ABSTRACT
The volatile aromatic components in cow’s or sheep’s milk Kashk samples collected from 11 regions of Iran were extracted by solid-phase micro-extraction and analyzed by GC/MS. Alkenes, aldehydes, free fatty acids, esters, terpenes, alcohols, sulfur compounds, and ketones were the most frequently used compounds in samples. Same volatile compounds were identified in sheep’s and cows’ milk Kashk, whereas the numbers of compounds were different. The results from principle component analysis (PCA), performed to distinguish flavor from different regions, showed that Kashk samples are divided into three groups, in which flavor of some regions in two groups is affected by ingredients.
Introduction
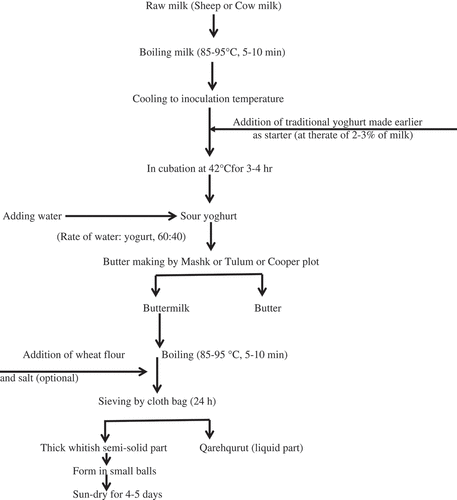
Fermented milk product originated from the raw milk will spoil after storage because of microbial action. A vast number of variations have changed the development of fermented dairy products. These variations include species of animals, heat treatment of raw milk, percentage of fat in the milk, fermentation temperature, and inoculation percentage.[Citation1] The request for traditional homemade food has improved in recent years and it has led to the growth of protected label of the origin (PDO). In fact, PDO is used for foods, which are manufactured in a defined geographical area with distinctive natural characteristic.[Citation2] Iran has many PDO products like Tarkhineh,[Citation3] Doogh,[Citation4] Siahmazgi cheese,[Citation5] Lighvan cheese,[Citation6] and dried Kashk. The Kashk is also produced in other countries under different names, Kishk (Lebanon, Syria), Zhum (Yemen), Kushuk (Iraq), Tarhana (Turkey), Kurut (Turkey).[Citation7] The dried Kashk has been produced in a wide geographical area especially in rural parts of Iran for many years. Dried Kashk is produced from cow’s and/or sheep’s milk but mostly produced from sheep. First the raw milk is boiled and then cooled and inoculated with traditional yoghurt made earlier as starter culture. The butter is isolated from sour yoghurt by Mashk, which is made from hide (sheepskin) and is used for butter making[Citation8] or Tulum (is a traditional device, which is made from wood of tree and depleted inside of tree) or cooper plot. Then buttermilk is boiled and sieved by cloth bag. Finally the thick whitish semi-solid part of buttermilk, which is sieved, is shaped in form of conic or cubic balls and then sun-dried for 3–4 days (). Furthermore, wheat meal or burghol (parboiled cracked wheat) is added, salt or some herb is optional according to issues of Iranian National Standards Organization.[Citation9] Traditional Kashk is dried, round or globular, cubic shape, white, creamy, and dirty yellow in color, and its scent is distinctive with salty and Umami like taste. Dried Kashk is a self-stable product mainly due to the low aw and high salt content and shelf life is about 4 years. Traditional dried Kashk can be consumed as fresh or used as a raw material for the production of liquid Kashk. Gocmen et al.[Citation10] reported that the method of drying had a significant effect on the number of active aroma compounds in Tarhana. They revealed that vacuum drying resulted in more aroma compounds in Tarhana than sun drying. Aldehydes like benzaldehyde, octanal, phenylacetaldehyde, E-2-Octenal were the greatest group of aroma compounds in both types of Tarhana, while the two methods of drying were differentiated in aromas, such as alcohols (geraniol), terpenes (terpinolene), and phenols (4-vinylguaiacol). In addition, the flavors of various cheeses were studied like Spanish soft cheese, which had acids, esters, ketones, alcohols, and aldehydes;[Citation11] Sicilian goat cheese like the former had alcohols, esters, ketones, and terpenes.[Citation12] Cheese flavor depends on proteolysis, lipolysis, and metabolism of lactose and citrate, largely due to enzymes from milk and microorganisms.[Citation2]
Volatile compounds are analyzed by gas chromatography–mass spectrometry (GC-MS). The technique of solid-phase micro-extraction (SPME) is used by small amount of samples and permits the isolation of volatile compounds from both in the solid and liquid matrices in a short time, and also in a simple way.[Citation11] There are no published studies on volatile compounds of traditional dried Kashk, though some studies have focused on contamination with Aflatoxin M1[Citation13] and chemical composition.[Citation4] Like other products, Kashk flavor is one of the most important criteria for determining consumer’s choice and acceptance. It was assumed that Kashk flavor may differ based on region, milk (cow or sheep’s milk), production producers (Mashk, Tulum, and cooper plot), and addition of wheat flour. The objective of this study was to characterize the volatile compounds in dried Kashk in different parts of Iran, which are produced from cow or sheep’s milk via different production producers by using of SPME/GC-MS technique.
Materials and methods
Sample collection
Dried Kashk samples were collected from 11 regions of Iran, including Kerman, Natanz (Esfahan), Birjand, Chalus, Ardebil, Najafabad (near to Esfahan), Shiraz, Quchan, Paveh (Kermanshah), Yazd, and Mashhad between July and August 2016. The collection of samples was done from different regions, produced by various apparatus (Mashk, Tulum, and cooper plot), originated from sheep or cow’s milk and with or without added wheat flour. Characteristic of different dried Kashk are showed in . The ages of the Kashk were between 3 and 6 months old. Samples were kept in sterile plastic bags and stored at 5°C until analysis.
Table 1. Characteristic of Kashk samples.
SPME-GC/MS
The volatile compounds of dried Kashk were extracted by SPME technique and analyzed by GC-MS. This technique is solvent free, cheap, easy to use, relatively fast, and needs a low volume of sample and also is sensitive enough for quality control purposes.[Citation14] The extraction was done according to Erkaya and Sengul[Citation15] with slight modification. For analysis of volatile compounds, 3 g of samples were grinned and placed in 20 mL vial and then sealed. The samples were heated and stirred for 10 min at 80°C to accelerate extraction equilibrium the headspace for volatile compounds in the Kashk matrix. Then volatile compounds extraction was carried out by inserting a 65 µm Divinylbenzene/Polydimethylsiloxane SPME fiber (Supelco, Bllefonte, PA, USA) into the vial and exposing to the headspace for 20 min at 80°C. A GC (Agilent 7890N) coupled into a mass selective detector (Agilent, 9575 C) was used to analyze the sample headspace components. The volatile compounds were desorbed by inserting the fiber into the injection port, which remained in the injector for 2 min at a temperature of 270°C, while helium was used as carrier gas at a flow rate of 1.0 mL/min. Temperature program was done according to Hayaloglu[Citation16] method with some modification. After the inserting the fiber, the oven temperature was held at 40°C for 6 min, and then increased at 4°C/min to 70°C, where it was held for 4 min. The temperature was then raised at 10°C/min to 250°C and was held for 10 min. Volatile compounds were separated by using a capillary column (30 m × 0.25 mm × 0.25 µm film thickness, Agilent).
Statistical analysis
All tests were carried out in duplicate and analyzed by SPSS statistical software program version 20. Mean values of volatile compound in different samples were compared with ANOVA and Duncan’s test with significance level of p < 0.05. Principle component analysis (PCA) and Hierarchical cluster analysis (HCA) were performed to test for significant differences between samples. PCA and HCA were carried out by using SPSS statistical program version 20.
Results and discussion
The most common volatile compounds in dried Kashk samples have been presented in . The numbers of volatile compounds that were detected in samples were quite different. In the samples 1–11, the numbers of detected compounds were 56, 53, 68, 67, 73, 34, 65, 30, 28, 58, and 70, respectively. Various alkanes, alcohols, aldehyds, alkenes, esters, FFAs, sulfur compounds, ketones, and terpenes were detected in dried Kashk, where the most frequent volatile compounds in samples were as follow: two sulfur compounds (carbon disulfide, disulfide dimethyl), two alkanes (tetradecane, hexadecane), one aldehyde (nonanal), one terpene (dl-limonene), and methyl naphthalene.
Table 2. Volatile compounds in kashk samples (The results were expressed as means of peak area% from duplicate analysis of each sample).
Alkanes
Due to high temperature used in Kashk production, alkanes were one of the most frequent compounds, which were detected in all samples and in most samples of Kashk from sheep’s milk showed higher concentration of these compounds than Kashk from cow’s milk. Erkaya and Sengul[Citation15] reported that they found alkanes like heptane, octane, decane, nonane, and undecane in all yoghurts, which are made from cow, buffalo, ewe, and goat’s milks, where the heptane had the highest concentration. Some of alkanes are most certainly produced by Staphylococcus xylosus, e.g. diacetyl and ethanol, while others are more likely to arise from lipid autoxidation, e.g. hexanal.[Citation17] The alkane compounds may rise in high temperature conditions by hemolytic cleavage of carbon–carbon bonds along the fatty acid chains.[Citation18] Therefore, it was the hypothesis that boiling milk and buttermilk may contribute in increasing of alkanes in samples. The contribution of alkanes to flavor is almost irrelevant due to their high thresholds.[Citation19] Tetradecane and hexadecane were found in most samples where the former had higher concentration in most samples.
Aldehydes
Aldehydes were detected in all samples and the numbers of these compounds were not affected by the type of milk and procedures, while they were higher in samples containing flour. Aldehydes can be formed directly from path of Millard (Amadori or Heyns) especially in thermally treated foodstuffs.[Citation20] Furthermore, aldehydes are the main products of autoxidation of unsaturated fatty acids: autoxidation proceeds via hydroperoxides, which in turn undergoes further degradation to hydrocarbons, alcohols, and carbonyl compounds and are characterized by green grass–like and herbaceous aromas.[Citation2] As far as using heat in producers of Kashk production, Millard reaction may occur. In addition, we found various unsaturated fatty acids in Kashk samples (data not shown). On the other hand, branched-chain amino acids (Leu, Ile, Val) are converted into specific aldehydes with malty flavors by Lactococcus and Lactobacillus.[Citation21] Furthermore, we isolated different Lactobacillus strains from Kashk precursor, buttermilk.[Citation8] Sample 5 had higher concentration of most aldehyde components than the others; it may be because of higher unsaturated fatty acids that were detected in this sample (data not shown). Nonanal, which has a threshold of 1 µg/kg,[Citation22] was found in all samples and most samples of Kashk that were produced from sheep’s milk had higher amount of this compound than those from cow’s milk. This compound may be produced by β-oxidation of unsaturated fatty acids.[Citation23] In addition, benzaldehyde was the one of the abundant compound, which was found in the Kashk from sheep’s milk but not in samples from cow’s milk. Benzaldehyde was one of the products, which is produced when milk is heated at 82°C for 30 minCitation24 or by oxidation of cinnamic acid or phenylacetaldehyde.[Citation25] Numerous studies reported that benzaldehyde has a bitter almond or sweet aroma,[Citation26] whereas presence of this component has also been reported in many cheeses.[Citation26–Citation29]
Free fatty acids
These important components of flavor were found in higher concentration in Kashk samples produced in Tulum. FFA originates primarily from the lipolysis of the milk fat. According to Urbach,[Citation30] the FFA which is produced by lipase originates more from the milk than from the lactic acid bacteria, meanwhile the milk fat must be partially hydrolyzed before the starter culture lipases become effective. It has been reported that the activity of lipase reduces dramatically at 100°C. Kashk production procedure includes two steps of boiling for milk, as well as the buttermilk. Therefore, it was assumed that FFA in the final product, dried Kashk, may originate from bacterial lipase activity than the activity of lipase originated from the milk. The sharpness of rancid flavor in Kashk originated from FFA is minimized by the formation of ethyl ester–like decanoic acid ethyl ester, dodecanoic acid ethyl ester.[Citation31] Decanoic acid showed the highest area value in 6 samples out of 11 while butanoic was the highest in sample 2. Furthermore, hexanoic, acid which gives a sharp tangy flavor was not detected in any cow’s milk Kashk. Different FFAs have been reported in various dairy products like cheese.[Citation32] Nishimoto et al.[Citation33] showed that lactic acid bacteria convert hippuric acid to benzoic acid. Benzoic acid that was found in two Kashk samples (3 and 11) may originate from activity of lactic acid bacteria in different steps of Kashk production procedures.
Esters
Low numbers of esters were identified in samples which were not affected by ingredients. Most esters have floral and fruity notes and may contribute to cheese aroma by minimizing the sharpness of fatty acids and the bitterness of amines.[Citation14] Some microorganisms produce ester but mainly yeasts are involved; however, some lactic acid bacteria, along with chemical reactions, can also be responsible for formation of these component.[Citation2] Biosynthesis of esters has two steps, first glycerides in milk fat are hydrolyzed by lipases and produce FFA and then esterification of FFA with an alcohol by esterase take place. It has been reported that esters may be produced in low water activity via esterification.[Citation34] In our Kashk samples with aw from 0.4 to 0.6, it was assumed that various esters, such as hexanoic acid, hexyl ester, decanoic acid and dodecanoic acid, ethyl ester may be produced by esterification. Ethyl esters like ethyl acetate and ethyl butanoate in semi-hard cheese[Citation35] and Kashar cheeseCitation16 have been reported previously.
Terpenes
Most terpenes identified were dl-limonene in all samples except No. 10 and gamma-terpinen in five samples. Similar to our results, Erkaya and Sengul[Citation15] showed that limonene was the highest terpenes in yogurt, which is made from cow, buffalo, ewe, and goat. Sample 6 had the highest concentration of terpenes compounds, which were produced from cow’s milk. Terpene content in dairy products is influenced by the feed given to the herd and especially by grazed herbage.[Citation36] Many terpenes exhibit characteristic odors and tastes, like in essential oils, fruits, vegetables, and culinary herbs. Terpenes, especially limonene, can derive directly from cow’s fodder.[Citation37] However, terpenes in milk and cheese have not been described among the characters impacting compounds.[Citation38] In addition, terpenes were identified in the volatile fraction of Kashar cheeses include α-pinene and ρ-cymene, which were the principal terpenes in the samples.[Citation16]
Sulfur compounds
Sulfur compounds like carbon disulfide were detected in 75% of samples, which are potent odorants in many fermented and ripened foods, contribute to flavors, and they are produced from the sulfur-containing amino acids like Met and Cys.[Citation21] We detected amino acids like Met in Kashk samples (data not published). Sulfur compounds also can be formed by the thermal degradation of protein and lipids.[Citation38] Sulfur compounds were detected in all samples except sample No. 8.
Ketones
Ketones are products of changes in the usual lipidic degradation by microbial pathways.[Citation2] Ketones were one of the lowest numbers of compounds detected in samples and just in sample 2 detected three ketones (acetone, hexanone, and nonanone). Nonanone was detected in four samples; this compound is produced by β-oxidation of FFA.[Citation39] Some research showed that ketone is one of the main compounds in butterCitation40,Citation41 and the concentration of compound is related to the amount of fat in the milk.[Citation42] Low numbers of samples contain ketones and the very low concentration of these compounds in Kashk samples were justified by the removal of most part of fat in our Kashk production procures.
Alcohols
Alcohols were identified in some samples and the numbers of these compounds were low. Alcohols play indirect role as flavorings. Furthermore, they play indirect roles as precursors for the preparation of other flavorings. For example, oxidation of alcohols may result in aldehydes. Many alcohols are the major metabolites of yeast fermentation.[Citation43] All samples contained low number of alcohols probably because of the lack of yeast fermentation.
Terpenoids
Camphor is found in two samples (No. 3 and 11), which are produced by Tulum (data not shown). Camphor is found in many aromatic plants as essential oil,[Citation44] so this volatile compound may originate from feed into milk and dairy products like Kashk.
Miscellaneous
Some other categories of compounds like benzene, toluene, furan, naphthalene, and dioxane were identified in some samples. Toluene may originate from the degradation of carotene.[Citation45] Xylene and ρ-Xylene, which are dried from feed, found in samples 4 and 9, respectively.[Citation23] Furthermore, different methyl naphthalene were detected in all samples except for No. 9. Menthol and maltol were detected only in one sample (No. 3 and 8, respectively). It has been reported that maltol may be produced by heating in high temperature.[Citation20]
PCA and HCA analysis
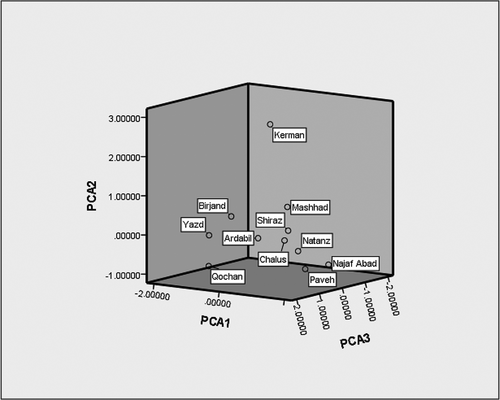
In order to found relation between the volatile compounds, PCA was applied, reveling separation and classification among three groups of compound. PCA can explain a new set of variable data in order to find how many components are necessary to show highest variance with a minimum loss of information. shows the results of KMO index and Bartlett’s sphericity test. According to data, KMO index is 0.750, whereas the significance of the Bartlett’s sphericity test was 0.000, i.e. lower than 0.05. These results confirmed the validity of extracted factors. Factor selection procedures based on eigenvalues carried out by the Kaiser–Guttmann rule (Kaiser Criterion) and the scree test. In this method, the component with eigenvalues greater than 1 is selected as PC. According to data, first three components have eigenvalues over 1.00, and the first principal component (PC1) had the highest eigenvalue of 4.179 and accounted for 46.437% of the variability in the data set. The second and third PC (PC2 and PC3) had eigenvalues of 1.971 and 1.337, and accounted for 21.899% and 14850% of the variance in the data. Together PC1, PC2, PC3 explain over 83.186% of the total variability in the data. The loading plot () established the relative importance of each group of volatile compounds. The scatter score plot of principle components showed the distinction among samples from different regions (). Combination of these two plots indicated the relationship between samples from various regions and their volatile compounds. The first component (PC1) was characterized by major levels of alkanes, aldeyhds, estesrs, sulphur components, and ketones while the alkanes and ketones were the highest by 0.931 and 0.906, respectively. These compound were most important in Natanz, Chalus, Ardebil, Najafabad, Shiraz, Paveh, and Mashhad. For the second principle component (PC2), the attribute alcohols and alkenes showed high and positive values. These compound were higher in Kerman. In addition, FFA was higher in third component (PC3) and was higher in Birjand, Quchan, and Yazd.
Table 3. KMO and Bartlett’s test.
Figure 2. Loading plot after principle components analysis of various componds by the three principle components (PC1, PC2, and PC3).
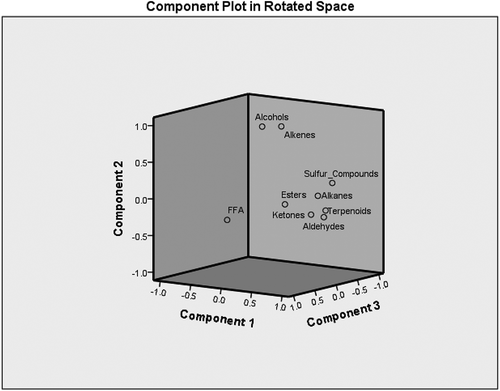
Figure 3. Score plot after principle component analysis of the individuals dried Kashk samples by the three principle components (PC1, PC2, and PC3).
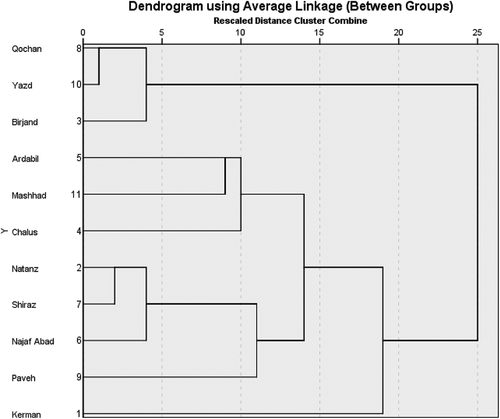
Furthermore, cluster analysis is a generic name for a wide range of exploratory multivariate techniques. It is used for classifying a data set in homogeneous groups The most frequent distinction between clustering techniques is whether the clusters come from a nested structure (hierarchical) or not (partitional). Results of agglomerative hierarchical clustering are represented graphically in a tree-like structure, which is called dendrogram. The dendrogram provides a simple way of visualizing the hierarchical structure of the clustering and the level at which each cluster is formed, as well as cluster. Atypical dendrogram of 11 samples is shown in . The results by HCA was similar to PCA and three main cluster can be identified by HCA. Kerman identified in cluster one and Natanz, Chalus, Ardebil, Najafabad, Shiraz, Paveh, and Mashhad were recognized as the most similar objects in the data set and were grouped in the cluster two. Birjand, Quchan, and Yazd were grouped in the cluster three, confirming their similarities.
Conclusion
Kashk that is produced from cow milk and sheep’ milk showed differences in their volatile profiles. As mentioned by this initial study, it is apparent that the volatile profile of Kashk represents a complexity of microbial, enzymatic, and spontaneous reactions. In industry liquid Kashk is more from dried Kashk, which is made from cow’s milk but this paper showed that samples with sheep’s milk had higher flavor compounds. GC-MS analysis by using the SPME technique showed that total 602 volatile compounds were detected from 11 traditional dried Kashk. In addition, aldehydes and alkenes were significantly more abundant in Kashk samples from sheep’s milk than cow’s milk. This study highlights that the aldehydes were higher level in samples that contained wheat and were not affected by types of milk, while FFAs were higher in Kashk samples that produced by Mashk and alkenes were higher in samples, which were made from sheep’s milk. PCA and HCA analysis have shown good separation of analyzed samples correlating with particular groups of volatile compounds. According to PCA and HCA, analysis identified three groups.
References
- Walstra, P.; Wouters, J. T. M.; Geurts, T. J. Dairy Science and Technology Taylor and Francis Group. CRC Press: Boca Raton. 2005, pp. 562.
- Gioacchini, A. M.; De Santi, M.; Guescini, M.; Brandi, G.; Stocchi, V. Characteristic of the Volatile Organic Compounds of Italian Fossa Cheese Y Solid Phase Microextraction Gas Chromatography/Mass Spectrometry. Rapid Communications in Mass Spectrometry 2010, 24, 3405–3412. DOI: 10.1002/rcm.4782.
- Vasiee, A. R.; Yazdi, F. T.; Mortazavi, A.; Edalatian, M. R. Isolation, Identification and Characterization of Probiotic Lactobacilli Spp. From Tarkhineh. International Food Research Journal 2014, 21, 2487–2492.
- Noori, A.; Keshavarzian, F.; Mahmoudi, S.; Yousefi, M.; Nateghi, L. Comparison of Traditional Doogh (Yogurt Drinking) and Kashk Characteristic (Two Traditional Iranian Dairy Products). Pelagia Research Library 2013, 3, 252–255.
- Farahani, G.; Ezzatpanah, H.; Abbasi, S. Characterization of Siahmazgi Cheese, an Iranian Ewes Milk Variety: Assessment of Physic-Chemical, Textural and Rheological Specifications during Ripening. LWT-Food science and Technology 2013, 58, 1–8.
- Lavasani, A. S.; Ehsani, M. R.; Mirdamadi, S.; Mousavi, M. A. E. Z. Changes in Physichochemical and Organoleptic Properties of Traditional Iranian Cheese Lighvan during Ripening. International Journal of Dairy Technology 2011, 65, 64–70.
- Tamime, A. Y.; O’Connor, T. P. Kishk-A Dried Fermented Milk/Cerial Mixture. International Dairy Journal 1994, 5, 109–128. DOI: 10.1016/0958-6946(95)92205-I.
- Iranmanesh, M.; Ezzatpanah, H.; Mojgani, N. Antibacterial Activity and Cholesterol Assimilation of Lactic Acid Bacteria Isolated from Traditional Iraninan Dairy Products. LWT-Food Science and Technology. Food science and technology 2013, 58, 1–5.
- Institute of Standards and Industrial Research of Iran. Karaj, Iran, Dried Kashk-Specification. 2005. ISIRI 1188.
- Gocmen, D.; Gurbuz, O.; Rouseff, R. L.; Smoot, J. M.; Dagdelen, A. F. Gas Chromatographic-Olfactometric Characterization of Aroma Active Compound in Sun-Dried and Vacuum-Dried Tarhana. European Food Research and Technology 2004, 218, 573–578. DOI: 10.1007/s00217-004-0913-6.
- Delgado, F. J.; Crespo, J. G.; Cave, R.; Parra, J. G.; Ramirez, R. Characterization by SPME-GC-MS of Volatile Profile of a Spanish Soft Cheese P.D.O. Torta Del Casar during Ripening. Food Chemistry 2009, 118, 182–189. DOI: 10.1016/j.foodchem.2009.04.081.
- Mondello, L.; Costa, R.; Tranchida, P. Q.; Chiofalo, B.; Zumbo, A.; Dugo, P.; Dugo, G. Determination of Flavor Components in Sicilian Goat Cheese by Automated HS-SPME-GC. Flavour and Fragrance Journal 2004, 20, 659–665. DOI: 10.1002/ffj.1529.
- Fallah, A. A.; Rahnama, M.; Jafari, T.; Saei-Dehkordi, S. S. Seasonal Variation of Aflatoxin M1 Contamination in Industrial and Traditional Iranian Dairy Products. Food Control 2011, 22, 1653–1656. DOI: 10.1016/j.foodcont.2011.03.024.
- Pinho, O.; Peres, C.; Ferreira, I. M. P. L. V. O. Solid Phase Microextraction of Volatile Compounds in Terrincho Ewe Cheese Comparison of Different Fibers. Journal of Chromatography 2003, 1–9. DOI: 10.1016/S0021-9673(03)01066-5.
- Erkaya, T.; Sengul, M. Comparison of Volatile Compounds in Yoghurt Made from Cows, Buffaloes, Ewes and Goats Milks. International Journal of Dairy Technology 2011, 240–246. DOI: 10.1111/j.1471-0307.2010.00655.x.
- Hayaloglu, A. A.;. Volatile Composition and Proteolysis in Traditionally Produced Mature Kashar Cheese. International Journal of Food Science and Technology 2009, 44, 1388–1394. DOI: 10.1111/j.1365-2621.2009.01968.x.
- Taylor, A. J.; Mottram, D. S. Flavour Science The royal society of chemistry, UK. 1996; pp. 142.
- Min, D. B.; Smouse, T. H. Flavor Chemistry of Lipid Foods The American Oil Chemists Society. Champaign, Illinois. 1989; pp. 41.
- Hui, Y. H.;. Handbook of Food Products Manufacturing Wiley: Hoboken, New Jersey, 2007; pp. 290.
- Taylor, A. J. Food Flavor Technology; CRC Press: UK, 2002; pp. 28.
- Ardo, Y.;. Flavour Formation by Amino Acid Catabolism. Biotechnology Advances 2006, 24, 238–242. DOI: 10.1016/j.biotechadv.2005.11.005.
- Zabbia, A.; Buys, E. M.; Kock, H. L. D. Undesirable Sulphur and Carbonyl Flavor Compound in UHT Milk. A Review. Critical Reviews in Food Science and Nutrition 2012, 52. DOI: 10.1080/10408398.2010.487166.
- Nogueira, M. C. L.; Lubachevsky, G.; Rankin, S. Z. A Study of Volatile Composition of Minas Cheese. LWT-Food Science and Technology. 2005, 555–563. DOI: 10.1016/j.lwt.2004.07.019.
- Fross, D. A.;. The Flavors of Dairy Fats. A Review. Journal of the American Oil Chemists Society 1971, 48, 702–710. DOI: 10.1007/BF02638525.
- Molimard, P.; Spinnler, H. Compounds Involved in the Flavor Surface of Mold-Ripened Cheese: Origins and Properties. Journal of Dairy Science 1996, 79, 169–184. DOI: 10.3168/jds.S0022-0302(96)76348-8.
- Sable, S.; Cottenceau, G. Current Knowledge of Soft Cheeses Flavor and Related Compounds. Journal of Agricultural and Food Chemistry 1999, 47, 4825–4836. DOI: 10.1021/jf990414f.
- Langler, J. E.; Libbey, L. M.; Day, E. A. Identification and Evaluation of Selected Compounds in Swiss Cheese Flavor. Journal of Agricultural and Food Chemistry 1967, 15, 386–391. DOI: 10.1021/jf60151a024.
- Parliament, T. H.; Kolar, M. G.; Rizzo, D. J. Volatile Components in Limberger Cheese. Journal of Agricultural and Food Chemistry 1982, 30, 1006–1008. DOI: 10.1021/jf00114a001.
- Kowalewska, J.; Zelazowska, H.; Babu,; Hammond, E. G.; Glatz, B. A.; Ross, F. The Isolation of Aroma Being Material from Lactobacillus Bulgaricus Culture in Cheese. Journal of Dairy Science 1985, 68, 2165–2171. DOI: 10.3168/jds.S0022-0302(85)81086-9.
- Urbach, G.;. Contribution of Lactic Acid Bacteria to Flavor Compound Formation in Dairy Products. International Dairy Journal 1995, 5, 887–903. DOI: 10.1016/0958-6946(95)00037-2.
- De Frutos, M.; Sanz, J.; Martinez-Castro, J. Characterization of Artisanal Cheeses by GC/MS Analysis of Their Medium Volatile (SDE) Fraction. Journal of Agricultural and Food Chemistry 1991, 39, 524–530. DOI: 10.1021/jf00003a019.
- Ur-Rehman, S.; Banks, J. M.; Brechany, E. Y.; Muir, D. D.; McSweeney, P. L. H.; Fox, P. F. Influence of Ripening Temperature on the Volatiles Profile and Flavour of Cheddar Cheese Made from Raw or Pasteurised Milk. International Dairy Journal 2000, 10, 55–65. DOI: 10.1016/S0958-6946(00)00019-4.
- Nishimoto, T.; Uyeta, M.; Taue, S. Precursor of Benzoic Acid in Fermented Milk. Journal of the Food Hygienic Society of Japan 1969, 10, 410–413. DOI: 10.3358/shokueishi.10.410.
- Lio, S. Q.; Holland, R.; Crow, V. L. Esters and Their Biosynthesis in Fermented Dairy Products: A Review. International Dairy Journal 2004, 14, 923–945. DOI: 10.1016/j.idairyj.2004.02.010.
- Verzera, A.; Ziino, M.; Condurso, C.; Romeo, V.; Zappala, M. Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry for Rapid Characterization of Semi Hard Cheeses. Analytical and Bioanalytical Chemistry 2004, 380, 930–936. DOI: 10.1007/s00216-004-2879-4.
- Nollet, L. M. L.; Toldra, F. Handbook of Dairy Foods Analysis Talor & Francis Group. CRC Press: Boca Raton. 2010; pp. 367.
- Viallon, C.; Verdier-Metz, I.; Denoyer, C.; Pradel, P.; Coulon, J. B.; Berdague, J. L. Desorbed Terpenes and Sesquiterpenes from Forages and Cheeses. Journal of Dairy Research 1999, 66, 319. DOI: 10.1017/S0022029999003520.
- Cadwallader, K. R.; Drake, M. A.; McGorrin, R. J. Flavour of Dairy Products American Chemical Society. Washington, DC. 2007, 127–130.
- Moio, L.; Piombino, P.; Addeo, F. Odour-Impact Compounds of Gorganzola Cheese. Journal of Dairy Research 2000, 67, 273–285. DOI: 10.1017/S0022029900004106.
- Christensen, T. C.; Holmer, G. GC⁄MS Analysis of Volatile Aroma Components in Butter during Storage in Different Catering Packaging. Milchwissenschaft 1996, 51, 134–139.
- Povplo, M.; Contarini, G. Comparision of Solid-Phase Microextraction and Purge-And Trap Methods for the Analysis of the Volatile Fraction of Butter. Journal of Chromatography 2003, 985, 117–125. DOI: 10.1016/S0021-9673(02)01395-X.
- Banks, J. M.; Brechany, E. Y.; Christie, W. W.; Hunter, E. A.; Muir, D. D. Volatile Components in Steam Distillates of Cheddar Cheese as Indicator Indices of Cheese Maturity, Flavour and Odour. Food Research International 1992, 2, 365–373. DOI: 10.1016/0963-9969(92)90111-H.
- Welsh, F. W.; Murray, W. D.; Williams, R. E. Microbiological and Enzymatic Production of Flavor and Fragrance Chemicals. Critical Reviews in Biotechnology 1989, 9, 105–169. DOI: 10.3109/07388558909040617.
- Chen, W.; Vermaak, I.; Viljolu, A. Camphor-A Fumigant during Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon-A Review. Molecules 2013, 18, 5434–5454. DOI: 10.3390/molecules18055434.
- Cakmakci, S.; Hayaloglu, A. Evaluation of the Chemical, Microbiological and Cvolatile Aroma Characteristics of Ispir Kaymak, a Traditional Turkish Dairy Product. International Journal of Dairy Technology 2011, 444–450. DOI: 10.1111/j.1471-0307.2011.00687.x.