ABSTRACT
Recently, propyl lactate has been firstly detected in Chinese Baijiu (Chinese liquor) as one of the odor-active components. In this study, the contents of propyl lactate in other different brewed alcoholic beverages were investigated, including wine, soju, sake, vodka, rum, tequila, whisky and beer, and analyzed by solid-phase microextraction and liquid–liquid extraction coupled with gas chromatography-mass spectrometry, determined by internal standard method followed by GC-MS with selective ion monitoring. The analyses indicated that propyl lactate was present in beer and tequila samples, while not detected in other brewed alcoholic beverages. The results showed that among the 40 beer samples from 11 different countries, 36 of them contained propyl lactate at concentrations in the range of 0.23–51.71 µg L−1. In addition, all of six tequila samples contained propyl lactate at concentrations in the range of 0.77–12.14 mg L−1, and the odor activity values (OAVs) of propyl lactate were greater than 1 in all of the tequila samples. These findings indicated that propyl lactate was widely distributed in beer and tequila; it was also an odor-active component of tequila according to OAVs.
Introduction
Brewed alcoholic beverages are highly appreciated by the combination of their desirable flavor and taste. The combined effects of hundreds of chemically diverse volatile compounds determine the aroma profile of wine as same as other brewed alcoholic beverages,[Citation1] such as Chinese Baijiu (or called Chinese liquor). According to the previous reports,[Citation2] 1737 volatile compounds in Chines Baijiu have been identified, including 431 esters, which are the largest families among the identified volatile compounds and are mainly produced through esterification of acid and alcohol compounds in fermentation and aging process.[Citation3,Citation4] Recently, study has showed that volatile esters have a huge impact on the sensory properties of Chinese Baijiu.[Citation5] Some of them produce desired and pleasant aromas, such as fruity, sweet or cognac aromas, and have been commonly detected in other brewed alcoholic beverages.
A recent study introduced 57 esters found in sake, [Citation6] which is a Japanese traditional alcoholic beverage.[Citation7] And it was also suggested that esters had effect on the flavor characteristics of sake.[Citation8] Furthermore, ethyl butanoate, ethyl hexanoate, ethyl 2-methylpropanoate, ethyl 2-methylbutanoate, 3-methylbutyl acetate, ethyl octanoate, ethyl decanoate, 2-phenylethyl acetate are considered as the most odor-active compounds in rum. [Citation9] Ethyl acetate is the major volatile composition in tequila.[Citation10,Citation11] Meanwhile, esters also have been found in vodka,[Citation12] whisky [Citation13] and beer.[Citation14–Citation16]
As odor-active compounds, esters usually constitute the most important contributors to the sweet-fruity notes of wine. Also, the relationship between esters and sensory perception of wine has been sufficiently studied.[Citation17–Citation24] Ethyl octanoate was found to increase red cherry aroma, the combination of ethyl octanoate and ethyl decanoate enhanced black cherry aroma of New Zealand Pinot Noir wines.[Citation18] Moreover, other esters, such as ethyl hexanoate, ethyl acetate, ethyl 3-methylbutanoate, were also commonly detected in wine as previous studies reported.[Citation19–Citation21] In general, volatile esters are widely recognized as a kind of odor-active compounds of brewed alcoholic beverages, thus it is valuable to study volatile ester in order to evaluate its contribution to the aroma profile of brewed alcoholic beverages.
Propyl lactate (grape-like fruity, milk aromas), a newly discovered volatile ester from Chinese Baijiu, only had been reported twice before in distilled calvados[Citation25] and Chinese rice wine[Citation26], has been quantified by internal standard (IS) method combined with gas chromatography-mass spectrometry (GC-MS) in our lab.[Citation27] The contents of propyl lactate ranged from 0.05 to 1.900 mg L−1 in 72 different Chinese Baijiu samples. Further research indicated the detection threshold of propyl lactate in 38% ethanol solution was 0.740 mg L−1, the odor activity values (OAVs) of propyl lactate were found to exceed 1 in 13 samples, which presented that propyl lactate was one of odor-active components in these Chinese Baijiu samples.
Similar to other esters, propyl lactate is possibly formed through the esterification of lactic acid with propanol, both of which have been reported during the fermentation of Chinese Baijiu. Additionally, the raw materials of Chinese Baijiu are mainly sorghum, combined with wheat, wheat bran, millet, rice and corn, which are more abundant than that of other brewed alcoholic beverages. Also, the fermentation process of Chinese Baijiu is different from other brewed alcoholic beverages. Thus, in terms of different raw materials and fermentation processes, whether propyl lactate exists in other brewed alcoholic beverages is still confused. Since there have been no studies conducted to investigate the presence and contribution of propyl lactate in other brewed alcoholic beverages, we stretched our work into other different representative brewed alcoholic beverages.
Recently, several pretreatment methods have been applied to identify and quantify the volatiles in brewed alcoholic beverages. Among them, liquid–liquid extraction (LLE) is an effective method for the gentle extraction of volatile components of liquid products such as fruit juices, wines and beer; solid-phase microextraction (SPME) can be considered as a fast, simple, affordable, sensitive, solvent-free and easy-to-automate technique to extract volatiles of liquor (14). So, in this study, LLE and SPME coupled with GC-MS were chosen to investigate propyl lactate of various brewed alcoholic beverages.
In this study, in order to find out whether propyl lactate only exist in Chinese Baijiu and evaluate its contribution to the aroma of other brewed alcoholic beverages based on OAVs, the contents of propyl lactate in 80 different brewed alcoholic beverage samples were quantified by IS method combined with GC-MS, including wine, soju, sake, vodka, rum, tequila, whisky and beer.
Materials and methods
Chemicals and reagents
Standards of methyl lactate (agentsC-MS, including wine, soju, sake, vodka, rum, tequila, whisky and beer., Shanghai, China. Methylene dichloride, ethanol, sodium chloride and sodium sulfate were supplied by Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China.
Samples
All brewed alcoholic beverages were purchased from the local market or airport shop and within expiration dates.
Preparation of samples
A stock solution of propyl lactate (1000.0 mg L−1) was prepared in absolute ethanol. A series of working standard solutions were prepared by diluting the stock solution with absolute ethanol. A working standard solution of the IS methyl lactate (100.0 mg L−1) was also prepared as described above. The above-mentioned solutions were all kept at −18°C in order to reduce the loss of sample.
Solid-phase microextraction
Propyl lactate from samples was extracted by a SPME fiber (50/30 μm DVB/CAR/PDMS) attached to a manual SPME holder (Supelco, Bellefonte, PA). Samples (10 mL) were introduced into a 40 mL vial sealed with a screw cap provided with a predrilled Teflonfaced septum. Except beer, the alcoholicity of samples was adjusted to 10°. The NaCl concentration of samples was adjusted to saturation. After thermal equilibration of the sample at 40°C for 30 min, the SPME fiber was exposed to the vial headspace for 30 min under agitation (600 rpm) and then introduced into the injector inlet for 5 min. All fibers were conditioned before use until no interfering peaks were obtained in blank runs.
Liquid-liquid extraction
Samples (15 mL) were spiked with 30 µL of the IS working solution. Except beer, the alcoholicity of samples was adjusted to 10°. The NaCl concentration of samples was adjusted to saturation and extracted three times with 5 mL methylene dichloride. Extract was dried with 2.5 g of anhydrous Na2SO4 overnight, concentrated to a final volume of 1 mL, and then stored at −18°C until analysis in order to prevent from the loss of sample.
GC-MS analysis
Samples and working standard solutions were analyzed by an Agilent 7890B gas chromatograph equipped with an Agilent 5977A mass selective detector (GC-MS) (Agilent Technologies, USA) and a capillary split/splitless injector system (Agilent Technologies, USA).
For GC, the analytical GC column was a DB-FFAP column (60 m × 0.25 mm internal diameter, 0.25 µm film thickness, Agilent Technologies, USA). The column carrier gas was helium at a constant flow rate of 1.0 mL/min. The injector temperature was set at 250°C; 1 µL of sample was injected in splitless mode. The temperature program of the oven was as follows: column temperature was ramped from 50°C (2 min) to 100°C at a rate of 6°C/min, then raised at 3°C/min up to 170°C (2 min) and raised at 10°C/min up to 200°C (2 min), finally raised at 15°C/min up to 230°C (15 min).
The MS was operated in electron impact mode at 70 eV. The temperature of the interface and the ion source were both set at 250°C. The identification of propyl lactate was conducted in Full Scan mode. The mass range was 45–350 amu with 0.2 s of scan time. The quantification of propyl lactate was conducted in selective ion monitoring (SIM) mode. Three ions for every analyte were selected in SIM mode. The dwell time of each ion was 0.05 s. The selected ions of propyl lactate and IS methyl lactate were as follows: propyl lactate (m/z 45, 75 and 117), methyl lactate (m/z 45, 61 and 89). All analyses were repeated in triplicate. Data processing was performed with the Agilent MassHunter Workstation software.
Propyl lactate identification
Identification of propyl lactate was achieved by comparing mass spectra, linear retention time (RT) of pure standard and NIST 11 library.
Method validation
The performance parameters assessed were: recovery, linearity, limit of detection (LOD), limit of quantitation (LOQ), and precision. Fifteen milliliter sample with 30 µL of the IS working solution and 30 µL stock solution of propyl lactate was done as the method mentioned in the section of LLE to determine recovery. The linearity was determined by evaluation of the regression curve. Calibration curve was created by five concentration levels of the working standard solutions. The LOD and LOQ were obtained from the lowest concentrations of the working standard solutions based on a signal-to-noise ratio of 3 and 10, respectively. The precision was assessed in sixfold at two different levels of the working standard solutions.
Propyl lactate quantification
The quantitative analysis of propyl lactate was performed in SIM mode to maximize sensitivity. Calibration curves were drawn by plotting the ratio between the analyte peak area and the IS peak area versus the ratio between their concentrations. The concentration of the IS methyl lactate was maintained constant at 3.0 mg L−1 in samples and working standard solutions, which were ready to be analyzed by GC-MS. Each point of the calibration curve was the mean of three replicates. The concentration of propyl lactate was calculated by the linear regression equation.
Statistical analysis
Two independent experiments were performed at least in triplicate. The concentrations of aroma compounds were expressed as mean ± standard deviation (SD). Statistical differences between different kinds of samples were obtained using the one-way ANOVA test with a post hoc Tukey test. p < 0.05 was accepted as statistically significant (SPSS Inc., Chicago, IL).
Results and discussion
Identification of propyl lactate
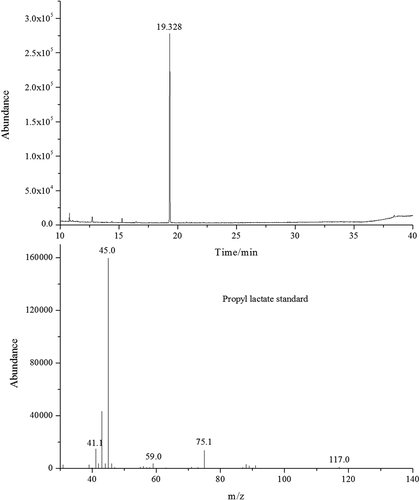
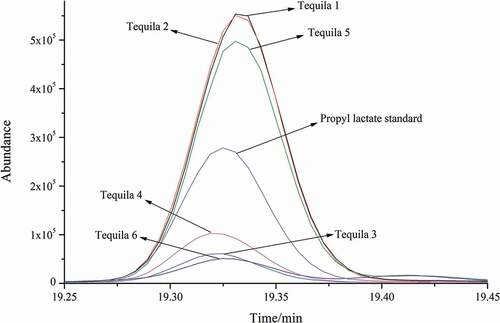
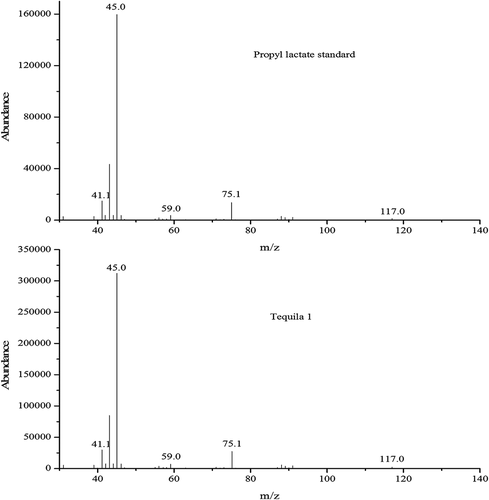
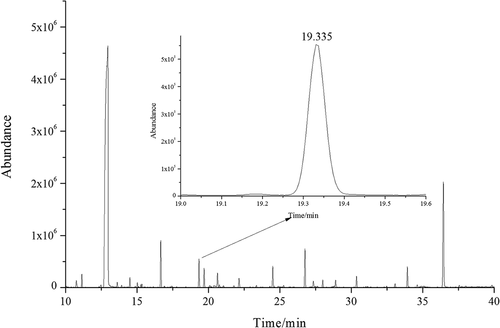
Working standard solutions and samples were analyzed by GC-MS with Full Scan mode. The total ion chromatogram (TIC) and mass spectrum of propyl lactate standard were shown in . TIC of sample (tequila 1) was drawn in . As shown in and , the RT (19.3 min) of unknown compound in sample (tequila 1) corresponded with propyl lactate standard. The partial TIC of propyl lactate standard and six tequila samples shown in were well overlapped, they all had nearly the same RTs. In addition, the mass spectrum of propyl lactate standard and unknown compound shown in matched with each other, which indicated the unknown compound in sample (tequila 1) was propyl lactate.
Figure 2. TIC of sample: Tequila 1 and its partial enlarged drawing (from 19 to 19.6 min) obtained from GC-MS.
Validation of the method
According to the mass spectrum of propyl lactate standard, the selected ions of propyl lactate were m/z 45, 75 and 117. Working standard solutions and samples were analyzed by GC-MS with SIM mode. The LOD and the LOQ of the method were 0.05 and 0.10 µg L−1, respectively. The IS curve equations in different concentration ranges were as follows: y = 13.21600x − 0.00409 (0.10 µg.L−1 < concentration of propyl lactate <500.00 µg L−1) and y = 10.19200x + 0.15368 (500.00 µg L−1 < concentration of propyl lactate < 50.00 mg.L−1). Specifically, y represented the concentration ratio between analyte (propyl lactate) and the IS (methyl lactate), and x represented the corresponding peak area ratio. The corresponding correlation coefficients (R2) were all up to 0.9998. The recovery was between 89.7% and 98.3% under the level of 1.00 µg L−1 and 10.00 mg L−1, the precision was all less than 5%.
Quantification of propyl lactate
The quantitative results of propyl lactate were listed in . As shown in , propyl lactate was not detected in all red wine samples as well as soju, sake, vodka, whisky and rum samples. However, all tequila samples contained propyl lactate, the contents of which ranged from 0.77 to 12.14 mg.L−1. With regard to beer samples, propyl lactate was detected in most beer samples except four samples, but the contents were far less than tequila samples, only from 0.23 to 51.71 µg L−1 (p < 0.05).
Table 1. The content of propyl lactate in 80 samples.
Calculation of the OAVS for propyl lactate
According to previous research, the detection threshold of propyl lactate in 38% ethanol solution was 0.740 mg L−1. However, the odor threshold can be affected by matrix, therefore, the influence of ethanol concentration on the volatility of an odorant should not be ignored. The ethanol concentration of tequila samples in this study was close to 38% and the ethanol concentration of beer samples was much less than 38%. So, the OAVs of propyl lactate in beer samples were merely a guideline and all far less than 1. In comparison, the OAVs of propyl lactate in tequila samples were all above 1. The OAV equaled to the ratio of the concentration of propyl lactate and its detection threshold value. If a compound has an OAV > 1.0, it could contribute to the flavor of a product. Therefore, the result indicated that propyl lactate made contribution to the aroma profile of tequila, because its concentrations clearly exceeded its odor thresholds in all tequila samples. In addition, propyl lactate was also detected in Chinese Baijiu and had much contribution to the aroma profile of Chinese Baijiu according to previous study.[Citation27] Propyl lactate showed grape-like fruity and milk aromas, which influenced on the fruity note of brewed alcoholic beverages.
Discussion
Combined with previous study,[Citation27] the contents of propyl lactate were generally highest in tequila samples (0.77–12.14 mg L−1), moderate in Chinese Baijiu samples (0.05–1.900 mg L−1), and lowest in beer samples (0.23–51.71 µg L−1) (p < 0.05). Different raw materials and fermentation processes may cause a big difference to the content of propyl lactate.
The raw materials of Chinese Baijiu are mainly sorghum, combined with wheat, wheat bran, millet, rice and corn, which are more abundant than that of other brewed alcoholic beverages, such as beer. Furthermore, Daqu is used as a kind of saccharifying agent and fermenting agent, which contains yeasts, molds, lactobacilli, bacteria and complex enzymes spontaneously proliferated on cooked wheat during the fermentation process of Chinese Baijiu. Hence, more abundant material base (raw materials and microorganism) is possible in the fermentation process of Chinese Baijiu. While plentiful material base and microorganism contribute to producing various kinds of volatiles, including organic acids and alcohols,[Citation28–Citation33] which are the substrate of esterification and produce esters during the fermentation and aging process of Chinese Baijiu.[Citation34–Citation36] Therefore, higher content of propyl lactate is likely to be detected in Chinese Baijiu. Indeed, the concentration of propyl lactate in Chinese Baijiu was higher than that in beer samples in this study. The main material of beer is malt, and single material with pure breed fermentation might cause lower concentration of propyl lactate.
As for tequila, a kind of Mexican liquor makes from fermented juices of an agave plant, which has higher content of sugars. In this study, the highest concentration of propyl lactate was observed for tequila samples. While the changes in the concentration of propyl lactate between tequila samples and beer samples might be due to the different contents and kinds of components between agave and malt or different microorganism. It was worthwhile to note that although the raw material of tequila was single, the propyl lactate concentration of tequila was higher than that of Chinese Baijiu. The reason may be that microbial competitive metabolism of esters and its intermediate products are becoming fiercer in terms of the plentiful material base and microorganism applied during the fermentation of Chinese Baijiu, which may have effect on the content of propyl lactate.
As for wine, soju, sake, vodka, rum and whisky, the main materials are grape, rice and sweet potato, rice, potato and corn, sugar cane, and wheat, respectively, which may too simple to provide propyl lactate. Furthermore, lack of the essential microorganism and enzyme for the metabolism of propyl lactate may lead to the result that propyl lactate was not detected in wine, soju, sake, vodka, rum and whisky.
Conclusion
In the present study, the propyl lactate was identified and quantified in various brewed alcoholic beverages. This was the first report to confirm the existence of proyl lactate in tequila and beer. SPME coupled with LLE method was applied to analyze propyl lactate in 80 samples from 8 types of brewed alcoholic beverages. Propyl lactate was quantified by IS method followed by gas chromatography-mass spectrometry with selected ion monitor. The results showed propyl lactate was only found in tequila and beer samples, the contents of which were from 0.77 to 12.14 mg.L−1 and 0.49 to 3.99 µg.L−1, respectively. OAV analysis indicated that propyl lactate made contribution to the formation of tequila’s aroma. These results could provide an experimental basis for further studies on the contribution of propyl lactate to the characteristic aroma profile of tequila and other brewed alcoholic beverages.
Additional information
Funding
References
- Alves, R. F.; Nascimento, A. M. D.; Nogueira, J. M. F. Characterization of the Aroma Profile of Madeira Wine by Sorptive Extraction Techniques. Analytica Chimica Acta 2005, 546, 11–21. DOI: 10.1016/j.aca.2005.05.012.
- Sun, B. G.; Wu, J. H.; Huang, M. Q.; Sun, J. Y.; Zheng, F. P. Recent Advances of Flavor Chemistry in Chinese Liquor Spirits (Baijiu). International Journal of Food Science and Technology 2015, 15, 1–8.
- González-Rompinelli, E. M.; Rodríguez-Bencomo, J. J.; García-Ruiz, A.; Moreno-Arribas, M. V.; Winery-Scale, A. Trial of the Use of Antimicrobial Plant Phenolic Extracts as Preservatives during Wine Ageing in Barrels. Food Control 2013, 33, 440–447. DOI: 10.1016/j.foodcont.2013.03.026.
- Wang, P. P.; Li, Z.; Qi, T. T.; Li, X. J.; Pan, X. Y. Development of a Method for Identification and Accurate Quantitation of Aroma Compounds in Chinese Daohuaxiang Liquors Based on SPME Using a Sol-Gel Fibre. Food Chemistry 2015, 169, 230–240. DOI: 10.1016/j.foodchem.2014.07.150.
- Fan, H. Y.; Fan, W. L.; Xu, Y. Characterization of Key Odorants in Chinese Chixiang Aroma-Type Liquor by Gas Chromatography–Olfactometry, Quantitative Measurements, Aroma Recombination, and Omission Studies. Journal of Agricultural and Food Chemistry 2015, 63, 3660–3668. DOI: 10.1021/jf506238f.
- Mimura, N.; Isogai, A.; Iwashita, K.; Bamba, T.; Fukusaki, E. Gaschromatography/Mass Spectrometry Based Component Profilingand Quality Prediction For Japanese Sake. Journal of Bioscience and Bioengineering 2014, 118, 406–414. DOI: 10.1016/j.jbiosc.2014.04.006.
- Takahashi, K.; Kabashima, F.; Tsuchiya, F. Comprehensive Two-Dimensional Gas Chromatography Coupled with Time-of-Flight Mass Spectrometry Reveals the Correlation between Chemical Compounds in Japanese Sake and Its Organoleptic Properties. Journal of Bioscience and Bioengineering 2016, 121, 274–280. DOI: 10.1016/j.jbiosc.2015.06.016.
- Yoshizaki, Y.; Yamato, H.; Takamine, K.; Tamaki, H.; Ito, K.; Sameshima, Y. Analysis of Volatile Compounds in Shochu Koji, Sake Koji, and Steamed Rice by Gas Chromatography-Mass Spectrometry. Journal of the Institute of Brewing 2010, 116, 49–55. DOI: 10.1002/(ISSN)2050-0416.
- Pino, J. A.; Tolle, S.; Gök, R.; Winterhalter, P. Characterisation of Odour-Active Compounds in Aged Rum. Food Chemistry 2012, 132, 1436–1441. DOI: 10.1016/j.foodchem.2011.11.133.
- Ceballos-Magaña, S. G.; De Pablos, F.; Jurado, J. M.; Mjesus, M.; Alcázar, A.; Muñiz-Valencia, R.; Gonzalo-Lumbreras, R.; Izquierdo-Hornillos, R. Characterisation of Tequila according to Their Major Volatile Composition Using Multilayer Perceptron Neural Networks. Food Chemistry 2013, 136, 1309–1315. DOI: 10.1016/j.foodchem.2012.09.048.
- Prado-Jaramillo, N.; Estarrón-Espinosa, M.; Escalona-Buendía, H.; Cosío-Ramírez, R.; Martín-del-Campo, S. T. Volatile Compounds Generation during Different Stages of the Tequila Production Process. A Preliminary Study. LWT Food Science and Technology 2015, 61, 471–483. DOI: 10.1016/j.lwt.2014.11.042.
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The Analysis of Vodka: A Review Paper. Food Analytical Methods 2015, 8, 2000–2010. DOI: 10.1007/s12161-015-0089-7.
- Poisson, L.; Schieberle, P. Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis. Journal of Agricultural and Food Chemistry 2008, 56, 5813–5819. DOI: 10.1021/jf800382m.
- Thompson-Witrick, K. A.; Rouseff, R. L.; Cadawallader, K. R.; Duncan, S. E.; Eigel, W. N.; Tanko, J. M.; O’Keefe, S. F. Comparison of Two Extraction Techniques, Solid-Phase Microextraction versus Continuous Liquid-Liquid Extraction/Solvent-Assisted Flavor Evaporation, for the Analysis of Flavor Compounds in Gueuze Lambic Beer. Journal of Food Science 2015, 80, C571–C576. DOI: 10.1111/1750-3841.12795.
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S. M. Insights on Beer Volatile Profile: Optimization of Solid-Phase Microextraction Procedure Taking Advantage of the Comprehensive Two-Dimensional Gas Chromatography Structured Separation. Journal of Separation Science 2015, 38, 2140–2148. DOI: 10.1002/jssc.v38.12.
- Langos, D.; Granvogl, M. Studies on the Simultaneous Formation of Aroma-Active and Toxicologically Relevant Vinyl Aromatics from Free Phenolic Acids during Wheat Beer Brewing. Journal of Agricultural and Food Chemistry 2016, 64, 2325–2332. DOI: 10.1021/acs.jafc.5b05606.
- Picard, M.; Tempere, S.; De Revel, G.; Marchand, S.; Sensory, A. Study of the Ageing Bouquet of Red Bordeaux Wines: A Three-Step Approach for Exploring A Complex Olfactory Concept. Food Quality and Preference 2015, 42, 110–122. DOI: 10.1016/j.foodqual.2015.01.014.
- Tomasino, E.; Harrison, R.; Breitmeyer, J.; Sedcole, R.; Sherlock, R. R.; Frost, A. Aroma Composition of 2-Year-Old New Zealand Pinot Noir Wine and Its Relationship to Sensory Characteristics Using Canonical Correlation Analysis and Addition/Omission Tests. Australian Journal of Grape and Wine Research 2015, 21, 376–388. DOI: 10.1111/ajgw.2015.21.issue-3.
- Chang, E. H.; Jeong, S. M.; Hur, Y. Y.; Koh, S. W.; Choi, I. M. Changes of Volatile Compounds in Vitislabrusca ‘Doonuri’ Grapes during Stages of Fruit Development and in Wine. Horticulture, Environment and Biotechnology 2015, 56, 137–144. DOI: 10.1007/s13580-015-0068-3.
- Marcq, P.; Schieberle, P. Characterization of the Key Aroma Compounds in a Commercial Amontillado Sherry Wine by Means of the Sensomics Approach. Journal of Agricultural and Food Chemistry 2015, 63, 4761–4770. DOI: 10.1021/acs.jafc.5b01418.
- Xiao, Z. B.; Liu, J. H.; Chen, F.; Wang, L. Y.; Niu, Y. W.; Feng, T. Comparison of Aroma-Active Volatiles and Their Sensory Characteristics of Mangosteen Wines Prepared by Saccharomyces Cerevisiae with GC-Olfactometry and Principal Component Analysis. Natural Product Research 2015, 29, 656–662. DOI: 10.1080/14786419.2014.981185.
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Silva, A.; Cela, R. Multiclass Semi-Volatile Compounds Determination in Wine by Gaschromatography Accurate Time-of-Flight Mass Spectrometry. Journal of Chromatography A 2016, 1442, 107–117. DOI: 10.1016/j.chroma.2016.03.005.
- Campo, E.; Cacho, J.; Ferreira, V. Solid Phase Extraction, Multidimensional Gas Chromatography Mass Spectrometry Determination of Four Novel Aroma Powerful Ethyl Esters. Assessment of Their Occurrence and Importance in Wine and Other Alcoholic Beverages. Journal of Chromatography A 2007, 1140, 180–188. DOI: 10.1016/j.chroma.2006.11.036.
- Slegers, A.; Angers, P.; Ouellet, É.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grownin Québec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. DOI: 10.3390/molecules200610980.
- Ledauphin, J.; Saint-Clair, J. F.; Lablanquie, O.; Guichard, H.; Founier, N.; Elisabeth Guichard, A. Identification of Trace Volatile Compounds in Freshly Distilled Calvados and Cognac Using Preparative Separations Coupled with Gas Chromatography-Mass Spectrometry. Journal of Agricultural and Food Chemistry 2004, 52, 5124–5134. DOI: 10.1021/jf040052y.
- Luan, J. S.;. Research on the Flavor in Rice Wine. China Brewing 2002, 6, 21–24.
- Wu, J. H.; Zheng, Y.; Sun, B. G.; Sun, X. T.; Sun, J. Y.; Zheng, F. P.; Huang, M. Q. The Occurrence of Propyl Lactate in Chinese Baijius (Chinese Liquors) Detected by Direct Injection Coupled with Gas Chromatography-Mass Spectrometry. Molecules 2015, 20, 19002–19013. DOI: 10.3390/molecules201019002.
- Chen, B.; Wu, Q.; Xu, Y. Filamentous Fungal Diversity and Community Structure Associated with the Solid State Fermentation of Chinese Maotai-Flavor Liquor. Int. J. Food Microbiol.2014, 179, 80–84. DOI: 10.1016/j.ijfoodmicro.2014.03.011.
- Lv, X. C.; Huang, Z. Q.; Zhang, W.; Rao, P. F.; Identification, N. L. Characterization of Filamentous Fungi Isolated from Fermentation Starters for Hong Qu Glutinous Rice Wine Brewing. Journal of General and Applied Microbiology 2012, 58, 33–42. DOI: 10.2323/jgam.58.33.
- Nahar, S.; Hossain, F.; Feroza, B.; Halim, M. A. Production of Glucoamylase by Rhizopussp. In Liquid Culture. Journal of Biological Sciences 2008, 40, 1693–1698.
- Chidi, B. S.; Rossouw, D.; Buica, A. S.; Bauer, F. F. Determining the Impact of Industrial Wine Yeast Strains on Organic Acid Production under White and Red Wine-Like Fermentation Conditions. South African Journal of Enology and Viticulture 2015, 36, 316–327. DOI: 10.21548/36-3-965.
- Fan, W. L.; Qian, M. C. Identification of Aroma Compounds in Chinese ‘Yanghe Daqu’ Liquor by Normal Phase Chromatography Fractionation Followed by Gas Chromatography/Olfactometry. Flavour Fragrance Journal 2006, 21, 333–342. DOI: 10.1002/ffj.1621.
- Liu, Y. P.; Huang, M. Q.; Zheng, F. P.; Chen, H. T.; Sun, B. G. Recent Advances in Extraction and Analysis of Volatile Flavor Compounds in Chinese Liquor. China Journal of Food Science 2010, 31, 437–441.
- Shen, F.; Li, F.; Liu, D.; Xu, H.; Ying, Y.; Li, B. Ageing Status Characterization of Chinese Rice Wines Using Chemical Descriptors Combined with Multivariate Data Analysis. Food Control 2012, 25, 458–463. DOI: 10.1016/j.foodcont.2011.11.019.
- Renault, P.; Coulon, J.; De Revel, G.; Barbe, J. C.; Bely, M. Increase of Fruity Aroma during Mixed T. delbrueckii/S. Cerevisiae Wine Fermentation Is Linked to Specific Esters Enhancement. International Journal of Food Microbiology 2015, 207, 40–48. DOI: 10.1016/j.ijfoodmicro.2015.04.037.
- Rojas, V.; Gil, J. V.; Piñaga, F.; Manzanares, P. Studies on Acetate Ester Production by Non-Saccharomyces Wine Yeasts. International Journal of Food Microbiology 2001, 70, 283–289. DOI: 10.1016/S0168-1605(01)00552-9.