?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed at investigating the physico-mechanical and microstructural properties of a novel edible film based on plasticized semolina flour with different plasticizer (sorbitol/glycerol, 3:1) contents (30, 40, and 50%, w/w). As plasticizer content increased, water vapor and oxygen permeability, tensile strength, and the elastic modulus of the semolina films decreased, while their water solubility, moisture content, and elongation-at-break increased significantly (p < 0.05). Semolina-based films exhibited excellent absorption of ultraviolet light, and the addition of plasticizers improved the optical properties of the resultant films. Fourier-transform infrared spectroscopy showed no significant effect on the structure of the protein. Thermogravimetric analysis also revealed that increasing plasticizer concentration has no remarkable influence on the magnitude of weight loss. Atomic force microscopy images showed that the surface roughness of the films was influenced by plasticizer concentrations. This study demonstrated that semolina flour protein has the potential to prepare edible films.
Introduction
Plastic has been extensively applied as packaging material; however, because of disposal and environmental problems due to the waste from these materials, petroleum-based plastics are presently being replaced by natural and compostable materials.[Citation1] Edible films and coatings have recently attracted increasing interest due to concerns with the environmental hazards, cost, and supply of petroleum.[Citation2] Materials available for forming edible films and coatings are generally classified as polysaccharides, proteins, and lipids.[Citation3]
Proteins have received considerable attention among biopolymer materials because of their renewability, availability, and safety. Meanwhile, transparent protein films can act as excellent oxygen barriers and provide certain mechanical properties.[Citation4] These films possess special nutritional and/or health-protective functions, as well as favorable film-forming properties. Moreover, the unique capacity of proteins to form networks and generate plasticity and elasticity is beneficial for preparing biopolymer-based packaging materials.[Citation5]
Among all the protein sources, wheat is considered to be a valuable candidate for packaging because of its low price, biodegradability, renewability, favorable film formation, and adhesive/cohesive properties.[Citation6] Semolina flour is a wheat product made up of complex carbohydrate and protein. Its main component is protein (highest gluten), which enables it to produce a cohesive adhesive film and lower oxygen and water vapor permeability (WVP).[Citation7] It also has the potential to improve the nutritional properties of edible films. Semolina is an extra-hard, translucent, and light-colored grain that exhibits antioxidant activity.[Citation8] In addition, semolina extracts suppress radical-induced liposome lipids through oxidation and display radical cation scavenging activity.[Citation9]
In general, protein-based film formulations require the addition of a plasticizing agent above a minimum threshold to reduce film fragility and to confer certain plastic properties.[Citation10] Plasticizers are low-molecular-weight nonvolatile substances applied to films to reduce biopolymer chain-to-chain interactions and improve film flexibility and stretchability.[Citation11] However, plasticizers also decrease the mechanical resistance and barrier properties of the films.[Citation12] Hydrophilic compounds such as polyols (sorbitol and glycerol) are commonly used as plasticizers in edible film formation. Glycerol improves film extensibility but reduces mechanical strength and water vapor barrier properties; films containing sorbitol also have reduced gas permeability.[Citation13]
To the best of our knowledge, no work has been conducted on how plasticizer type (sorbitol and glycerol) affects the structure and properties of semolina-based films produced by the casting method. Hence, the objective of this work was to examine the influence of plasticizer mix (sorbitol and glycerol) concentrations on the physical (solubility, barrier to water vapor, oxygen, and color), mechanical (tensile strength [TS], elongation-at-break [EAB], and elastic modulus [EM]), thermal, and structural characteristics of novel plasticized semolina flour films.
Materials and methods
Materials
Semolina flour (14.2% protein and 18.5% gluten) was purchased from the local market in Tehran, Iran, and stored in a cool, dry place for the experiments. Food-grade sorbitol and glycerol were obtained from SIM and Liangtraco Company (Sdn. Bhd. Penang, Malaysia), respectively. Magnesium nitrate for humidity control was also purchased from Sigma-Aldrich (Kuala Lumpur, Malaysia), while sodium hydroxide (NaOH) was procured from Merck Chemicals Co. (Darmstadt, Germany).
Semolina film preparation
Semolina flour (4 g) was dispersed in 80 mL of distilled water (based on water or water/ethanol) at room temperature by simple magnetic stirring; the pH of the dispersion was adjusted to 8 with 1 M NaOH. Plasticizers comprising a blend of sorbitol and glycerol in a 3:1 ratio were added into the dispersions to yield different final plasticizer concentrations of 30%, 40%, and 50% (w/w, semolina basis); films without plasticizers served as a control. Subsequently, the semolina film-forming dispersions (FFDs) were heated at 85°C for 30 min under constant stirring. They were then cooled at room temperature, and thereupon, 90 g of the dispersion was cast on Perspex plates fitted with rims around the edge to yield a 16 × 16 cm2 film-forming area. Finally, the films were dehydrated in an oven at 40°C for 16 h, and the dried films were peeled and stored at 23 ± 2°C and 50 ± 5% relative humidity (RH) until examination.
Characterization of the film-forming dispersions and films
ζ-Potential
The ζ-potential of the semolina FFDs was determined using a Zeta sizer Nano-ZS90 (Malvern Instruments Ltd, Worcestershire, UK). The analysis was performed at a scattering angle of 90° with samples diluted to different concentrations with deionized distilled water. All the experiments were conducted at 25°C.
Thickness
In order to measure the film thickness, a digital micrometer was employed (Model No. 2046–08; Mitutoyo, Tokyo, Japan). For this purpose, the thickness of the films was measured in 10 randomly selected locations around each film sample.
Film solubility
The solubility of the semolina film was measured according to the method reported in the literature.Citation[13] Three pieces of film (2.5 × 2.5 cm) were dehydrated in desiccators with phosphorus pentoxide (0% RH) at 25°C for 3 days and then accurately weighed (to the nearest 0.0001 g) to obtain their initial dry weight (Wi). Then, the samples were submerged in deionized water (80 mL, 18 MX) and gently shaken (40 rpm) for 1 h at room temperature. The remaining pieces of film were separated using filter paper (Whatman No. 1) and dried in an oven for 24 h at 60°C until of a constant weight to determine the weight of dry matter which had not dissolved in water (Wf). The weight of the water-soluble material was calculated by subtracting the weight of insolubilized dry matter to that of the initial dry matter. The film solubility (FS%) was calculated using Eq. (1):
Wi = initial dry film weight (g)
Wf = final dry film weight (g).
Moisture content (MC)
The pieces of film were dried at 105°C for 24 h (until equilibrium weight was attained). Weight difference was determined after drying, and MC was obtained using the following equation:
where Wi and Wf are the initial and final masses of the dried samples (mg), respectively. Three replicates were considered for each sample.
Water vapor permeability
WVP values of the films were measured gravimetrically according to ASTM E96-05 method.[Citation14] Film samples were sealed over the circular opening of a permeation cell containing 6 mL distilled water; the cells were then placed in a desiccator containing silica gel. The water transported through the films was monitored every day for 7 days by the weight loss of the glass permeation cell. The measured WVP of the films was determined using Eq. (3):
where WVTR is the water vapor transmission rate (g), L is the average film thickness (m), ΔP is the partial water vapor pressure variance (Pa) through two sides of the film, and A is the area of the cup (m2). Three replicates of each film were tested.
Oxygen permeability (OP)
OP measurements were performed on films with Mocon Oxtran 2/21 (Minneapolis, USA) at 25°C and 55% RH using oxygen (21%) as the test gas in accordance with ASTM standard method D3985-05.[Citation15] Film specimens were placed on an aluminum foil mask that allowed an exposure area of 5 cm2. Three replicates of each film were evaluated.
Film color
Color measurement of the films was performed using a colorimeter (Minolta CM-3500D, Minolta Co. Ltd., Osaka, Japan). The parameters L* (luminosity), a* (red to green), and b* (yellow to blue) were measured. Five points were selected for each film.
Light transmittance
The barrier properties of the semolina films against ultraviolet (UV) and visible light were measured at a selected wavelength between 190 and 1100 nm, using a UV-1650 spectrophotometer (Model PC, Shimadzu, Kyoto, Japan). Film strips of 60 × 4 mm were placed in a spectrophotometer test cell. An empty test cell was used as the reference.
Mechanical properties
A texture analyzer (TA-XT2, Stable Micro Systems, Surrey, UK) was used to measure TS, EM, and EAB in accordance with ASTM D882-10.[Citation16] Film strips of 100 × 20 mm were conditioned in an environmental chamber for 48 h at 23 ± 2°C and 53 ± 2% RH before testing. Each type of film was tested by at least seven replicates.
Fourier-transform infrared with attenuated total reflection (FTIR-ATR)
FTIR spectra of the films were recorded using the attenuated total reflection (ATR) method in Smart iTR (Thermo Scientific, Madison, USA). The thin films were directly applied onto the ZnSe ATR cell. For each spectrum, 64 consecutive scans at 4 cm−1 resolution were averaged to reduce spectral noise.
Decomposition temperature
Samples (~15 mg) were scanned using a thermo-gravimetric analyzer (TGA-1, Perkin Elmer, Massachusetts, USA) from 40 to 800°C at a heating rate of 10°C/min under nitrogen environment.
Atomic force microscopy (AFM)
The surface topography of the films was measured using an atomic force microscope (Dimension Edge, Bruker, Madison, WI, USA) in contact operation mode. Two statistical parameters, associated with sample roughness (average roughness (Ra) and root-mean-square roughness (Rq)), were calculated using Nanoscript software (Veeco Instruments, Plainview, NY, USA) according to ASME B46.1.14.
Statistical analysis
Analysis of variance and Duncan post-hoc tests (at 5% significance level) were used to detect differences among the mean values of the semolina film properties. Statistical analyses were conducted using SPSS version 22.0.
Results and discussion
ζ-Potential
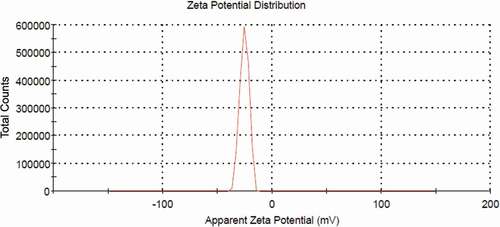
Micro- and nanoparticle charge is one of the main factors determining the physical stability of both emulsions and suspensions and can be quantified by measuring their so called “ζ-potential”.[Citation17] Generally, when all the particles have a large positive or negative ζ-potential (where the positivity and negativity are greater or lower than +30 mV and −30 mV), they will repel each other and, as a consequence, the suspension becomes stable. On the other hand, when the particles have low ζ-potential values (close to 0 mV), the tendency for flocculation increases.[Citation18] In this sense, the film-forming solutions (FFS) with higher ζ-potential will be the most stable one during the film drying step.[Citation19] In the present study, semolina FFS present negative values of ζ-potential about −25.5 mV (), confirming their anionic structure as well as stability of FFS. The result is in agreement with the value obtained by Ladjal-Ettoumi et al.,[Citation20] with a ζ-potential of approximately −20 mV for legume protein isolates (peas, chickpeas, and lentils) at pH 6.
Film thickness
The semolina films had thickness values within the range of 0.132–0.144 mm (). Increasing the concentration of plasticizer (sorbitol/glycerol) during film preparation caused significant increase (p < 0.05) in the thickness of the resulting film. Similarly, Ahmadi et al.[Citation21] reported significantly increased thickness of psyllium hydrocolloid-based edible film with a higher glycerol concentration, which is ascribed to the higher adsorption of moisture that resulted in swelling and, consequently, increased thickness. In contrast, Dick et al.[Citation22] reported that different concentrations of glycerol did not have a significant effect on the thickness of the edible film produced from chia seed mucilage.
Table 1. Physical and barrier properties of semolina films incorporated with various sorbitol/glycerol concentrations.
Solubility in water and moisture content
The solubility of edible film is essential when selecting a film for a specific purpose. The solubility of semolina film plasticized with various concentrations of sorbitol/glycerol increased significantly (p < 0.05) from 37.51 (at 30% plasticizer concentration) to 45.14% (at 50% plasticizer concentration) (). In sorbitol/glycerol-plasticized semolina films, the plasticizer decreased the interactions among the biopolymer molecules and increased the solubility of the films; meanwhile, the solubility was more affected by the hydrophilic nature of the plasticizer used than by its concentration.[Citation23] The highest solubility was achieved when the maximum concentration of the plasticizer (50%, w/w) was used in the films.
As can be seen from , the MC of the semolina films was affected by the plasticizer content and increased with increasing concentrations of sorbitol/glycerol, which may be correlated with the water-holding capacity of the plasticizer (due to their hygroscopic nature).[Citation24] Ghasemlou et al.[Citation23] reported a similar behavior for edible film based on kefiran, in which the level of glycerol enhancement exceeded 15%. These authors demonstrated that without glycerol, the hydroxyl groups of kefiran polymers are surrounded by water molecules through hydrogen bonds; adding a small amount of glycerol resulted in an interaction with the kefiran–water system without changing the MC. However, the film properties changed at glycerol concentrations above 15% because of the formation of glycerol–kefiran and glycerol–water interactions.[Citation23]
WVP
Semolina-based edible films with no plasticizer are too sticky and brittle to remove from the surface of a glass plate; it is necessary, therefore, to use a plasticizer. indicates the WVP values of the semolina films with various concentrations of sorbitol/glycerol (3:1). The lowest concentration of plasticizer (i.e. 30%) in the semolina films exhibited the highest WVP, which may be attributed to the poor chain association and formation of numerous void spaces and cavities in the films.[Citation25] A similar behavior was demonstrated by Ghanbarzadeh et al.,[Citation26] who observed that pure zein film (without plasticizer) has the highest WVP and increasing the amounts of glycerol and sorbitol decreases WVP from 1.95 (control film) to 1.57 and 1.82, respectively. In our opinion, the increase in the number of voids in the structure of the film can increase the WVP.
Regarding the WVP result, the film based on semolina (range 8.97–8.01 × 10−7 g. m/m2. d. Pa) had lower permeability than achira flour film (4.57 × 10−5 g. m/m2. d. Pa) and amaranth flour film (2.67 × 10−5 g. m/m2. d. Pa) because of the high amount of gluten in semolina, which causes compact, tight, and semi-crystallization structure film, so molecules and gases will have less chance of permeability. This is true since there is less free volume in crystalline regions compared to amorphous regions. Molecules and gases are not able to move around freely in crystalline areas because they are tightly composed.[Citation27,Citation28]
Oxygen barrier properties
Barriers to oxygen in a packaging system can increase the shelf-life of food products and also improve the quality of food.[Citation29] The OP values of semolina films with various concentrations of sorbitol/glycerol are summarized in . The OP of the semolina film with 30% plasticizer was 2.24 ± 0.095 [cc.mil/(m2.day.atm)], which decreased to 2.03 ± 0.04 [cc.mil/(m2.day.atm)] after increasing the plasticizer content up to 50% (w/w), thus revealing improved oxygen barrier properties of the semolina films with increasing plasticizer content. These results may be attributed to the fact that sorbitol is composed of six hydroxyl groups, so it can form a larger number of hydrogen bonds with the protein and starch than glycerol, which in turn can decrease OP of a film.[Citation30] Moreover, according to Siracusa[Citation31], permeate diffusion across a film is influenced by the film thickness, in which an increase in film thickness may result in a decrease in O2 permeability, as observed in the present study.
Film color
Color and transparency are essential parameters in many applications, especially in food packaging, where visual inspection of the contents is of key relevance and could influence the opinion of consumers.[Citation27] gives the L*, a*, and b* color values of the semolina films affected by various plasticizer concentrations. As can be seen in , with the exception of green-red (a*) value, significant differences (p < 0.05) were observed for lightness (L*) and yellow-blue shade (b*) values between the semolina-based films plasticized with different amounts of sorbitol/glycerol. Increasing plasticizer concentration in the semolina films (up to 50%, w/w) resulted in increased L- and b-values (). So, the addition of plasticizers to the semolina films improved the optical properties of the films, which is similar to those previously reported on color parameters as a result of adding glycerol.[Citation21,Citation23]
Table 2. Apparent colorimetric parameters of semolina films incorporated with various sorbitol/glycerol concentrations.
Light transmittance
The ability of the biodegradable materials to absorb UV light is important for extending the shelf-life of fatty foods which are susceptible to the oxidative degradation induced by UV radiation.[Citation22] Three zones have been identified for the UV region, namely UVC (180–280 nm), UVB (280–320 nm), and UVA (320–400 nm). Light transmission of the semolina-based films together with some synthetic films at selected wavelengths is shown in . A notable barrier property was demonstrated for the semolina films in the UVC range (180–280 nm) and negligible transmission was noted at 200–280 nm for films.
Table 3. Light transmission (%) values of semolina films incorporated with various sorbitol/glycerol concentrations.
Protein-based films are generally known to have excellent UV barrier properties due to the presence of a high content of aromatic amino acids which can absorb UV light.[Citation32] According to Aitken and Learmonth,[Citation33] barrier properties of proteins against UV radiation are associated with the presence of UV-absorbing chromophore, especially aromatic amino acids tyrosine and tryptophan, and to a lesser extent, phenylalanine and disulfide bonds. On the other hand, some synthetic polymer films, such as oriented polypropylene and low-density polyethylene, did not prevent the passage of the UV range of 200–280 nm[Citation34] (see ).
Mechanical properties
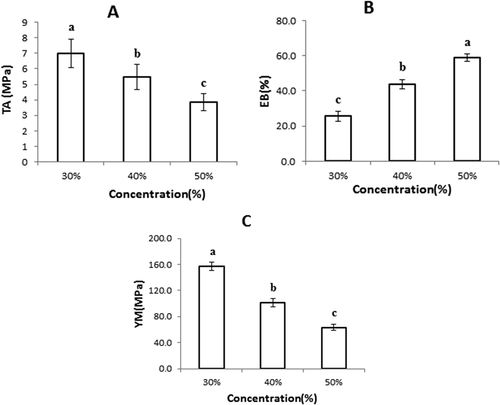
shows the TS, EAB, and EM of the semolina-based films plasticized with different contents of plasticizer. Generally, the TS of the films was inversely correlated with the concentration of the plasticizer, while EAB was positively correlated.[Citation35] Likewise, in this study, increasing the concentration of sorbitol/glycerol in the semolina films from 30% to 50% (w/w) decreased the TS and EM of the films from 7.17 to 3.85 MPa and 156.82 to 63.44 MPa, respectively (Fig. 2a and 2b); but EAB was increased from 25.67% to 58.78% (Fig. 2c). This behavior could be related to the structural modifications of starch and protein networks when plasticizer was incorporated. The matrix of the film becomes less dense, facilitating movement of polymer chains under stress, which decreases the film resistance. Plasticizers interfere with polymeric chain association, which facilitates their slipping and enhances film flexibility. They also decrease the rigidity of the network, produce a less-ordered film structure, and increase the ability of polymer chain movement.[Citation36] The addition of plasticizer to film has a large effect on film mechanical properties, such as increasing film flexibility and resilience.[Citation22] Similar trends were observed on plasticized tara gum[Citation35] or chia seed mucilage-based edible films.[Citation22]
FTIR spectra
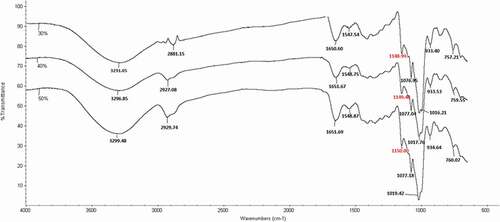
The FTIR spectra of the semolina-based edible films plasticized with different contents of sorbitol/glycerol are shown in . The broad band at 3600–3200 cm−1 is a result of the O-H stretching vibration associated with free and inter- and intramolecular bound hydroxyl groups and the peaks in the region 3000–2800 cm−1 is a result of C-H stretching.[Citation35] Different plasticizer contents resulted in no remarkable difference of absorbance in these regions. The absorption peaks which correspond to the stretching vibrations of amide bonds associated with the protein network covers a range between 1200 and 1700 cm−1.[Citation37] The band situated at the wavenumber of ~1077 cm−1 was found in all film samples corresponding to the sorbitol/glycerol (-OH group) which was added as a plasticizer (especially glycerol) and is related to carbohydrate (starch).[Citation38] The spectra of plasticized semolina films of the same general shape as nonsignificant shifts in the wavenumber of the ether bonds are present in the region of 1100–900 cm−1. It has been previously reported that plasticizers had no significant interaction with the protein.[Citation39]
Thermal behavior
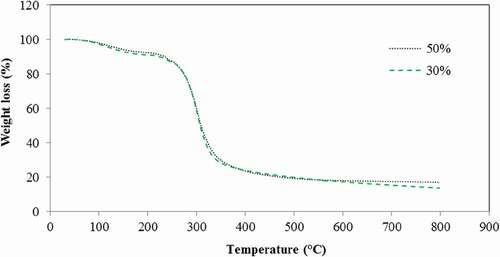
The thermal degradation behavior of the semolina-based films with different plasticizer concentrations is shown in . Semolina films exhibited three main stages of weight loss with a first peak in the range of 50–100°C (<100°C), which was associated with the vaporization of loosely bound water[Citation40]; a second one occurred at 120–290°C and corresponded to the evaporation of low-molecular-weight compounds in the films such as plasticizer compounds;[Citation41] and a third stage of weight loss assigned to the thermal decomposition of the protein that occurred above 290°C. Overall, these results suggested that increasing glycerol concentration has no remarkable influence on the magnitude of weight loss.
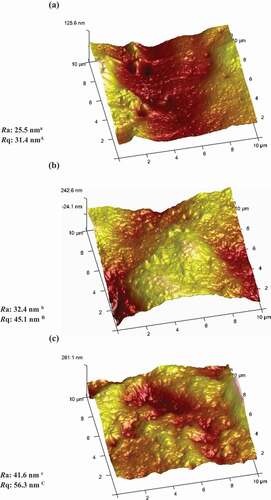
Atomic force microscopy
The AFM technique is a powerful tool for studying surfaces and has been used to provide qualitative and quantitative information about biopolymers at the nanometer scale that are often inaccessible by any other experimental technique. AFM can be useful for identifying structural differences of films prepared by different methods.[Citation42]
shows the surface morphologies and the corresponding results of roughness parameters (Ra and Rq) of the films with various plasticizer concentrations. The 30% (w/w) plasticizer-containing film exhibits a relatively rough surface, with Ra and Rq values of 25.5 and 31.4 nm, respectively (Fig. 5a). The film surface became rougher as plasticizer content was increased. According to the AFM images, semolina films, including 50% (w/w) plasticizer (Fig. 5c), were rougher, as indicated by higher Ra and Rq values (41.6 and 56.3 nm, respectively) (p < 0.05). These results are in agreement with those of Ghasemlou et al.[Citation23] for kefiran-based films containing various plasticizer types. These authors described that the coalescence and creaming of oil droplets may increase the surface roughness of the films. Other researchers obtained similar results, which show that pure Soluble soybean polysaccharide (SSPS) films were the smoothest and showed the lowest values of all roughness parameters. The film surfaces became increasingly rough as glycerol was incorporated.[Citation43]
Conclusion
This work contributes to the understanding of the plasticizing influences of sorbitol/glycerol (3:1) at three different levels on the properties of semolina-based protein films. This study demonstrated a relationship between plasticizer contents in semolina films and their solubility, MC, WVP, and OP. Moreover, semolina flour films exhibited high UVC light barrier properties. Increasing plasticizer concentration in the semolina films improved flexibility and decreased film resistance. Semolina films containing a high concentration of glycerol were slightly yellowish in color but still had a transparent appearance. Finally, semolina flour protein is an interesting ingredient for the design of new FFS. This research has revealed that such edible films plasticized with sorbitol/glycerol have the potential to make new edible films with required specifications. Further studies should be performed to modify semolina film attributes regarding improvement of water solubility and WVP.
Disclosure statement
The authors have no conflicts of interest to disclose. This article does not contain any studies with human or animal subjects.
References
- Jafarzadeh, S.; Alias, A.; Ariffin, F.; Mahmud, S. Characterization of Semolina Protein Film with Incorporated Zinc Oxide Nano Rod Intended for Food Packaging. Polish Journal of Food and Nutrition Sciences 2017, 67(3), 183–190.
- Janjarasskul, T.; Krochta, J. M. Edible Packaging Materials. Annual Review of Food Science and Technology 2010, 1, 415–448. DOI: 10.1146/annurev.food.080708.100836.
- Chang, C.; Nickerson, M. T. Effect of Plasticizer-Type and Genipin on the Mechanical, Optical, and Water Vapor Barrier Properties of Canola Protein Isolate-Based Edible Films. European Food Research and Technology 2014, 238, 35–46. DOI: 10.1007/s00217-013-2075-x.
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effects of Plasticizers on the Properties of Edible Films from Skin Gelatin of Bigeye Snapper and Brownstripe Red Snapper. European Food Research and Technology 2006, 222, 229–235. DOI: 10.1007/s00217-005-0004-3.
- Voon, H. C.; Bhat, R.; Easa, A. M.; Liong, M. T.; Karim, A. A. Effect of Addition of Halloysite Nanoclay and SiO2 Nanoparticles on Barrier and Mechanical Properties of Bovine Gelatin Films. Food and Bioprocess Technology 2012, 5, 1766–1774. DOI: 10.1007/s11947-010-0461-y.
- Jafarzadeh, S.; Alias, A. K.; Ariffin, F.; Mahmud, S.; Najafi, A.; Sheibani, S. Characterization of a New Biodegradable Edible Film Based on Semolina Loaded with Nano Kaolin. International Food Research Journal 2017, 24, 1.
- Sissons, M.; Abecassis, J.; Marchylo, B.; Carcea M. Durum wheat chemistry and technology, 2nd ed. St. Paul, MN: AACC International. 2012
- Onyeneho, S. N.; Hettiarachchy, N. S. Antioxidant Activity of Durum Wheat Bran. Journal of Agricultural and Food Chemistry 1992, 40, 1496–1500. DOI: 10.1021/jf00021a005.
- Jafarzadeh, S.; Ariffin, F.; Mahmud, S.; Alias, A. K.; Najafi, A.; Ahmad, M. Characterization of Semolina Biopolymer Films Enriched with Zinc Oxide Nano Rods. Italian Journal of Food Science 2017, 29, 2.
- Hernandez-Izquierdo, V. M.; Krochta, J. M. Thermoplastic Processing of Proteins for Film Formation – A Review. Journal of Food Science 2008, 73, 30–39. DOI: 10.1111/j.1750-3841.2007.00636.x.
- Araujo-Farro, P. C.; Podadera, G.; Sobral, P. J.; Menegalli, F. C. Development of Films Based on Quinoa (Chenopodium Quinoa, Willdenow) Starch. Carbohydrate Polymers 2010, 81, 839–848. DOI: 10.1016/j.carbpol.2010.03.051.
- Karbowiak, T.; Hervet, H.; Leger, L.; Champion, D.; Debeaufort, F.; Voilley, A. Effect of Plasticizers (Water and Glycerol) on the Diffusion of a Small Molecule in K-Carragennan Biopolymer Films for Edible Coating Application. Biomacromolecules 2006, 7, 2011–2019. DOI: 10.1021/bm060179r.
- Gontard, N.; Guilbert, S.; Cuq, J. L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties Using Response Surface Methodology. Journal of Food Science 1992, 57, 190–195. DOI: 10.1111/jfds.1992.57.issue-1.
- ASTM. Standard Test Method for Water Vapor Transmission of Materials (E 96-05); Philadelphia: PA, USA, 2005.
- ASTM. Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor (D 3985-05); Philadelphia: PA, USA, 2005.
- ASTM. Standard Test Method for Tensile Properties of Thin Plastic Sheeting (D 882-19); Philadelphia: PA, USA, 2010.
- Sabbah, M.; Di Pierro, P.; Esposito, M.; Giosafatto, C. V. L.; Mariniello, L.; Porta, R. Stabilization of Charged Polysaccharide Film Forming Solution by Sodium Chloride: Nanoparticle Z-Average and Zeta-Potential Monitoring. Journal of Biotechnology & Biomaterials 2016. DOI: 10.4172/2155-952X.1000e128.
- Carneiro-da-Cunha, M. G.; Cerqueira, M. A.; Souza, B. W.; Teixeira, J. A.; Vicente, A. A. Influence of Concentration, Ionic Strength and pH on Zeta Potential and Mean Hydrodynamic Diameter of Edible Polysaccharide Solutions Envisaged for Multinanolayered Films Production. Carbohydrate Polymers 2011, 85, 522–528. DOI: 10.1016/j.carbpol.2011.03.001.
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, Antioxidant and Antimicrobial Properties of Chitosan–Cinnamon Leaf Oil Films as Affected by Oleic Acid. Food Hydrocolloids 2014, 36, 256–264. DOI: 10.1016/j.foodhyd.2013.10.003.
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophysics 2016, 11, 43–51. DOI: 10.1007/s11483-015-9411-6.
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.-S.; Jahandideh, F. Development and Characterization of a Novel Biodegradable Edible Film Obtained from Psyllium Seed (Plantago Ovata Forsk). Journal of Food Engineering 2012, 109, 745–751. DOI: 10.1016/j.jfoodeng.2011.11.010.
- Dick, M.; Costa, T. M. H.; Gomaa, A.; Subirade, M.; De Oliveira Rios, A.; Flôres, S. H. Edible Film Production from Chia Seed Mucilage: Effect of Glycerol Concentration on Its Physicochemical and Mechanical Properties. Carbohydrate Polymers 2015, 130, 198–205. DOI: 10.1016/j.carbpol.2015.05.040.
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Rheological and Structural Characterisation of Film-Forming Solutions and Biodegradable Edible Film Made from Kefiran as Affected by Various Plasticizer Types. International Journal of Biological Macromolecules 2011, 49, 814–821. DOI: 10.1016/j.ijbiomac.2011.07.018.
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Water Vapour Permeability, Thermal and Wetting Properties of Whey Protein Isolate Based Edible Films. International Dairy Journal 2010, 20, 53–60. DOI: 10.1016/j.idairyj.2009.07.008.
- Wang, Q.; Padua, G. W. Properties of Zein Films Coated with Drying Oils. Journal of Agricultural and Food Chemistry 2005, 53, 3444–3448. DOI: 10.1021/jf047994n.
- Ghanbarzadeh, B.; Oromiehie, A. R.; Musavi, M.; Rezayi, K.; Razmi, E.; Milani, J. Investigation of Water Vapour Permeability, Hydrophobicity and Morphology of Zein Films Plasticized by Polyols. Iranian Polymer Journal 2006, 15, 691–700.
- Andrade-Mahecha, M. M.; Tapia-Blacido, D. R.; Menegalli, F. C. Development and Optimization of Biodegradable Films Based on Achira Flour. Carbohydrate Polymers 2012, 88(2), 449–458. DOI: 10.1016/j.carbpol.2011.12.024.
- Tapia-Blácido, D. R.; do Amaral Sobral, P. J.; Menegalli, F. C. Optimization of amaranth flour films plasticized with glyceroland sorbitol by multi-response analysis. LWT-Food Science andTechnology 2011 44(8), 1731–1738.
- Jafarzadeh, S.; Ariffin, F.; Mahmud, S.; Alias, A. K.; Hosseini, S. F.; Ahmad, M. Improving the Physical and Protective Functions of Semolina Films by Embedding a Blend Nanofillers (Zno-Nr and Nano-Kaolin). Food Packaging and Shelf Life 2017, 12, 66–75. DOI: 10.1016/j.fpsl.2017.03.001.
- Ghanbarzadeh, B.; Oromiehie, A. R.; Musavi, M.; Falcone, P. M.; Rad, E. R. Study of Mechanical Properties, Oxygen Permeability and AFM Topography of Zein Films Plasticized by Polyols. Packag Technology and Sciences 2007, 20, 55–163. DOI: 10.1002/pts.750.
- Siracusa, V.;. Food Packaging Permeability Behaviour: A Report. International Journal of Polymer Science 2012, 2012, 1–11. DOI: 10.1155/2012/302029.
- Hoque, M. S.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of Blend Film Based on Cuttlefish (Sepia pharaonis) Skin Gelatin and Mungbean Protein Isolate. International Journal of Biological Macromolecules 2011, 49, 663–673. DOI: 10.1016/j.ijbiomac.2011.06.028.
- Aitken, A.; Learmonth, M. P. Protein Determination by UV Absorption. In The Protein Protocols Handbook; Walker, J. M., ed; Human Press Inc: Totowa, NJ, 1996.
- Ramos, Ó. L.; Reinas, I.; Silva, S. I.; Fernandes, J. C.; Cerqueira, M. A.; Pereira, R. N.; Vicente, A. A.; Pocas, M. F.; Pintado, M. E.; Malcata, F. X. Effect of Whey Protein Purity and Glycerol Content upon Physical Properties of Edible Films Manufactured Therefrom. Food Hydrocolloids 2013, 30, 110–122. DOI: 10.1016/j.foodhyd.2012.05.001.
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H. J.; Zhong, F. Physicochemical and Thermomechanical Characterization of Tara Gum Edible Films: Effect of Polyols as Plasticizers. Carbohydrate Polymers 2014, 111, 359–365. DOI: 10.1016/j.carbpol.2014.04.005.
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. Journal of Texture Studies 2013, 44(3), 176–186.
- Pérez, L. M.; Piccirilli, G. N.; Delorenzi, N. J.; Verdini, R. A. Effect of Different Combinations of Glycerol and/or Trehalose on Physical and Structural Properties of Whey Protein Concentrate-Based Edible Films. Food Hydrocolloids 2016, 56, 352–359. DOI: 10.1016/j.foodhyd.2015.12.037.
- Bergo, P.; Sobral, P. J. A. Effects of Plasticizer on Physical Properties of Pigskin Gelatin Films. Food Hydrocolloids 2007, 21, 1285–1289. DOI: 10.1016/j.foodhyd.2006.09.014.
- Embuscado, M. E.; Huber, K. C. Edible Films and Coatings for Food Applications; springer: Dordrecht, The Netherlands, 2009; pp. 295–314.
- Dang, K. M.; Yoksan, R. Development of Thermoplastic Starch Blown Film by Incorporating Plasticized Chitosan. Carbohydrate Polymers 2015, 115, 575–581. DOI: 10.1016/j.carbpol.2014.09.005.
- Reddy, J. P.; Rhim, J.-W. Characterization of Bionanocomposite Films Prepared with Agar and Paper-Mulberry Pulp Nanocellulose. Carbohydrate Polymers 2014, 110, 480–488. DOI: 10.1016/j.carbpol.2014.04.056.
- Ghanbarzadeh, B.; Oromiehie, A. R.; Musavi, M.; Falcone, P. M.; Rad, E. R. Study of Mechanical Properties, Oxygen Permeability and AFM Topography of Zein Films Plasticized by Polyols. Packaging Technology and Science 2007, 20(3), 155–163. DOI: 10.1002/pts.750.
- Tjik, S.; Maghsoudlou, Y.; Khodaian, F.; Jafari, S. M.; Ghasemlou, M.; Alami, M. Soluble Soybean Polysaccharide: A New Carbohydrate to Make a Biodegradable Film for Sustainable Green Packaging. Carbohydrate Polymers 2013, 97, 817–824. DOI: 10.1016/j.carbpol.2013.05.037.