values (140.74 ± 2.81–478.49 ± 6.40 µg/mL). Such variations in antimicrobial activity of essential oils may due to difference in chemical composition of essential oils. Moreover, the MIC values and inhibition zones show that Pastrulla multocida and E. coli were the most sensitive bacterial strains presenting larger inhibition zones (10.79 ± 0.14–24.21 ± 0.34 mm and 7.57 ± 0.19–16.80 ± 0.33 mm, respectively) and smaller MIC values (35.18 ± 0.77–281.47 ± 1.96 µg/mL and 70.36 ± 1.82–337.78 ± 4.52 µg/mL, respectively). S. aureus and Bacillus subtilis were the least sensitivity bacterial strains presenting smaller inhibition zones (9.87 ± 0.18–14.15 ± 0.27 mm and 8.47 ± 0.15–14.47 ± 0.25 mm, respectively) and greater MIC values (98.52 ± 1.96–168.88 ± 1.96 µg/mL and 98.52 ± 1.96–281.47 ± 1.96 µg/mL, respectively). F. solani and Alternaria alternate were the most sensitive fungal strains that exhibited the larger inhibition zones (10.12 ± 0.24–23.00 ± 0.46 mm and 16.24 ± 0.32–21.64 ± 0.34 mm, respectively) and least MIC values (56.29 ± 2.40–337.12 ± 2.42 µg/mL and 79.36 ± 1.34–140.74 ± 2.81 µg/mL, respectively). Aspergillus niger and Aspergillus flavus were the least sensitive fungal strains and have shown the smaller inhibition zones (8.80 ± 0.21–12.08 ± 0.30 mm and 11.62 ± 0.18–13.64 ± 0.42 mm, respectively) and greater MIC values (281.46 ± 7.03–562.94 ± 8.07 µg/mL and 225.17 ± 3.50–281.46 ± 3.62 µg/mL, respectively). These experimental results are in good agreement with previous studies.[Citation6,Citation50] The GC–MS analyses of essential oils have shown that monoterpenes and oxygenated monoterpenes were the major components of Boswellia serrata oleo gum resin essential oils. These compounds might be responsible for their antimicrobial activity. It is also previously reported that monoterpenes and oxygenated monoterpenes might be responsible for antimicrobial activity of Boswellia serrata oleo gum resin essential oil.[Citation6] In another study, it was reported that the antimicrobial potential of Boswellia serrata oleo gum resin essential oil is not due to a single compound. It was due to the synergistic effect of several compounds.[Citation50] GC–MS analysis of steam distilled essential oil represented higher amounts of α-thujene, camphene, β-pinene, myrcene, limonene, m-cymene, and cis-verbenol as compared to hydro and supercritical fluid CO2 extracted essential oil. These compounds may be responsible for the higher antimicrobial activity of Boswellia serrata oleo gum resin steam distilled essential oil ().
Table 2. Antimicrobial activity of Boswellia serrata oleo gum resin essential oils isolated through different extraction methods.
Table 3. GC–MS analysis of Boswellia serrata oleo gum resin essential oils isolated by different extraction methods.
Chemical composition of essential oil
The chemical composition of essential oils isolated through different extraction methods were analyzed by GC–MS and their results are given in . A total of 26 compounds were identified in essential oils with α-pinene (80.86–74.20%) as major compound, followed by α-thujene (5.224–2.06%), β-pinene (3.30–0.94%), α-thujone (2.46–0.55%), trans-pinocarveol (5.31–2.25%), and cis-verbenol (0.17–2.09%). A much similar chemical composition with α-pinene as major compound is reported in the literature.[Citation51] Essential oil was mainly consisted of monoterpenes (84.41–91.31%) and oxygenated monoterpenes (8.69–14.73%). In monoterpenes, α-pinene, α-thujene, β-pinene, and limonene were the main compounds, while in oxygenated monoterpenes, α-thujone, trans-pinocarveol, α-campholenal, and cis-verbenol were the main compounds. These results are in accordance with previous study which reported that Boswellia serrata bark essential oil contained α-pinene as major compound, followed by β-pinene, cis-verbenol, and trans-pinocarveol.[Citation52] It was also reported that monoterpene hydrocarbons were the major class of compounds present in Boswellia serrata essential oil. In another study, it was found that monoterpene hydrocarbons and oxygenated monoterpenes were the dominant class of compounds present in Indian Boswellia serrata oleo gum resin essential oil.[Citation6] A different study found α-thujene as a major compound in commercially available Boswellia serrata oleo gum resin essential oils from India.[Citation41] Such variations in chemical composition of essential oils may be due to difference in agro-climatic conditions, extraction methods/conditions, seasons, and geographical variations.[Citation44–Citation46] It is observed that the extraction methods significantly affected the chemical composition of Boswellia serrata oleo gum resin essential oils (). The components that varied with extraction methods were α-pinene, 2,4(10)-thujadien, sabinene, 4-methyl-anisole, and α-thujone (highest in hydro-distilled essential oil: 80.86%, 0.76%, 1.27%, 1.98%, and 2.46%, respectively); α-thujene, camphene, β-pinene, myrcene, limonene, m-cymene, and cis-verbenol (highest in steam distilled essential oil: 5.24%, 1.66%, 3.30%, 1.25%, 1.93%, 1.55%, and 2.09%, respectively); and verbenene, α-campholenal, and trans-pinocarveol (1.48%, 2.10%, 5.31%, respectively, premier in supercritical fluid CO2 extracted essential oil) ().
Conclusion
In conclusion, extraction methods significantly affected the essential oil yield, chemical composition, antioxidant, and antimicrobial activities of Boswellia serrata oleo gum resin essential oils. Maximum essential oil yield was observed in hydro-distillation extraction method. Essential oil extracted using supercritical fluid CO2 has shown highest antioxidant activity. The variations in antioxidant and antimicrobial activities of essential oils may be due to difference in chemical composition of essential oils. Essential oil isolated through steam distillation method has shown superior antimicrobial activities. This might be due to higher concentrations of α-thujene, camphene, β-pinene, myrcene, limonene, m-cymene, and cis-verbenol in steam distilled essential than hydro and supercritical fluid CO2 extracted essential oils. The findings of the present study provide the insights for future research on optimization of experimental variables involved in various extraction techniques. It would be interesting to assess the effects of temperature and pressure on essential oil quality in relevance to biological activities.
References
- Maupetit, P.;. New Constituents in Olibanum Resinoid and Essential Oil. Perfumer and Flavorist 1984, 9(6), 19–37.
- Khan, I. A.; Abourashed, E. A. Leung’s Encyclopedia of Common Natural Ingredients: Used in Food, Drugs and Cosmetics. New Jersey, USA: John Wiley & Sons. 2011, 1, 36–40.
- Singh, S.; Khajuria, A.; Taneja, S.; Khajuria, R.; Singh, J.; Johri, R.; Qazi, G. The Gastric Ulcer Protective Effect of Boswellic Acids, a Leukotriene Inhibitor from Boswellia serrata, in Rats. Phytomedicine 2008, 15(6), 408–415, DOI:10.1016/j.phymed.2008.02.017.
- Mothana, R. A.; Hasson, S. S.; Schultze, W.; Mowitz, A.; Lindequist, U. Phytochemical Composition and in Vitro Antimicrobial and Antioxidant Activities of Essential Oils of Three Endemic Soqotraen Boswellia Species. Food Chemistry 2011, 126(3), 1149–1154, DOI:10.1016/j.foodchem.2010.11.150.
- Menon, M.; Kar, A. Analgesic and Psychopharmacological Effects of the Gum Resin of Boswellia serrata. Planta Medicine 1971, 19(02), 333–341, DOI:10.1055/s-0028-1099651.
- Gupta, M.; Rout, P.; Misra, L.; Gupta, P.; Singh, N.; Darokar, M.; Saikia, D.; Singh, S.; Bhakuni, R. Chemical Composition and Bioactivity of Boswellia serrata Essential Oil in Relation to Geographical Variation Roxb. Plant Biosystems 2016, 151(4), 623–629.
- Khan, M. A.; Singh, M.; Khan, M. S.; Najmi, A. K.; Ahmad, S. Caspase Mediated Synergistic Effect of Boswellia serrata Extract in Combination with Doxorubicin against Human Hepatocellular Carcinoma. BioMed Research International (E-Journal) 2014, 2014, 11.
- Dar, M. Y.; Shah, W. A.; Mubashir, S.; Rather, M. A. Chromatographic Analysis, Anti-Proliferative and Radical Scavenging Activity of Pinus wallichina Essential Oil Growing in High Altitude Areas of Kashmir, India. Phytomedicine 2012, 19(13), 1228–1233, DOI:10.1016/j.phymed.2012.07.015.
- Wang, S.-Y.; Kuo, Y.-H.; Chang, H.-N.; Kang, P.-L.; Tsay, H.-S.; Lin, K.-F.; Yang, N.-S.; Shyur, L.-F. Profiling and Characterization Antioxidant Activities in Anoectochilus formosanus Hayata. Journal of Agricultural and Food Chemistry 2002, 50(7), 1859–1865, DOI:10.1021/jf0113575.
- Vági, E.; Rapavi, E.; Hadolin, M.; Vasarhelyine Peredi, K.; Balázs, A.; Blázovics, A.; Simándi, B. Phenolic and Triterpenoid Antioxidants from Origanum majorana L. Herb and Extracts Obtained with Different Solvents. Journal of Agricultural and Food Chemistry 2005, 53(1), 17–21, DOI:10.1021/jf048777p.
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Cereals and Cereal Products. In Food Chemistry, 4th ed.; Springer: Berlin, 2009; pp 670–745.
- Scallan, E.; Hoekstra, R. M.; Angulo, F. J.; Tauxe, R. V.; Widdowson, M.-A.; Roy, S. L.; Jones, J. L.; Griffin, P. M. Foodborne Illness Acquired in the United States – Major Pathogens. Emerging Infectious Disease 2011, 17, 1.
- Hussain, A. I.; Anwar, F.; Hussain Sherazi, S. T.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum Basilicum) Essential Oils Depends on Seasonal Variations. Food Chemistry 2008, 108(3), 986–995.
- Celiktas, O. Y.; Kocabas, E. H.; Bedir, E.; Sukan, F. V.; Ozek, T.; Baser, K. Antimicrobial Activities of Methanol Extracts and Essential Oils of Rosmarinus officinalis, Depending on Location and Seasonal Variations. Food Chemistry 2007, 100(2), 553–559, DOI:10.1016/j.foodchem.2005.10.011.
- Hyldgaard, M.; Mygind, T.; Meyer, R. L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Frontiers in Microbiology 2012, 12 (3), 1–24.
- Miguel, M. G.;. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15(12), 9252–9287, DOI:10.3390/molecules15129252.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils – A Review. Food and Chemical Toxicology 2008, 46(2), 446–475, DOI:10.1016/j.fct.2007.09.106.
- Mertens, M.; Buettner, A.; Kirchhoff, E. The Volatile Constituents of Frankincense-a Review. Flavour and Fragrance Journal 2009, 24 (6), 279–300.
- Sadgrove, N.; Jones, G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture 2015, 5(1), 48–102, DOI:10.3390/agriculture5010048.
- Wang, H.-W.; Liu, Y.-Q.; Wei, S.-L.; Yan, Z.-J.; Lu, K. Comparison of Microwave-Assisted and Conventional Hydrodistillation in the Extraction of Essential Oils from Mango (Mangifera Indica L.) Flowers. Molecules 2010, 15(11), 7715–7723, DOI:10.3390/molecules15117715.
- Moradalizadeh, M.; Samadi, N.; Rajaei, P. Comparison of Hydrodistillation, Microwave Hydrodistillation and Solvent Free Microwave Methods in Analysis of the Essential Oils from Aerial Parts of Haplophyllum robustum Bge. By GC/MS Method. International journal of Advanced Biological and Biomedical Research 2013, 1(9), 1058–1067.
- Gañán, N.; Brignole, E. A. Supercritical Carbon Dioxide Fractionation of T. minuta and S. officinalis Essential Oils: Experiments and Process Analysis. Journal of Supercritical Fluids 2013, 78, 12–20. DOI:10.1016/j.supflu.2013.03.019.
- Kokoska, L.; Havlik, J.; Valterova, I.; Sovova, H.; Sajfrtova, M.; Jankovska, I. Comparison of Chemical Composition and Antibacterial Activity of Nigella Sativa Seed Essential Oils Obtained by Different Extraction Methods. Journal of Food Protection 2008, 71(12), 2475–2480, DOI:10.4315/0362-028X-71.12.2475.
- Van Vuuren, S.; Kamatou, G.; Viljoen, A. Volatile Composition and Antimicrobial Activity of Twenty Commercial Frankincense Essential Oil Samples. South African Journal of Botany 2010, 76(4), 686–691, DOI:10.1016/j.sajb.2010.06.001.
- Okoh, O.; Sadimenko, A.; Afolayan, A. Comparative Evaluation of the Antibacterial Activities of the Essential Oils of Rosmarinus officinalis L. Obtained by Hydrodistillation and Solvent Free Microwave Extraction Methods. Food Chemistry 2010, 120(1), 308–312, DOI:10.1016/j.foodchem.2009.09.084.
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of Essential Oils of Clove Buds Extracted with Supercritical Carbon Dioxide and Other Three Traditional Extraction Methods. Food Chemistry 2007, 101(4), 1558–1564, DOI:10.1016/j.foodchem.2006.04.009.
- Khan, R. A.; Khan, M. R.; Sahreen, S.; Ahmed, M. Assessment of Flavonoids Contents and in Vitro Antioxidant Activity of Launaea procumbens. Chemistry Central Journal 2012, 6(1), 1–11, DOI:10.1186/1752-153X-6-43.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and their Scavenging Effects on Superoxide Radicals. Food Chemistry 1999, 64(4), 555–559, DOI:10.1016/S0308-8146(98)00102-2.
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the Volatile Composition of Essential Oils of Some Lamiaceae Spices and the Antimicrobial and Antioxidant Activities of the Entire Oils. Journal of Agricultural and Food Chemistry 2006, 54(5), 1822–1828, DOI:10.1021/jf051922u.
- Chan, E.; Lim, Y.; Omar, M. Antioxidant and Antibacterial Activity of Leaves of Etlingera Species (Zingiberaceae) in Peninsular Malaysia. Food Chemistry 2007, 104(4), 1586–1593, DOI:10.1016/j.foodchem.2007.03.023.
- Singh, G.; Marimuthu, P.; De Heluani, C. S.; Catalan, C. A. Antioxidant and Biocidal Activities of Carum nigrum (Seed) Essential Oil, Oleoresin, and their Selected Components. Journal of Agricultural and Food Chemistry 2006, 54(1), 174–181, DOI:10.1021/jf0518610.
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant Activity of Anthraquinones and Anthrone. Food Chemistry 2000, 70(4), 437–441, DOI:10.1016/S0308-8146(00)00108-4.
- Rashid, S.; Rather, M. A.; Shah, W. A.; Bhat, B. A. Chemical Composition, Antimicrobial, Cytotoxic and Antioxidant Activities of the Essential Oil of Artemisia indica Willd. Food Chemistry 2013, 138(1), 693–700, DOI:10.1016/j.foodchem.2012.10.102.
- Sarker, S. D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42(4), 321–324, DOI:10.1016/j.ymeth.2007.01.006.
- Dabur, R.; Ali, M.; Singh, H.; Gupta, J.; Sharma, G. A novel antifungal pyrrole derivative from Datura metel leaves. Die Pharmazie-An International Journal of Pharmaceutical Sciences 2004, 59(7), 568–570.
- Hanif, M. A.; Nawaz, H.; Ayub, M. A.; Tabassum, N.; Kanwal, N.; Rashid, N.; Saleem, M.; Ahmad, M. Evaluation of the Effects of Zinc on the Chemical Composition and Biological Activity of Basil Essential Oil by Using Raman Spectroscopy. Industrial Crops and Products 2017, 96, 91–101. DOI:10.1016/j.indcrop.2016.10.058.
- Adams, R. P.;. Identification of Essential Oil Components by GC/MS; Allured Publ. Corp: Carol Stream, IL, 1995.
- Adams, R.;. Essential Oil Components by Quadrupole GC/MS; Allured Publishing Corp: Carol Stream, IL, 2001.
- Bagheri, H.; Manap, M. Y. B. A.; Solati, Z. Antioxidant Activity of Piper nigrum L. Essential Oil Extracted by Supercritical CO2 Extraction and Hydro-Distillation. Talanta 2014, 121, 220–228. DOI:10.1016/j.talanta.2014.01.007.
- Ferreira, S. R.; Nikolov, Z. L.; Doraiswamy, L.; Meireles, M. A. A.; Petenate, A. J. Supercritical Fluid Extraction of Black Pepper (Piper Nigrun L.) Essential Oil. Journal of Supercritical Fluids 1999, 14(3), 235–245, DOI:10.1016/S0896-8446(98)00092-8.
- Singh, B.; Kumar, R.; Bhandari, S.; Pathania, S.; Lal, B. Volatile Constituents of Natural Boswellia serrata Oleo‐Gum‐Resin and Commercial Samples. Flavour and Fragrance Journal 2007, 22(2), 145–147, DOI:10.1002/(ISSN)1099-1026.
- Al-Harrasi, A.; Al-Saidi, S. Phytochemical Analysis of the Essential Oil from Botanically Certified Oleogum Resin of Boswellia Sacra (Omani Luban). Molecules 2008, 13(9), 2181–2189, DOI:10.3390/molecules13092181.
- Ali, N.; Wurster, M.; Arnold, N.; Teichert, A.; Schmidt, J.; Lindequist, U.; Wessjohann, L. Chemical Composition and Biological Activities of Essential Oils from the Oleogum Resins of Three Endemic Soqotraen Boswellia Species. Records of Natural Products 2008, 2(1), 6–12.
- Mita, E.; Tsitsimpikou, C.; Tsiveleka, L.; Petrakis, P. V.; Ortiz, A.; Vagias, C.; Roussis, V. Seasonal Variation of Oleoresin Terpenoids from Pinus halepensis and Pinus pinea and Host Selection of the Scale Insect Marchalina Hellenica (Homoptera, Coccoidea, Margarodidae, Coelostonidiinae). Holzforschung 2002, 56(6), 572–578, DOI:10.1515/HF.2002.087.
- Hussain, A. I.; Anwar, F.; Sherazi, S. T. H.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum Basilicum) Essential Oils Depends on Seasonal Variations. Food Chemistry 2008, 108(3), 986–995, DOI:10.1016/j.foodchem.2007.12.010.
- Raut, J. S.; Karuppayil, S. M. A Status Review on the Medicinal Properties of Essential Oils. Industrial Crops and Products 2014, 62, 250–264. DOI:10.1016/j.indcrop.2014.05.055.
- Mothana, R. A. A.;. Anti-Inflammatory, Antinociceptive and Antioxidant Activities of the Endemic Soqotraen Boswellia elongata Balf. F. and Jatropha unicostata Balf. F. In Different Experimental Models. Food and Chemical Toxicology 2011, 49(10), 2594–2599, DOI:10.1016/j.fct.2011.06.079.
- Jung, H.-Y.; Sawayanagi, T.; Wongkaew, P.; Kakizawa, S.; Nishigawa, H.; Wei, W.; Oshima, K.; Miyata, S.-I.; Ugaki, M.; Hibi, T. Candidatus Phytoplasma Oryzae’, a Novel Phytoplasma Taxon Associated with Rice Yellow Dwarf Disease. International Journal of Systematic and Evolutionary Microbiology 2003, 53(6), 1925–1929, DOI:10.1099/ijs.0.02531-0.
- Huang, D.; Ou, B.; Prior, R. L. The Chemistry Behind Antioxidant Capacity Assays. Journal of Agricultural and Food Chemistry 2005, 53(6), 1841–1856, DOI:10.1021/jf030723c.
- Camarda, L.; Dayton, T.; Di Stefano, V.; Pitonzo, R.; Schillaci, D. Chemical Composition and Antimicrobial Activity of Some Oleogum Resin Essential Oils from Boswellia spp. (Burseraceae). Annali Di Chimica 2007, 97(9), 837–844, DOI:10.1002/adic.200790068.
- Verghese, J.; Joy, M.; Retamar, J.; Malinskas, G. G.; Catalan, C.; Gros, E. A Fresh Look at the Constituents of Indian Olibanum Oil. Flavour and Fragrance Journal 1987, 2(3), 99–102, DOI:10.1002/ffj.2730020304.
- Kasali, A. A.; Adio, A. M.; Oyedeji, A. O.; Eshilokun, A. O.; Adefenwa, M. Volatile Constituents of Boswellia serrata Roxb. (Burseraceae) Bark. Flavour and Fragrance Journal 2002, 17(6), 462–464, DOI:10.1002/(ISSN)1099-1026.