ABSTRACT
This study reports the phenolic composition, antioxidant activity, and capacity of Physalis angulata and Newbouldia laevis leaves to inhibit enzymes (phosphodiesterase-5′ [PDE-5′], arginase, acetylcholinesterase [AChE], and angiotensin-I converting enzyme [ACE]) linked to erectile dysfunction. High-performance liquid chromatography–diode array detector analysis of the aqueous extracts revealed the presence of phenolic acids (caffeic, ellagic, chlorogenic, and gallic acids) and flavonoids (quercetin, rutin, isoquercitrin, kaempferol, and quercitrin). N. laevis exhibited significantly higher inhibitory effects on PDE-5′, arginase, and ACE activities compared to P. angulata. There was no significant (P < 0.05) difference in the AChE inhibitory activities of both extracts. Furthermore, P. angulata exhibited lower radical scavenging and chelating abilities compared to N. laevis. These findings revealed that P. angulata and N. laevis leaves are good candidates for the development of functional foods with potentials to improve erectile function.
Introduction
Today, the use of plant materials as functional foods and nutraceutics for the treatment of different diseases is on the increase all over the world.[Citation1] These could be linked to their phytochemical constituents, which include phenolic acids, flavonoids, and alkaloids. These phytochemicals have several health benefits with little or no side effect compared to synthetic products.[Citation1,Citation2] Physalis angulata is the most common specie in the genus Physalis L. (family; Solanacaea) and highly distributed across regions of the world.[Citation3,Citation4] Newbouldia laevis (family: Bignoniaceae) is commonly known as a boundary tree which grows mainly in the tropics in Africa.[Citation5] The leaves of these plants are usually used in preparations of local dishes, as spices, and also consumed as vegetables in many Nigerian homes. These plants are also used for the management of diabetes, malaria, hepatitis, and inflammation.[Citation5–Citation8] However, despite several reports on their biological activities, there is a dearth of information on their effects on biomolecules that mediates erectile dysfunction (ED).
ED is a vascular disease, which occurs in men and involves inability to have penile erection sufficient for sexual satisfaction.[Citation9] ED is a public health problem due to its prevalence amongst young and old men with an estimate of 300 million patients by 2025.[Citation10,Citation11] Several risk factors have been attributed to ED, including vascular, neurological, hormonal psychological disorders, as well as hypertension, diabetes, heart diseases, and oxidative stress.[Citation9,Citation12] Moreover, some enzymes such as phosphodiesterase-5′ (PDE-5′), arginase, acetylcholinesterase (AChE), and angiotensin-I converting enzyme (ACE) have been identified to be upregulated in penile corpus cavernosa tissues in ED patients and also used as therapeutic points in the management of this condition.[Citation13–Citation15] Although synthetic inhibitors of PDE-5', arginase, AChE and ACE are effective, however, they pose several harmful effects.[Citation1,16] Hence, the use of herbal remedies, functional foods, and nutraceutics has been reported to be of good use.[Citation1] In this study, phenolic constituents of P. angulata and N. laevis were determined as the effects of their aqueous extracts on PDE-5', arginase, AChE and ACE activities.
Materials and methods
Sample collection and preparation
Fresh P. angulata and N. Laevis leaves were collected from a local farm around Federal University of Technology Akure. The leaves were identified at the Department of Biology, Federal University of Technology Akure, Ondo state. A voucher specimen was deposited in the Herbarium. The leaves were dried at room temperature and pulverized. Twenty gram each was extracted by maceration at room temperature with 100 mL of distilled water. After 48 h, the extracts were filtered, freeze-dried, and kept in sealed vials at −4°C prior to subsequent analysis.
Chemicals and reagents
PDE-5′ (from Spodoptera frugiperda) was acquired from Merck Millipore; hippuryl-l-histidyl l-leucine (HHL), p-nitrophenylphenylphosphonate, acetylthiocholine iodide, 5,5′-dithiobio-(2-nitrobenzoic acid), AChE (from electric eel, type VI-S), arginase (from bovine liver), and ACE (from rabbit lung) were purchased from Sigma-Aldrich Co. (Steinheim, Germany). All other reagents used were of analytical grade and glass distilled water was used.
Quantification of phenolic compounds by HPLC–DAD
The quantification of phenolic compounds in the extract was carried out according to the method described by Akomolafe et al.[Citation17] using high-performance liquid chromatography coupled with diode array detector (HPLC–DAD). The peaks observed in the chromatogram were confirmed by comparing its retention time with those of reference standards and by DAD spectra (200–600 nm). Calibration curve for gallic acid: Y = 12683x + 1176.5 (r = 0.9993), chlorogenic acid: Y = 13074x + 1267.9 (r = 0.9991), caffeic acid: Y = 11983x + 1371.0 (r = 0.9998), ellagic acid: Y = 13571x + 1257.4 (r = 0.9995), quercitrin: Y = 13509x + 1264.7 (r = 0.9999), isoquercitrin: Y = 12854x + 1186.1 (r = 0.9993), rutin: Y = 12983x + 1321.6 (r = 0.9995), quercetin: Y = 13582x + 1196.5 (r = 0.9997), and kaempferol: Y = 12930x + 1265.8 (r = 0.9994). The analysis was done at ambient temperature and in triplicate. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the responses and the slope using three independent analytical curves. LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
PDE-5′ inhibition assay
The PDE-5′ assay was determined using the method of Kelly and Butler[Citation18] with slight modification. The method was based on the spectrophotometric measurement of the color intensity of p-nitrophenol produced by the catalytic action of PDE-5′ at 405 nm. The control experiment was carried out without the addition of extracts. The PDE-5′ inhibitory effect of the extracts was calculated and expressed as percentage inhibition.
Arginase inhibition assay
The method of Kaysen and Strecker[Citation19] was followed to determine the effects on arginase activity. This method is based on the spectrophotometric measurement of urea produced from arginine at 450 nm. The control experiment was carried out without the addition of the extracts. The arginase inhibitory capacity of the extracts was calculated and expressed as percentage inhibition.
AChE inhibition assay
The AChE inhibition of the extracts was assessed by a colorimetric method.[Citation20] The AChE activity was measured by JENWAY UV–visible spectrophotometer from the absorbance changes at 412 nm for 3.0 min at 25°C, using acetylthiocholine iodide (100 μL of 0.05 mM aqueous solution) as substrate. The enzyme inhibitory activities of the extracts were expressed as percentage inhibition.
ACE inhibition assay
The effect of the extracts on ACE activity was determined according to the method of Ademiluyi et al.[Citation21] This method was based on the measurement of hippuric acid (Bz-Gly) produced from the substrate (HHL) by the activity of ACE. The hippuric acid produced was redissolved with distilled water and measured at 228 nm.
Measurement of the radicals scavenging capacity of the extracts
The 1,1-diphenyl-2 picrylhydrazyl (DPPH) radical scavenging capacity of P. angulata and N. laevis extracts was determined using the method of Gyamfi et al.,[Citation22] while the ability of the extracts to scavenge hydroxyl radical produced from Fe2+/H2O2-induced decomposition of deoxyribose was carried out using the method of Halliwell and Gutteridge.[Citation23] The radical scavenging activities of the extracts were subsequently calculated as percentage inhibition. The method of Minotti and Aust[Citation24] and Puntel et al.[Citation25] was used to determine the Fe2+ chelating ability of the extracts. The Fe2+ chelating ability was subsequently calculated and expressed as percentage Fe chelation ability.
Statistical analysis
All the analyses were carried out in triplicates. The results are expressed as mean ± SD. Statistical comparisons were investigated by one-way ANOVA followed by Duncan’s post-hoc test for multiple comparisons. IC50 values were calculated using nonlinear regression analysis.
Result and discussion
Phenolic composition
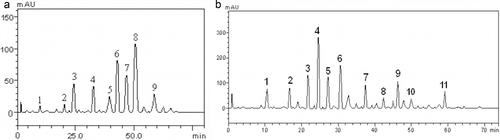
The phenolic components of the extracts derived from P. angulata and N. laevis were quantified via HPLC–DAD by comparing their retention time and peaks with standard phenolic compounds under the same conditions. The result in and shows that some phenolic acids (ellagic acid, caffeic acid, chlorogenic acid, and gallic acid) and flavonoids (kaempferol, isoquercitrin, rutin, quercitrin, and quercetin) were detected in both extracts. However, catechin (50.13 ± 0.03 mg/g) and epicatechin (87.59 ± 0.03 mg/g) were present in N. laevis but were not detected in P. angulata. Furthermore, N. laevis extract showed higher levels of gallic acid (49.71 ± 0.03 mg/g), chlorogenic acid (70.86 ± 0.02 mg/g), caffeic acid (146.08 ± 0.02 mg/g), ellagic acid (69.82 ± 0.01 mg/g), rutin (69.82 ± 0.01 mg/g), isoquercitrin (62.76 ± 0.02 mg/g), and kaempferol (49.32 ± 0.03 mg/g) compared to P. angulata. Quercitrin (26.37 ± 0.01 mg/g) and quercetin (26.51 mg/g) were significantly lower in N. laevis compared to P. angulata (42.91 ± 0.02 and 56.74 ± 0.02 mg/g), respectively (). The phenolic composition of P. angulata and N. laevis extracts revealed that both plants contain appreciable levels of flavonoids and phenolic acids. Several reports have shown that these compounds have multiple biological effects and play a critical role in the treatment and management of degenerative diseases such as diabetes, cancer, cardiovascular, and Alzheimer’s disease.[Citation26–Citation29] Moreover, some flavonoid-rich foods have been reported to be effective in the treatment of ED.[Citation30] The presence of flavonoids and phenolic acids identified in P. angulata and N. laevis extracts could be linked to the reported biological activities and also could serve as nutraceticals with great potentials in improving erectile function.
Table 1. Phenolic composition of Physalis angulata and Newbouldia laevis leaf extracts (mg/g).
Figure 1. (A) Representative high-performance liquid chromatography profile of Physalis angulata extract. Gallic acid (peak 1), chorogenic acid (peak 2), caffeic acid (peak 3), ellagic acid (peak 4), rutin (peak 5), quercitrin (peak 6), isoquercitrin (peak 7), quercitrin (peak 8), and kaempferol (peak 9). (B) Representative high-performance liquid chromatography profile of Newbouldia laevis leaf extract. Gallic acid (peak 1), catechin (peak 2), chorogenic acid (peak 3), caffeic acid (peak 4), ellagic acid (peak 5), epicatechin (peak 6), rutin (peak 7), quercitrin (peak 8), isoquercitrin (peak 9), quercitrin (peak 10), and kaempferol (peak 11).
PDE-5′ inhibition assay
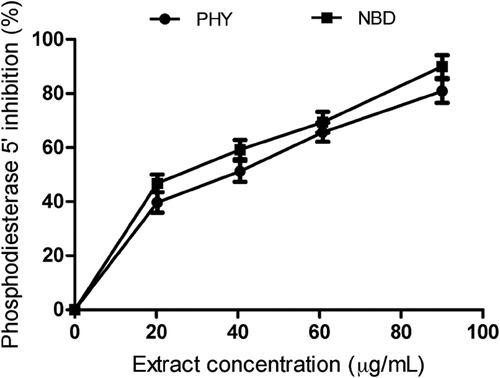
The effect of P. angulata and N. laevis extracts on PDE-5′ activity was investigated at different concentrations (20.27–90.10 µg/mL). N. laevis extract had the highest inhibitory effect with IC50 value of 44.25 ± 1.54 µg/mL compared to P. angulata extracts (IC50 = 48.98 ± 1.23 µg/mL) ( and ). Sildenafil citrate (PDE-5′ inhibitor) showed higher PDE-5′ inhibitory effect than the extracts (). PDE-5′ is localized in the corpus cavernosum of penile tissues and plays a major role in penile detumescence, inhibition of platelet, and vasodilation.[Citation31] The observed inhibition of PDE-5′ activities by N. laevis and P. angulata extracts suggests that these plants contain bioactive compounds with promising potentials for the management and treatment of ED, as this could increase cellular concentrations of cyclic-guanosine monophosphate in penile tissues thereby enhancing vasodilation and smooth muscle relaxation.
Table 2. IC50 values for the inhibition of PDE-5′ Arginase, AChE, and ACE activities, as well as DPPH, OH radical scavenging, and Fe2+ chelating capacities of Physalis angulata and Newbouldia laevis leaf extracts.
Arginase inhibitory activity
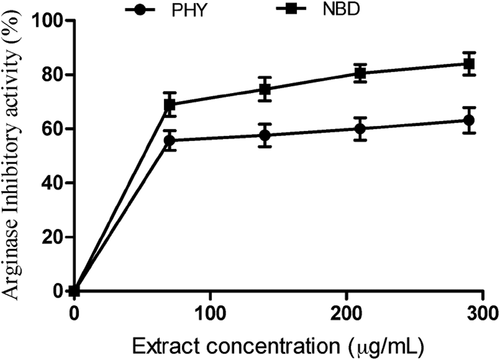
revealed arginase inhibitory activities of P. angulata and N. laevis extracts in vitro. Nevertheless, N. laevis (IC50 = 134.92 ± 3.23 µg/mL) exhibited better inhibitory effect on arginase activities compared to P. angulata (IC50 = 177.92 ± 4.51 µg/mL) (). Nѡ-hydroxy-l-arginine (IC50 = 0.75 ± 0.20 µg/mL) exhibited significantly higher inhibitory activity on arginase compared to N. Laevis and P. angulata (). Recent investigations have identified arginase as a biomarker for the treatment of sexual dysfunction in men.[Citation32,Citation33] There is upregulation of arginase activity and low nitric oxide (NO) levels in the corpus cavernosa tissues of ED patients. This condition has been associated with reduced vasorelaxation of smooth muscles and impaired erection.[Citation34] However, inhibition of arginase improves NO biosynthesis, smooth muscle relaxation and restores penile erection in ED patients.[Citation35] This result agrees with the report of Kim et al.[Citation32] which revealed that Scutellaria indica inhibited arginase activities in vitro. Moreover, functional foods have been shown to be effective in the treatment of ED.[Citation1] Oboh et al.[Citation35] established the link between the inhibition of arginase activity exhibited by Moringa oleifera leaves and their phenolic constituents. Moreover, structure–function relationship has shown that quercitrin and quercetin which were identified in P. angulata and N. laevis extracts are potent inhibitors of arginase activity and their inhibitory effect was attributed to their hydrophobic interactions with the active site of the enzyme, thereby increasing the pool of arginine and biosynthesis of NO.[Citation36]
AChE inhibitory activity
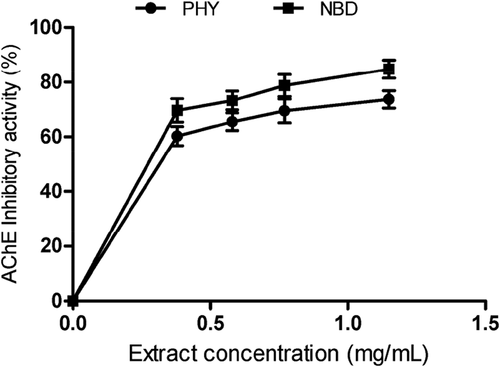
P. angulata and N. laevis extracts showed inhibitory effects on AChE activity in a dose-dependent manner as illustrated in . Moreover, the IC50 values in revealed that there was no significant difference (P > 0.05) between the inhibitory effects of P. angulata (0.60 ± 0.04 mg/mL) and N. laevis (0.53 ± 0.05 mg/mL) extracts on AChE activity. The AChE inhibitory activity of the extracts was significantly lower than prostigmine (positive control) as shown in . Furthermore, the release of NO in the corpus cavernosum tissue has been shown to be dependent on cholinergic nerves.[Citation37] Previous research have demonstrated that cholinergic nerves are present in the penile tissues and are capable of releasing acetylcholine, which binds to muscarinic receptors and stimulate NO synthesis via activation of endothelial NO synthase.[Citation13] In , we observed that P. angulata and N. laevis extracts reduced AChE activity which can increase the pool of acetylcholine in penile tissues. Our findings revealed that the use of P. angulata and N. laevis may be a novel approach to alleviate psychogenic ED as cerebral impulse has been reported to initiate the release of NO and acetylcholine.[Citation38]
ACE activity
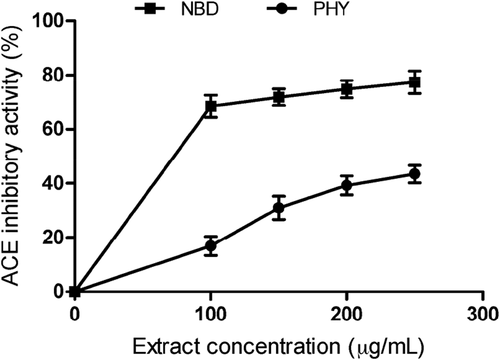
Angiotensin II (Ang II) is a peptide which stimulates smooth muscle contraction and contributes to the development of ED.[Citation39,Citation40] This peptide is formed in the renin–angiotensin systemic pathway in a reaction catalyzed by ACE.[Citation41] High levels of Ang II have been observed in the corpus cavernosum of ED patients.[Citation42] Previous reports have also shown that drugs that can reduce Ang II levels via inhibition of ACE activity could improve erectile function.[Citation13,Citation43] reveals the effect of the extracts on ACE activity. P. angulata and N. laevis reduced ACE activity. The IC50 in revealed that P. angulata extract (269.69 ± 2.30 µg/mL) had significantly (P < 0.05) lower inhibitory effect on ACE activity compared to N. laevis (130.00 ± 3.11 µg/mL). However, lisinopril (IC50 = 0.22 ± 0.03 µg/mL) exhibited significantly (P < 0.05) higher inhibitory activity than the extracts (). This result suggests that the extracts may contain potent ACE inhibitors that are capable of improving erectile response as well as sexual satisfaction in ED patients.
Antioxidant activity
Evidence from previous studies has revealed that oxidative stress mediated through free radicals may impair cavernosal function in ED patients.[Citation44] Free radicals especially superoxide react with NO to form radicals such as peroxynitrite thereby impairing transmission of NO and smooth muscle relaxation which could lead to penile flaccidity.[Citation45–Citation47] However, antioxidants have been reported to play an important role in erectile function due to their ability to mop up free radicals and chelate transition metals.[Citation48,Citation49] In this study, the capacities of P. angulata and N. laevis to scavenge free radicals and chelate Fe2+ were determined. Interestingly, both extracts scavenged DPPH and OH radicals and were able to chelate Fe2+ in a dose-dependent manner. The IC50 values in showed that the highest DPPH radical scavenging activity was exhibited by N. laevis (2.66 ± 0.10 mg/mL). P angulata showed an IC50 of 3.62 ± 0.25 mg/mL. Both extracts also scavenged OH radicals and prevented the degradation of deoxyribose. The result obtained in revealed that there was no significant (P > 0.05) difference between the OH radical scavenging activity of P angulata (0.52 ± 0.04 mg/mL) and N. laevis (0.42 ± 0.07 mg/mL), while P. angulata extract exhibited a lower Fe chelating ability (). The observed OH radical scavenging and metal chelating capacities of the extracts may prevent oxidative stress and protect the penile tissues against oxidative damage induced by-products of lipid peroxidation. Fe has been implicated as a catalyst and mediator in the reaction involving peroxyl radicals and lipid molecules which triggers a chain reaction leading to lipid peroxidation.[Citation44]
Conclusion
This study revealed that P. angulata and N. laevis leaves contain appreciable levels of phenolic acids and flavonoids particularly chlorogenic acid, caffeic acid, epicatechin, ellagic acid, rutin, and quercetin. The aqueous extracts showed inhibitory effects on PDE-5′, arginase, AChE, and ACE activities and exhibited radical scavenging and metal chelating activities which could be linked to their phenolic constituents. The phenolic composition, enzyme inhibitory properties, and antioxidant activities of these plants suggest their potentials as functional foods and/or nutraceuticals for the management of ED. However, aqueous extract of N. laevis exhibited higher potentials to improve erectile function compared to P. angulata.
References
- Ho, C. C.; Tan, H. M. Rise of Herbal and Traditional Medicine in Erectile Dysfunction Management. Current Urology Reports 2011, 12, 470–478. DOI: 10.1007/s11934-011-0217-x.
- Zhang, Q.; Cui, H. Simultaneous Determination of Quercetin, Kaempferol, and Isorhamnetin in Phytopharmaceuticals of Hippophaerhamnoides L. By High-Performance Liquid Chromatography with Chemiluminescence Detection. Journal of Separation Science 2005, 28, 1171–1178. DOI: 10.1002/(ISSN)1615-9314.
- Medina-Medrano, J. R.; Almaraz-Abarca, N.; González-Elizondo, M. S.; Uribe-Soto, J. N.; González-Valdez, L. S.; Herrera-Arrieta, Y. Phenolic Constituents and Antioxidant Properties of Five Wild Species of Physalis (Solanaceae). Botanical Studies 2015, 56, 24. DOI: 10.1186/s40529-015-0101-y.
- Kusumaningtyasa, R. W.; Lailya, N.; Limandha, P. Potential of Ciplukan (Physalis Angulata L.) As Source of Functional Ingredients. Procedia Chemistry 2015, 14, 367–372. DOI: 10.1016/j.proche.2015.03.050.
- Usman, H.; Osuji, J. C. Phytochemical and in Vitro Antimicrobial Assay of the Leaf Extract of Newbouldia Laevis. Africa Journal of Traditional Complementary and Alternative Medicine 2007, 4, 476–480. DOI: 10.4314/ajtcam.v4i4.31240.
- Mahalakshmi, A. M.; Ramesh, B. N. Physalis Angulata, L. A Ethanopharmacological Review. Indo American Journal Pharmaceutical Research 2014, 4(3), 1479–1486.
- Adnyana, I. K.; Yulinah, E.; Maeistuti, N.; Setiawan, F. Evaluation of Ethanolic Extracts of Mullaca (Physalis Angulata L.) Herbs for Treatment of Lupus Disease in Mice Induced Pristane. Procedia Chemistry 2014, 13, 186–193. DOI: 10.1016/j.proche.2014.12.025.
- Tankeo, S. B.; Tane, P.; Kuete, V. In Vitro Antibacterial and Antibiotic Potentiation Activities of the Methanol Extracts from Beilschmiedia Acuta, Clausena Anisata, Newbouldia Laevis and Polyscias Fulva against Multidrug-Resistant Gram Negative Bacteria. BMC Complementary and Alternative Medicine 2015, 15, 412. DOI: 10.1186/s12906-015-0944-5.
- Huang, S. A.; Lie, J. D. Phosphodiesterase-5 (PDE5) Inhibitors in the Management of Erectile Dysfunction. Pharmacy and Therapeutics 2013, 38(7), 407–419.
- Ayta, I. A.; McKinlay, J. B.; Krane, R. J. The Likely Worldwide Increase in Erectile Dysfunction between 1995 and 2025 and Some Possible Policy Consequences. BJU international 1999, 84, 50–56. DOI: 10.1046/j.1464-410x.1999.00142.x.
- Kandeel, F. R.; Koussa, V. K.; Swerdloff, R. S. Male Sexual Function and Its Disorders: Physiology, Pathophysiology, Clinical Investigation, and Treatment. Endocrine Reviews 2001, 22(3), 342–388. DOI: 10.1210/edrv.22.3.0430.
- Selvin, E.; Burnett, A. L.; Platz, E. A. Prevalence and Risk Factors for Erectile Dysfunction in the U.S. American Journal of Medicine 2007, 120, 151–157. DOI: 10.1016/j.amjmed.2006.06.010.
- Andersson, K. E.;. Mechanisms of Penile Erection and Basis for Pharmacological Treatment of Erectile Dysfunction. Pharmacological Review 2011, 63(4), 811–859. DOI: 10.1124/pr.111.004515.
- Lorenzen, J. M.; Ückert, S.; Scheller, F.; Haller, H.; Kuczyk, M. A. Effects of Arginase Inhibitors on the Contractile and Relaxant Responses of Isolated Human Penile Erectile Tissue. World Journal of Urology 2009, 27, 805–810. DOI: 10.1007/s00345-009-0405-1.
- Corbin, J. D.;. Mechanisms of Action of PDE5 Inhibition in Erectile Dysfunction. International Journal of Impotence Research 2004, 16, S4–S7. DOI: 10.1038/sj.ijir.3901205.
- Bivalacqua, T. J.; Burnett, A. L.; Hellstrom, W. J.; Champion, H. C. Overexpression of Arginase in the Aged Mouse Penis Impairs Erectile Function and Decreases eNOS Activity: Influence of in Vivo Gene Therapy of Anti-Arginase. American Journal of Physiology and Heart Circulatory Physiology 2007, 292, H1340–H1351. DOI: 10.1152/ajpheart.00121.2005.
- Akomolafe, S. F.; Oboh, G.; Oyeleye, S. I.; Boligon, A. A. Aqueous Extract from Ficus Capensis Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Prevent Oxidative Stress in Rats’ Penile Tissue. NFS Journal 2016, 4, 15–21. DOI: 10.1016/j.nfs.2016.06.001.
- Kelly, S. J.; Butler, L. G. Enzymic Hydrolysis of Phosphonate Esters. Reaction Mechanism of Intestinal 5′-Nucleotide Phosphodiesterase. Biochemistry. 1977, 16, 1102–1104. DOI: 10.1021/bi00625a011.
- Kaysen, G.; Strecker, H. J. Purification and Properties of Arginase of Rat Kidney. Biochemical Journal 1973, 133(4), 779–788. DOI: 10.1042/bj1330779.
- Perry, N. S.; Houghton, P. J.; Theobald, A.; Jenner, P.; Perry, E. K. In-Vitro Inhibition of Human Erythrocyte Acetylcholinesterase by Salvia Lavandulaefolia Essential Oil and Constituent Terpenes. Journal of Pharm. Pharmacology 2000, 52(7), 895–902. DOI: 10.1211/0022357001774598.
- Ademiluyi, A. O.; Oyeleye, S. I.; Oboh, G. Biological Activities, Antioxidant Properties and Phytoconstituents of Essential Oil from Sweet Basil (Ocimum Basilicum L.) Leaves. Comparative Clinical Pathology 2016, 25, 169–176. DOI: 10.1007/s00580-015-2163-3.
- Gyamfi, M. A.; Yonamine, M.; Aniya, Y. Free-Radical Scavenging Action of Medicinal Herbs from Ghana: Thonningiasanguinea on Experimentally Induced Liver Injuries. General Pharmacology 1999, 32, 661–667. DOI: 10.1016/S0306-3623(98)00238-9.
- Halliwell, B.; Gutteridge, J. M. C. Formation of a Thiobarbituric-Acid-Reactive Substance from Deoxyribose in the Presence of Iron Salts. FEBS Letters 1981, 128, 347–352. DOI: 10.1016/0014-5793(81)80114-7.
- Minotti, G.; Aust, S. D. An Investigation into the Mechanism of Citrate Fe2+-Dependent Lipid Peroxidation. Free Radical Biology and Medicine 1987, 3, 379–387. DOI: 10.1016/0891-5849(87)90016-5.
- Puntel, R. L.; Nogueira, C. W.; Rocha, J. B. T. Krebs Cycle Intermediates Modulate Thiobarbituric Acid Reactive Species (TBARS) Production in Rat Brain in Vitro. Neurochemical Research 2005, 30, 225–235. DOI: 10.1007/s11064-004-2445-7.
- Rangel-Huerta, O. D.; Pastor-Villaescusa, B.; Aguilera, C. M.; Gi, A.; Systematic, A. Review of the Efficacy of Bioactive Compounds in Cardiovascular Disease: Phenolic Compounds. Nutrients 2015, 7, 5177–5216. DOI: 10.3390/nu7075177.
- Ravishankar, D.; Rajora, A. K.; Greco, F.; Osborn, H. M. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. International Journal of Biochemistry and Cell Biology 2013, 45, 2821–2831. DOI: 10.1016/j.biocel.2013.10.004.
- Shodehinde, S. A.; Adefegha, S. A.; Oboh, G.; Oyeleye, S. I.; Olasehinde, T. A.; Nwanna, E. E.; Adedayo, B. C.; Boligon, A. A. Phenolic Composition and Evaluation of Methanol and Aqueous Extracts of Bitter Gourd (Momordica Charantia L) Leaves on Angiotensin-I-Converting Enzyme and Some Pro-Oxidant-Induced Lipid Peroxidation in Vitro. Journal of Evidence-Based Complementary and Alternative Medicine 2016, 21, NP67–76. DOI: 10.1177/2156587216636505.
- Oboh, G.; Nwanna, E. E.; Oyeleye, S. I.; Olasehinde, T. A.; Ogunsuyi, O. B.; Boligon, A. A. In Vitro Neuroprotective Potentials of Aqueous and Methanol Extracts from Heinsia Crinita Leaves. Food Science and Human Wellness 2016, 5, 95–102. DOI: 10.1016/j.fshw.2016.03.001.
- Cassidy, A.; Franz, M.; Rimm, E. B. Dietary Flavonoid Intake and Incidence of Erectile Dysfunction. American Journal of Clinical Nutrition 2016, 103, 534–541. DOI: 10.3945/ajcn.115.122010.
- Rosen, R. C.; Kostis, J. B. Overview of Phosphodiesterase 5 Inhibition in Erectile Dysfunction. The American Journal of Cardiology 2003, 92, 9–18. DOI: 10.1016/S0002-9149(03)00824-5.
- Kim, S. W.; Cuong, T. D.; Hung, T. M.; Ryoo, S.; Lee, J. H.; Min, B. S. Arginase II Inhibitory Activity of Flavonoid Compounds from Scutellaria Indica. Archives Pharmacal Research 2013, 36, 922–926. DOI: 10.1007/s12272-013-0125-3.
- Segal, R.; Hannan, J. L.; Liu, X.; Kutlu, O.; Burnett, A. L.; Champion, H. C.; Kim, J. H.; Steppan, D.; Berkowitz, D. E.; Bivalacqua, T. J. Chronic Oral Administration of the Arginase Inhibitor 2(S)-Amino-6-Boronohexanoic Acid (ABH) Improves Erectile Function in Aged Rats. Journal of Andrology 2012, 33, 1169–1175. DOI: 10.2164/jandrol.111.015834.
- Sakai, Y.; Masuda, H.; Kihara, K.; Kurosaki, E.; Yamauchi, Y.; Azuma, H. Involvement of Increased Arginase Activity in Impaired Cavernous Relaxation with Aging in the Rabbit. Journal of Urology 2004, 172, 369–373. DOI: 10.1097/01.ju.0000121691.06417.40.
- Oboh, G.; Ademiluyi, A. O.; Ademosun, A. O.; Olasehinde, T. A.; Oyeleye, S. I.; Boligon, A. A.; Athayde, M. L. Phenolic Extract from Moringa Oleifera Leaves Inhibits Key Enzymes Linked to Erectile Dysfunction and Oxidative Stress in Rats’ Penile Tissues. Biochemistry Research International 2015, 2015, 175950.
- Da Silva, E. R.; Maquiaveli, C. D. C.; Magalh˜ Aes, P. P. The Leishmanicidal Flavonols Quercetin and Quercitrin Target Leishmania (Leishmania) Amazonensis Arginase. Experimental Parasitology 2012, 130(3), 183–188. DOI: 10.1016/j.exppara.2012.01.015.
- Ayajiki, K.; Hayashida, H.; Tawa, M.; Okamura, T.; Toda, N. Characterization of Nitrergic Function in Monkey Penile Erection in Vivo and in Vitro. Hypertension Research 2009, 32, 685–689. DOI: 10.1038/hr.2009.84.
- Dean, R. C.; Lue, T. F. Physiology of Penile Erection and Pathophysiology of Erectile Dysfunction. Urology Clinics of North America 2005, 32(4), 379–395. DOI: 10.1016/j.ucl.2005.08.007.
- Fraga-Silva, R. A.; Montecucc, F.; Mach, F.; Santos, R. A.; Stergiopulos, N. Pathophysiological Role of the Renin–Angiotensin System on Erectile Dysfunction. European Journal Clinical Investigation 2013, 43(9), 978–985. DOI: 10.1111/eci.12117.
- Odubanjo, V. O.; Olasehinde, T. A.; Oyeleye, S. I.; Oboh, G.; Boligon, A. A. Seed Extracts from Myristica Fragrans (Nutmeg) and Moringa Oleifera (Drumstick Tree) Inhibits Enzymes Relevant to Erectile Dysfunction and Metal‐Induced Oxidative Damage in Rats’ Penile Tissues. Journal of Food Biochemistry 2018, 42.
- Oboh, G.; Olasehinde, T. A.; Ademosun, A. O. Inhibition of Enzymes Linked to Type-2 Diabetes and Hypertension by Essential Oils from Peels of Orange and Lemon. International Journal of Food Properties 2017, 20, 586–594. DOI: 10.1080/10942912.2017.1303709.
- El Melegy, N. T.; Ali, M. E.; Awad, E. M. Plasma Levels of Endothelin-1, Angiotensin II, Nitric Oxide and Prostaglandin E in the Venous and Cavernosal Blood of Patients with Erectile Dysfunction. BJU International 2005, 96, 1079–1086. DOI: 10.1111/j.1464-410X.2005.05780.x.
- Fogari,R.; Zoppi, A.; Poletti, L.; Marasi, G.; Mugellini, A.; Corradi, L. Sexual Activity in Hypertensive Men Treated with Valsartan or Carvedilol: A Crossover Study. American Journal of Hypertension 2001, 14(1), 27–31. DOI: 10.1016/S0895-7061(00)01214-0.
- Agarwal, A.; Virk, G.; Ong, C.; Plessis, S. Effect of Oxidative Stress on Male Reproduction. World Journal Mens Health 2014, 32, 1–17. DOI: 10.5534/wjmh.2014.32.1.1.
- Minhas, S.; Jeremy, J. Y.; Jones, R. A.; Ralph, D.; Rees, R. W.; Persad, R. A. Oxygen Free Radicals and the Penis. Experts opinion on pharmacotherapy 2002, 3, 889–897. DOI: 10.1517/14656566.3.7.889.
- Masood, A. K.; Thompson, C. S.; Mumtaz, F. H.; Mikhailidis, D. P.; Morgan, R. J.; Bruckdorfer, R. K.; Naseem, K. M. The Effect of Nitric Oxide and Peroxynitrite on Rabbit Cavernosal Smooth Muscle Relaxation. World Journal of Urology 2001, 19, 220–224. DOI: 10.1007/s003450000162.
- Ertemi, H.; Mumtaz, F. H.; Howie, A. J.; Mikhailidis, D. P.; Thompson, C. S. Effect of Angiotensin II and Its Receptor Antagonists on Human Corpus Cavernous Contractility and Oxidative Stress: Modulation of Nitric Oxide Mediated Relaxation. The Journal of Urology 2011, 185, 2414–2420. DOI: 10.1016/j.juro.2011.02.2645.
- Azadzoi, K. M.; Schulmanmi, R. N.; Aviram, M.; Siroky, M. B. Oxidative Stress in Arteriogenic Erectile Dysfunction: Prophylactic Role of Antioxidants. The Journal of Urology 2005, 174, 386–393. DOI: 10.1097/01.ju.0000161209.39959.67.
- Oboh, G.; Ademiluyi, A. O.; Oyeleye, S. I.; Olasehinde, T. A.; Boligon, A. A. Modulation of Some Markers of Erectile Dysfunction and Malonaldehyde Levels in Isolated Rat Penile Tissue with Unripe and Ripe Plantain Peels: Identification of the Constituents of the Plants Using HPLC. Pharmaceutical biology 2017, 55, 1920–1926. DOI: 10.1080/13880209.2017.1340966.