ABSTRACT
Similarity evaluation of complicated chromatographic profiles is a potential instrument for the identification and quality control of herbal medicinal products to ensure the prospective biological activity. The present study was implemented to evaluate the quality of Sonchus brachyotus DC. sampled using high-performance liquid chromatography (HPLC) fingerprinting coupled with a suite of chemometrics methods. By this means, the coefficients of correlation of similarity of 10 batches extract of S. brachyotus DC. were >0.98. The 10 batches extract of Sonchus brachyotus DC. showed stable antioxidant capacity in vitro. The results of total antioxidant capacity were in accordance and completed well for the quality evaluation of Sonchus brachyotus DC. Thus, HPLC fingerprinting coupled with chemometrics techniques provides a greatly flexible and dependable method for the quality assessment of the extract of Sonchus brachyotus DC.
Introduction
Disquisitions on herbal medicines have been involved in several pharmacopeias. Hence, modern methods depicting the identification and quantification of active ingredients in the plant raw material may be of service to proper standardization of herbs and their formulations, the World Health Organization (WHO) has stressed the demand to assure the quality of medicinal plant products making use of modern controlled techniques and putting proper standards into use.[Citation1,Citation2] Fingerprinting technique is a mighty and broadly instrument for the quality control of polymerous herbal medicines and their derivative products. In addition, fingerprint analysis has been brought in and adopted by WHO as a method for the evaluation of herbal medicines[Citation3], and it is also demanded by the State Food and Drug Administration of China to standardize injections manufactured from traditional Chinese medicines and their rough materials.[Citation4]
Sonchus brachyotus DC. belongs to the Sonchus family which can be discovered in China, Japan, Mongolia, and Russia’s far east. Sonchus brachyotus DC. is a natural wild vegetable with a long history and rich nutrition, which contains a great deal of constant and trace elements, and these elements participate in the body’s metabolism and maintain good health; in addition, taking in these trace elements will have an evident effect on human health and development for the long-term. When Sonchus brachyotus DC. was used as a medicine for the whole herbs, which was known as lost grass, it has the function of heat-clearing and detoxifying, swelling and empyema, stasis, and analgesia.[Citation5] Sonchus Brachyotus DC. is a wild plant used for ornamental, edible, and medicinal purposes and has important ecological and economic value.[Citation6] A prevenient study discovered that Sonchus brachyotus DC. has antimicrobial activities against a quantity of pathogenic microorganisms.[Citation7] Sonchus brachyotus DC. can induce apoptosis of A549 cells and inhibit its growth and proliferation, which is a potential medicine and food plant for the prevention and inhibition of tumor growth.[Citation8] Our group also proved that the extract form Sonchus brachyotus DC. could exhibit antimicrobial activity against Escherichia coli, Enterobacter cloacae, Klebsiella pneumonia, Salmonella enteric, Staphylococcus aureus, and Micrococcus luteus; this is especially so in the case of Escherichia coli.[Citation9] Xia et al.[Citation7] provided evidence indicated that the methanol extract from Sonchus brachyotus DC. was capable of antioxidant ability. However, how to realize the control by the antioxidant capacity stability of the extract from Sonchus brachyotus DC. is necessary.
Chinese medicines and their derivative products are widely used as therapeutic products in many countries. Most Chinese medicine formulae comprise a complex mixture of different herbs, each of which may consist of hundreds of chemical components. Consequently, it is necessary to define all of the active chemical constituents of Chinese medicine extracts to guarantee safety, reliability, and repeatability for clinical efficacy and pharmacological studies.[Citation10] The medicinal significance of the plant is a reflection of its chemical composition since the plant includes many bioactive material.[Citation11] Hence, the target of the present study is to exploit phytochemical bolting and high-performance liquid chromatography (HPLC) fingerprinting of Sonchus Brachyotus DC., which may be utilized as a marker for quality assessment and standardization of the medicine.
Materials and method
Reagents
Analytical-grade ethanol (Beijing, China) was utilized for sample preparation. Methanol and acetonitrile of HPLC grade was purchased from the Thermo Fisher Scientific (Beijing, China). High-purity water was gained utilizing a Millipore Milli-Q water purification system (MA, USA) and analytical-grade methanoic acid (Beijing, China) was used for preparation of mobile phase. Ephedrine (Purity 98%) was purchased from Chinese pharmaceutical biological products inspection institute.
Preparation of standard solutions and plant material
Ephedrine was precisely weighed and dissolved in water, and then diluted to proper concentration ranges for backup. All the samples of Sonchus brachyotus DC. were collected from Beijing, P. R. China, in May 2015. All specimens were authenticated by Xiu-Mei Li (Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, P. R. China). A voucher specimen was deposited at the Feed Research Institute (Specimen number: P20150501).
Preparation of sample preparation
All the samples of Sonchus brachyotus DC. were collected from Shandong (China). The aerial parts of the dried plants were gained and then chosen through a 60-mesh sieve. Each sample dust (1.00 g) was precisely weighed and derivative utilizing 30 mL 3:1 (v/v) ethanol-water solution and changing pH to 5 by ultrasonic extraction for 30 min at 55°C and 700 w. After centrifugation at 5000 g for 3–5 min, the eluate was evaporated by rotary evaporation at 40 ± 2°C. Before utilize, 1 g of the extract was weighed and dissolved in 10 mL of 3:1 (v/v) ethanol-water and filtered through a 0.22-μm membrane filters.
Instrumental conditions
An HPLC system being constitutive of an Agilent 1200 series pump, an autosampler, a binary pump, and a 1200 series UV-vis diode array detector was utilized for all analyses. HPLC separation was implemented on a Kromasil C18 column (250 × 4.6 mm i.d.; 5 μm).
Data analysis
Data analysis was implemented utilizing the professional software Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (version 2004 A). The coefficients of correlation of total chromatographic profiles among samples were computed, and the simulative mean chromatogram was computed and produced. All data on the total antioxidant capacity (TAC) of 10 batches are shown as means ± SD of three independent experiments. Data were statistically compared utilizing one-way ANOVA with Tukey post hoc, and p < 0.05 was considered statistically significant.
Assay of TAC
The ferric reducing antioxidant power (FRAP) was evaluated according to Benzie et al. [Citation12] utilizing a HewlettPackard 8453 diode array spectrophotometer. The method is based on the reduction of the Fe3+-TPTZ complicated to the ferrous form at low pH. This reduction is supervised by surveying the absorption variation at 593 nm. Succinctly, 180 mL of working FRAP reagent prepared daily was mixed with 5 μL of identical concentrations sample; the absorbance at 593 nm was obtained after a 3–5 min incubation at 37°C. FRAP values were gained by comparing the absorption variation in the measurement mixture with those gained from increasing concentrations of Fe3+ and expressed as mmol of Fe2+ equivalents per g of the extract from Sonchus Brachyotus DC.
Results and discussion
Optimization of HPLC conditions
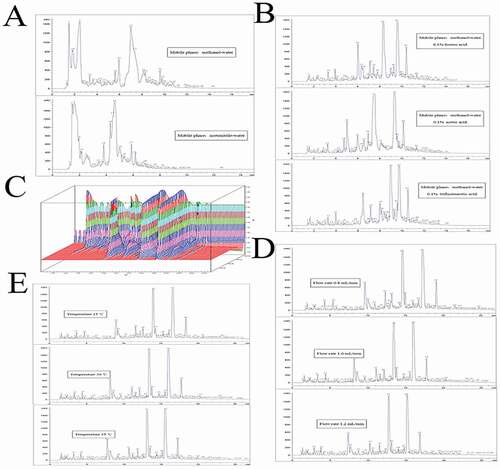
HPLC fingerprinting stresses the systematic characterization of components of samples and converged on the identification and evaluation of the stability of the constituents in complicated systems.[Citation13–Citation15] This may not be adequate to control the entire quality of herbal preparations as a result of the lack of quantitative information of active compounds.[Citation16] For this reason, it would be better to detect the main bioactive components comtemporaneously, and the employment of HPLC fingerprinting is specially suggested. [Citation17] Some combinative methods utilizing HPLC fingerprints and quantitative detection of bioactives have been arranged and were implemented to assess the quality of herbal preparation.[Citation18–Citation20] In order to gain the chromatograms with better separation of adjacent peaks and more peaks within a short time, measure wavelength, mobile phase, organic acid, flow rate, and column temperature were researched. As demonstrated in ), compared with acetonitrile, methanol had better resolutions and more peaks, the number of peaks is 44 and 45, respectively, and the toxicity of acetonitrile is stronger than that of methanol; hence, a methanol-water system was selected to be utilized as the mobile phase. In addition, organic acid could effectively improve the shape of peaks, when organic acids are 0.1% formic acid, 0.1% acetic acid, and 0.1% trifluoroacetic acid, and the number of peaks is 63, 61, and 59, respectively. Thus 0.1% formic acid was added to the methanol-water system to further modify the peak shape ()). The gradient elution procedure was as follows: from 0 to 8 min, solvent A linearly varied from 5% to 25%; from 8 to 10 min, solvent A linearly varied from 25% to 35%; from 10% to 20 min, solvent A linearly varied from 35% to 60%; from 20 to 24 min, solvent A linearly varied from 60% to 80%; and from 24 to 27 min, solvent A linearly maintained 80%. Moreover, in order to gain a great deal of detectable peaks on the HPLC chromatogram, a full-scan experiment was implemented of the extract of Sonchus brachyotus DC. from 200 to 400 nm. As demonstrated in , 260 nm was chosen as the detection wavelength, as a result that more characteristic peaks could be gained. Furthermore, for increasing the analysis efficiency, the flow rate is 0.8, 1.0, and 1.2 mL/min, and the number of peaks is 77, 80, and 78, respectively, so the flow rate was optimized as 1.0 mL/min ()). Different column temperatures (25–35°C) were also researched for their effects on the separation of the extract from Sonchus Brachyotus DC., and the number of peaks is 72, 80, and 75, respectively, and it could be reviewed that the separation effect was better when the column was immersed into a column oven kept at 30°C compared with 25°C or 35°C. The column was positioned in a column heater set at 30°C (as shown in )), and the injection volume was 10 μL.
Figure 1. HPLC conditions of the extract of Sonchus Brachyotus DC. Kromasil C18 column (250 × 4.6 mm i.d.; 5 μm). (A) Mobile phase; (B) organic acid; (C) 3D wavelength scan chromatogram of the extract of Sonchus Brachyotus DC.; (D) flow rate; and (E) column temperature.
Further, a significant factor in fingerprint development is the selection of stationary phase. In the present work, a particle-based C18 column which broadly measured herbal fingerprinting HPLC column was utilized. The efficiency of these columns is highly reliance on their particle size (normally 3–5 μm). When the particle size is halved, the pressure quadruples, proposing that smaller particle sizes strongly increase the backpressure. As the present HPLC instrumentation can withstand up to 400 bar, higher efficiencies and more peaks by reducing particle size and increasing the flow rates are blocked.[Citation21]
Method validation
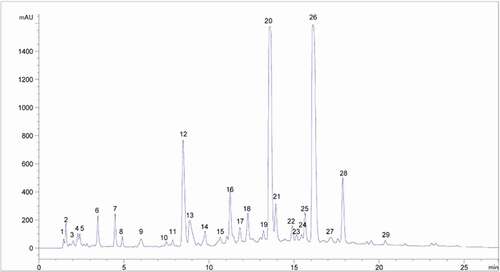
HPLC fingerprint analysis is not a quantitative method. The parameters assessed and validation aspects are various from common assaying methods.[Citation22,Citation23] The HPLC fingerprint analysis is specific and may be utilized for qualitative measurement and compositive assessment of Chinese herbal medicine. Utilizing the HPLC method, samples were analyzed in the optimum circumstances.[Citation24] The average chromatogram from the samples was considered as the standardized characteristic fingerprint of Sonchus Brachyotus DC. Peaks that existed in all samples with reasonable heights and great resolution are appointed as ‘‘common peaks’’ for identification of the extract. In our study, among all the peaks surveyed, 29 common peaks within 27 min, which appeared in all samples that were detected for fingerprint parameters assessment based on chromatographic trajectory; intensity and stability of fingerprint peaks were marked, as shown in . Among these constituents, peak 12 (ephedrine) pointed out a steady content; hence, it was selected to calculate the relative retention time (RRT) and the relative peak area (RPA). The figuring formulas of RRT and RPA were RRT = RTpeak/RTpeak12 and RPA = PApeak/PApeak12, respectively. In addition, the other 28 peaks also showed relatively high absorption intensities and correlative differences in the field of retention time, and were also selected as characteristic peaks for the measurement and evaluation of extract of Sonchus Brachyotus DC.
Figure 2. A representative fingerprint extract of Sonchus Brachyotus DC. Peaks 1–29 were used as common characteristic peaks.
To assure the precision of the method, six replicated measurement of the same sample were implemented in 1 day. The RSD of RRT and RPA of 29 common peaks were not more than 3.01% and 4.94%, respectively. Six independent sample solutions of Sonchus brachyotus DC. were prepared in parallel and analyzed for assessment of repeatability, by assessing that the RSD of RRT and RPA of 29 common peaks were all <0.86% and < 4.99%, respectively. A stability test was performed by analyzing the same sample over intervals of 0, 2, 4, 6, 8, 12, and 24 h. During this period, the solution was deposited at room temperature. The RSD of RRT and RPA of 29 common peaks did not exceed 2.05% and 4.98%, respectively. The similarity of these consequences to those from the repeatability and the injection precision pointed out that the sample maintained steady state during this period. The data for precision, repeatability, and stability of the suggested method are listed in ; From the validation measurement mentioned above, the total analytical procedure was discovered to be accurate and applicable, and hence was deemed suitable for analysis of a good deal of extract of Sonchus brachyotus DC. set up in the present work.
Table 1. Precision, repeatability, and stability data of the proposed method.
Quality control of the extract of Sonchus Brachyotus DC
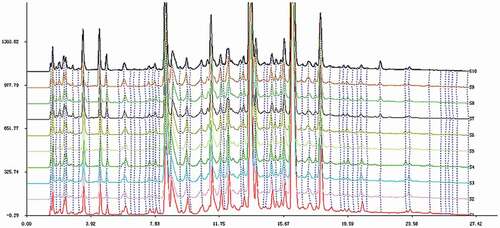
In this study, 10 batches (from Shandong) of Sonchus brachyotus DC. (labeled as S1–S10) prepared as mentioned above were assessed to confirm batch-to-batch consistency under the chosen chromatographic conditions to set up HPLC fingerprint chromatograms. The superposition graph () was gained by utilizing the software ‘‘Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine,’’ reported by the Chinese Pharmacopoeia Commission (Version 2004A, Beijing, China), and was utilized to analyze the similarity of chromatographic and spectral patterns; the Mean Chromatographic Fingerprint could be created by this software which was suggested by State Food and Drug Administration (Beijing, China)[Citation25], and the similarity values between the fingerprints of each sample were figured.
TAC
An ideal antioxidant drug must have a prominent effect. Therefore, we researched the TAC of the crude extract from Sonchus Brachyotus DC. With identical concentrations 200 μg/mL of the extract from Sonchus Brachyotus DC., evaluating TAC of the extract from Sonchus brachyotus DC. for 10 batches. The TAC for different batches of the extract from Sonchus brachyotus DC. is extremely similar and steady (), pointing out that good quality control to ensure the stability of antioxidant effects of the extract from Sonchus Brachyotus DC.
Table 2. The similarities (correlation coefficients) and the TAC of 10 batches.
Conclusion
In this study, the chromatographic fingerprints demonstrated similar component and full sets of detectable compositions, in which not only were majority of the common characteristic peaks emerge but also the peak-to-peak spatial distribution were steady and consistent. Moreover, similarities among 10 batches of samples were >0.98, pointing out that the samples from same location had sufficient similarity in chemical component to show the quality and stability of the extract from Sonchus Brachyotus DC. The consistent and distinction of fingerprints of a category of samples becomes a promising method for sample comparison and quality evaluation. Hence, fingerprint analysis coupled with chemometric paths is an innovative, responsible, and undemanding approach that can provide a potent and significant way to elaborately implement quality evaluation of Sonchus Brachyotus DC. Given that the validation parameters meet the standards, the approach may be applied for conventional quantitative determination of Sonchus Brachyotus DC. The proposed approach is accurate and repeatable for quantitative bioanalyses of Sonchus Brachyotus DC. Stability tests showed that Sonchus brachyotus DC. is steady in practical conditions. The simplicity, rapid nature, and low cost of this approach allow laboratories to perform conventional analysis of Sonchus brachyotus DC. using HPLC instrumentation. Moreover, our data also demonstrate that Sonchus brachyotus DC. could be utilized as a resource for the development of novel drugs to scavenge reactive oxygen species. In addition, quality evaluation can also be applied to other edible and medicinal plants.
Abbreviations used
| RRT | = | relative retention time |
| RPA | = | relative peak area |
| WHO | = | World Health Organization |
| FRAP | = | ferric reducing antioxidant power |
| TAC | = | total antioxidant capacity. |
Additional information
Funding
References
- Chaudhay, R. R.; Herbal Medicine for Human Health. Regional publication.SEARO, No. 20. WTO: New Delhi, 1992, 1–80.
- WHO.1998.Quality Control Method for Medicinal Plant Material.World Health Organization: Geneva. 2301–2315.
- WHO. Guidelines for the Assessment of Herbal Medicines; World Health Organization: Geneva, 1991.
- State Food and Drug Administration. Guidance for Experimental Research on HPLC Fingerprint of Traditional Chinese Injections (Draft). Beijing, 2000. Retrived from: http://www.sda.gov.cn/WS01/CL0237/15768.html
- Huo, J. The Determination of Traces of Element in the Radixes of Sonchus Brachyotus DC. With Microwave Digestion Icp-Aes Method. Journal of Tianjin University of Science & Technology 2003, 18(1), 33–35.
- Shi, L. R.; Liu, Z. H. Influences of Drought Stress on Antioxidative Activity and Osmoregulation Substance of Sonchus Brachyotus DC. Acta Agrestia Sinica 2010, 18, 5, 673–677.
- Xia, D. Z.; Yu, X. F.; Zhu, Z. Y.; Zou, Z. D. Antioxidant and Antibacterial Activity of Six Edible Wild Plants (Sonchus Spp.) In China. Natural Product Research 2011, 25(20), 1893–1901. DOI: 10.1080/14786419.2010.534093.
- Yan-Yun, H. E.; Liu, X. M.; Zhang, C. Y.; Hua, Z. Y. Apoptosis of Lung Cancer Line A549 Induced by Sonchus Branchyotus DC. Aqueous Extracts. Natural Product Research & Development 2014, 26(9), 1380–1384.
- Li, X. M.; Liu, J.; Du, Y. K.; Wen, Z. G.; Wang, J. T.; Yang, P. L. Antibacterial Activity and Mechanism of the Ethanol Extracts from Sonchus Brachyotus DC. International Journal of Food Properties 2016, 20(3) 2923–2931.
- Gao, X.; Yang, X. W.; Marriott, P. J. Evaluation of Coptidis Rhizoma-Euodiae Fructus Couple and Zuojin Products Based on HPLC Fingerprint Chromatogram and Simultaneous Determination of Main Bioactive Constituents. Pharmaceutical Biology 2013, 51(11), 1384–1392. DOI: 10.3109/13880209.2013.793719.
- Sunil, K.; Paras, S.; Amit, J.; Mukesh, S. S. Preliminary Phytochemical Screening and HPTLC Fingerprinting of Nicotiana Tabacum Leaf. Journal of Pharmacy Research 2010, 3, 5, 1144–1145.
- Benzie, I. F.; Strain, J. J. Ferric Reducing Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods in Enzymology 1999, 299, 1, 15–27.
- Drasar, P.; Moravcova, J. Recent Advances in Analysis of Chinese Medical Plants and Traditional Medicines. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences 2004, 812(1–2), 3–21. DOI: 10.1016/j.jchromb.2004.09.037.
- Liu, A. H.; Lin, Y. H.; Yang, M.; Guo, H.; Guan, S. H.; Sun, J. H.; Guo, D. A. Development of the Fingerprints for the Quality of the Roots of Salvia Miltiorrhiza and Its Related Preparations by HPLC-DAD and LC-MSn. Journal Chromatogr B Analyt Technol Biomed Life Sci 2007, 846(1–2), 32–41. DOI: 10.1016/j.jchromb.2006.08.002.
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO Press: Geneva, Switzerland, 2007.
- Li, Y.; Wu, T.; Zhu, J. H.; Wan, L.; Yu, Q.; Li, X.; Cheng, Z.; Guo, C. Combinative Method Using HPLC Fingerprint and Quantitative Analysis for Quality Consistency Evaluation of an Herbal Medicinal Preparation Produced by Different Manufacturers. Journal of Pharmaceutical & Biomedical Analysis 2010, 52(4), 597–602. DOI: 10.1016/j.jpba.2010.01.018.
- Lu, Y.; Chen, D. F. Analysis of Schisandra Chinensis and Schisandra Sphenanthera. Journal of Chromatography A 2009, 1216(11), 1980–1990. DOI: 10.1016/j.chroma.2008.12.003.
- Tang, D. Q.; Yang, D.; Tang, A.; Gao, Y.; Jiang, X.; Mou, J.; Yin, X. Simultaneous Chemical Fingerprint and Quantitative Analysis of Ginkgo Biloba Extract by HPLC-DAD. Analytical & Bioanalytical Chemistry 2010, 396(8), 3087–3095. DOI: 10.1007/s00216-010-3536-8.
- Wei, H.; Sun, L. N.; Tai, Z.; Gao, S.; Xu, W.; Chen, W. A Simple and Sensitive HPLC Method for the Simultaneous Determination of Eight Bioactive Components and Fingerprint Analysis of Schisandra Sphenanthera. Analytica Chimica Acta 2010, 662(1), 97–104. DOI: 10.1016/j.aca.2009.12.039.
- Xu, L. N.; Han, X.; Qi, Y.; Xu, Y.; Yin, L.; Peng, J.; Liu, K.; Sun, C. Multiple Compounds Determination and Fingerprint Analysis of Lidanpaishi Tablet and Keli by High Performance Liquid Chromatography. Analytica Chimica Acta 2009, 633(1), 136–148. DOI: 10.1016/j.aca.2008.11.043.
- Tistaert, C.; Dejaegher, B.; Heyden, Y. V. Chromatographic Separation Techniques and Data Handling Methods for Herbal Fingerprints. Analytica Chimica Acta 2011, 690(2), 148–161. DOI: 10.1016/j.aca.2011.02.023.
- Xie, P.; Feasible, A. Strategy for Applying Chromatography Fingerprint to Assess Quality of Chinese Herbal Medicine. Traditional Chinese Drug Research & Clinical Pharmacology 2001, 12(3), 141–151.
- Ji, Y. B.; Xu, Q. S.; Hu, Y. Z.; Heyden, Y. Development, Optimization and Validation of a Fingerprint of Ginkgo Biloba Extracts by High-Performance Liquid Chromatograph. Journal of Chromatography A 2005, 1066(1–2), 97–104. DOI: 10.1016/j.chroma.2005.01.035.
- Li, X. M.; Luo, X. G.; Ma, N.; Li, K.; Li, W.; Ma, D. Y.; Zhang, T. C. Quality and Antitumour Activity Evaluation of Extract of Hypericum Ascyron. Biomedical Chromatography 2015, 29(1), 47–52. DOI: 10.1002/bmc.3169.
- Liang, Y. Z.; Xie, P.; Chan, K. Quality Control of Herbal Medicines. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences 2004, 812(1–2), 53–70. DOI: 10.1016/S1570-0232(04)00676-2.