ABSTRACT
Hovenia acerba Lindl. is a perennial tree of Rhamnaceae family. In the present study, four solvents (80% methanol, ethyl acetate, n-hexane, and water) were used to extract the compounds of Hovenia acerba Lindl. peduncles (HAP). The total contents of phenolics and tannins (TPC and TTC), as well as their antioxidant activities (DPPH, ABTS, and FRAP) of these four different extracts including 80% methanol extract (ME), ethyl acetate extract (EE), n-hexane extract (HE) and water extract (WE) were compared. The antioxidant activities of HAP showed positive correlations with TPC and TTC, indicating that phenolics maybe a major contributor to the antioxidant activities of HAP. The significant difference between antioxidant activities and TPC/TTC implied the respective contribution extent of phenolics for the antioxidant activities of four solvent extracts. Besides, a total of 117 compounds, including 7 oligosaccharides, 11 organic acids, 41 phenolics, 1 steroids, 25 terpenes, 11 fatty acids, 9 amino acids, and 12 other compounds were identified or tentatively characterized in HAP for the first time. Among them, 23 bioactive constituents, including 4 organic acids, 5 phenolic acids, and 14 flavonoids, were quantified. The comparison of characterization and quantitation of 4 extracts demonstrated that 80% methanol showed the widest range of selectivity for both HAP flavonoid aglycones and glycosides, while ethyl acetate preferred to extract the flavonoids aglycone from HAP. Secondly, water and 80% methanol were excellent solvents for the extraction of phenolic acids. And both ethyl acetate and 80% methanol perform well at the extraction of terpenes and steroids.
Introduction
Hovenia acerba Lindl. (HA), Hovenia trichocarpa (HT), and Hovenia dulcis Thunb. (HD), belonging to Rhamnaceae family, are perennial trees with deciduous large-leaves. They are native species in East Asia, including China, Japan, and Korea. Although the leaves, inflorescences, and flowers of HA, HT, and HD are different in plant taxonomy, they belong to the same genus (Hovenia), the peduncles of them are the main edible parts and their functional activities are similar. Herein, HA and HD are more widely cultivated and consumed as fruit or herb with extensively studied health effects such as accelerating detoxification of ethanol, hepatoprotective, antioxidative, antimicrobial, anti-hyperlipidaemia, and antidiabetic properties.[Citation1–Citation3] In traditional medicine formula, the peduncles of Hovenia acerba Lindl. (HAP) and Hovenia dulcis Thunb. (HDP) are generally used as the febrifuge, laxative, and diuretic agent, which nowadays have been also developed as functional food and dietary supplement such as fermented juice, vinegar, syrup, or capsule.[Citation3] In last several years, enormous studies were focused on the health benefits of common vegetables and fruits on the basis of their certain bioactive constituents such as phenolics, polyphenols, and saponins. HDP and HAP are also rich in phenolic compounds, polysaccharides, sterenes, and terpenes which are verified as a group of predominated bioactive compounds. Representatively, different polysaccharide fractions were separated and purified from HDP and proved with significant hepatoprotective and immunostimulatory activities.[Citation4] And all of the flavonoids, organic acids, and some other low-molecular-weight compounds including amino acids, terpenes, and its glycosides derivatives in HDP were thought as a potential contributor to antioxidant activity.[Citation1,Citation5–Citation9] However, the chemical ingredients analysis of Hovenia genus plant especially of HA is still scarce. In the present study, LC-QTOF-MS (liquid chromatography orthogonal acceleration quadrupole time-of-flight mass spectrometry) and LC-QqQ-MS (liquid chromatography triple quadrupole tandem mass spectrometry) are employed to better analyse the full profile of small-size macronutrients and phytochemicals in HAP.[Citation10]
Moreover, the chemical compositions and bioactivities of plant extract varied with different extraction solvents. One of the main causes is that the selectivity of solvents on extracting specific compounds is diverse, which is positively correlated with the similarity between the polarity of targeted compounds and solvents. Exemplarily, higher contents of chlorogenic acid in Helianthus annuus L. were found in the aqueous alcohol and water extracts rather than that in ethyl acetate extract, which was due to the higher similarity between chlorogenic acid and methanol/water.[Citation11] Similarly, higher catechin and lower epicatechin contents were found in 80% acetone extract of Hordeum vulgare L than that in 80% methanol extract.[Citation12] Moreover, a previous study on Matricaria pubescens showed that the extraction yield of phenolics ranged from 5.05% to 34.68% with different extraction solvents (50% acetone, 100% acetone, water, and 50% ethanol), and 50% ethanol ranked first in not only the extract yield but also the total phenolic content (TPC) and antioxidant activities, followed by those of 50% acetone, water, and 100% acetone.[Citation13] As well, it was reported that the TPC of 90% methanol and water extracts of Helianthus annuus (2522.5 mg GAE/100 g DW and 2455.8 mg GAE/100 g DW, respectively) were significantly higher than those of 50% ethanol (1760.7 mg GAE/100g DW) and ethyl acetate (141.5 mg GAE/100 g DW).[Citation11] The study in Tetrastigma hemsleyanum also reported that 80% methanol extract higher contents of phytochemicals with greater antioxidant activities than those of ethyl acetate and hexane.[Citation14] These reports above suggested that solvents could significantly affect the targeted compound content of plant extracts.
Generally, hexane is usually used to extract the lipophilic bioactive ingredients (carotenoids, tocopherols, phenolic lipids, phytosterols, and polyunsaturated fatty acids). However, it is not suitable to extract the hydrophilic compounds such as non-aromatic organic acids, flavonoids, and phenolic acids. On the other hand, ethyl acetate is relatively low hydrophilic even if it is less hydrophobic than hexane, thus it prefers to extract the compounds in polarity between hexane and methanol such as low-polar phenolics (flavonoid aglycones and phenolic acid esters) and steroids with fewer impurities such as organic acids and saccharides. Though several bioactive studies of HAP extracts have been conducted, few studies focused on the effects of different solvents on the extraction of individual chemical constituents of HAP. Therefore, to unveil the full ingredient profiles and the main bioactivity contributors of HAP, as well as to investigate the effects of different solvents on the individual chemical constituents of HAP, solvents in various polarity including ethyl acetate and n-hexane, as well as water and 80% methanol should be applied.
In this study, a total of four different solvents (80% methanol, ethyl acetate, n-hexane, and water) were used to extract the chemical compounds of HAP. The in vitro antioxidant activities (DPPH, ABTS, and FRAP) of these four extracts (80% methanol extract, ME; ethyl acetate extract, EE; hexane extract, HE; and water extract, WE) were also compared. Meanwhile, a combination of UPLC-ESI-QTOF-MS/MS and HPLC-ESI-QqQ-MS/MS method was established to clarify the specific structures of full compound profiles as well as the certain quantity of the main phenolics in these four HAP extracts. And the structure–function relationships between phenolics and the antioxidant activities of HAP extracts were emphatically discussed.
Materials and methods
Plant materials
Hovenia acerba Lindl. peduncles were purchased from a local grocery store in Nanchang, Jiangxi Province, China, in April 2015. HAPs were collected, washed, cut into small pieces, and ground with a commercial blender (Philips, Netherlands) to obtain a homogeneous paste. Approximately 100 g of this paste was air-dried at 40°C with an electro-thermostatic blast oven (Cimo, China) and ground into a fine powder. These materials were stored at −80°C prior to analysis.
Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH), Folin-Ciocalteu reagent, gallic acid, 1,3,5-tri(2-pyridyl)-2,4,6-triazine (TPTZ), 2,2ʹ-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid; ABTS), catechin and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma Co. (St. Louis, MO, USA). All the standards including citric acid, malic acid, abscisic acid, p-hydroxybenzoic acid, ferulic acid, isoferulic acid, quinic acid, chlorogenic acid, protocatechuic acid, apigenin, naringenin, quercetin, taxifolin, gallocatechin, epigallocatechin, myricetin, ampelopsin, rutin, naringenin-7-O-glucoside, chrysin, genistein, and epicatechin were purchased from Shanghai Aladdin Reagents Co. (Shanghai, China). Methanol, ethyl acetate, n-hexane, acetonitrile, and hydrochloric acid (HCl) were purchased from Merck (Darmstadt, Germany). Formic acid was purchased from ACROS Organics (Morris, IL, USA). Sodium acetate, ferric chloride hexahydrate (FeCl3·6H2O), potassium persulfate (K2S2O8), sodium phosphate monobasic, and sodium phosphate dibasic were purchased from Xilong Chemical Co. (Guangzhou, China).
Sample extraction
Briefly, the air-dried HAP powder (2.0 g) was accurately weighed and extracted with 40 mL solvent (80% methanol, ethyl acetate, n-hexane, or water) by ultrasonic extraction for 30 min (400 w, 22 Hz, repeated twice, GA92-IID, Wuxi, China). The mixture was centrifuged at 4200 rpm for 15 min (Sorvall SL16 centrifuge, Thermo Scientific, USA). The extraction was repeated for 3 times and the supernatants were combined, topped up to 120 mL, and filtered through a 0.45-µm PTFE membrane filter (Agilent Technologies, USA). The filtrate was used as a crude extract for the further analysis of LC-MS/MS, TPC, TTC, and antioxidant activities.
Total phenolic contents
The TPC of the extract was estimated using the Folin-Ciocalteu method.[Citation15] In brief, 25 μL gallic acid standard or sample was mixed with 125 μL 0.2 M Folin-Ciocalteu reagents in a 96-well plate and incubated for 6 min at 37°C in a MCO-15AC constant temperature incubator (SANYO Electric Co., Ltd., Osaka, Japan). Then, 125 μL saturated sodium carbonate (Na2CO3) solution was added and allowed to react for 15 min at 37°C before the absorbance was recorded at 765 nm using a Synergy HTX microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The TPC was expressed as milligram gallic acid equivalent per gram of dry weight HAP (mg GAE/g DW). The linearity range of the calibration curve was 0.025–1.6 mg/mL (R2 = 0.9992).
Total tannin contents
The total tannin content (TTC) was determined by vanillin-sulphuric acid colorimetric method.[Citation15] Briefly, 40 μL extract of HAP or standard was mixed with 200 μL of vanillin reagent (containing 1% vanillin and 2.5 M H2SO4 in methanol) in a 96-well microplate (Synergy HTX, BioTek Instruments, Inc., Winooski, VT, USA) and allowed to stand for 20 min at room temperature. The absorbance was recorded at 515 nm and the result was expressed as milligram catechin equivalents per gram of dry weight HAP (mg CAE/g DW) using the calibration curve of (+)-catechin. The linearity range of the calibration curve was 0.0125–1.0 mg/mL (R2 = 0.9947).
Antioxidant activities
DPPH radical scavenging activity: the DPPH antioxidant activity was determined by a microplate reader (Synergy HTX, BioTek Instruments, Inc.,Winooski, VT, USA) scaled down from a previous method.[Citation6] Briefly, 200 μL of a methanolic solution of DPPH (300 μg/mL) was mixed with 15 μL of HAP extract or a standard solution (12.5–1000 μM Trolox) in a 96-well plate. The mixture was allowed to react for 30 min at room temperature before the absorbance was recorded at 490 nm. DPPH antioxidant activities were calculated as micromole of Trolox equivalent per gram of dry weight HAP (μmol TE/g DW, R2 = 0.9989).
ABTS radical scavenging activity: ABTS radical scavenging activity was evaluated adapted from the previous method.[Citation10] The stock solutions included 0.2 mL 7.4 mM ABTS+ solution, 0.2 mL 2.6 mM oxidant solution (potassium persulfate), and 0.1 mL 10 mM Trolox solution. ABTS working solution was prepared by reacting ABTS+ stock solution with oxidant solution in a ratio of 1:1 (v/v) for 12 h before use. The mixed ABTS+ solution was diluted with 80% methanol to the absorbance of 0.700 ± 0.050 at 406 nm. Approximately 10 μL of sample or Trolox standard was added to 200 μL of diluted ABTS+ solution, and absorbance was measured at 734 nm after 6 min reaction at room temperature. Trolox was used for preparing a calibration curve for a concentration range of 12.5–1000 μM. The antioxidant activities were expressed as micromole Trolox equivalents per gram of dry weight HAP (μmol TE/g DW, R2 = 0.9994).
FRAP assay: the FRAP assay was conducted according to Peng et al.[Citation10] Briefly, 10 μL of FeSO4 standard or extract was added to a 96-well plate and react with 200 μL of ferric-TPTZ reagent [prepared by mixing 300 mM acetate buffer, pH 3.6, 10 mM TPTZ in 40 mM HCl with 20 mM FeCl3∙6H2O at a ratio of 10:1:1 (v/v/v)]. The mixture was incubated at 37–C for 30 min, and the absorbance was measured at 595 nm. The standard curve was linear between 25 and 1600 μM FeSO4. The results were expressed as micromole FeSO4 equivalents per gram of dry HAP (μmol FE/g DW, R2 = 0.9990).
Qualitative analysis by LC-ESI-QTOF-MS/MS
Liquid chromatographic conditions: the Agilent 1290 infinity series UPLC system, consisted of a degasser, a binary pump Bin Pump SL, a thermostated HiP-ALS autosampler, a TCC SL column oven and a DAD detector (Agilent Technologies, USA), was used for the analysis of HAP extracts. Separation was done in an Eclipse XDB-C18 column (4.6 mm × 250 mm, 5 μm, Agilent Technologies, USA). The column was thermostatically controlled at 40°C and the flow rate was 0.7 mL/min. The mobile phase consisted of two solvents: water (A, 0.1% acetic acid) and methanol (B, 0.1% acetic acid). The UV absorbance of the peaks was recorded over a range of 190–600 nm and monitored at 280, 320, and 360 nm. The injection volume was 5 μL, and the linear gradient solvent system was as follows: 0–7 min, 5–40% B; 7–25 min, 40–65% B; 25–30 min, 65–80% B; 30–35 min, 80% B; 35–60 min, 80–95% B; 60–65 min, 95–100% B; 65–70 min, 100–5% B.
Mass Spectrometric Conditions: a 6538 Accurate-Mass QTOF LC/MS system (Agilent Technologies, USA) was equipped with an orthogonal electrospray ionization source (ESI). The ESI source was operated in negative ions mode, and full scan mass spectral data were acquired from m/z 100 to 1700. The optimum source parameters were as follows: capillary voltage (+ 4.0 kV); drying gas flow (10.0 L/min); drying gas temperature (350°C); nebulizing gas pressure (40 psi). Moreover, the pseudomolecular ions [M-H]− were selected as precursor ions and subjected to MS/MS analysis. The collision energy was set at 20 eV, and the fragmentor voltage was 175 V, using nitrogen as a collision gas.
Quantitative analysis by LC-ESI-QqQ-MS/MS
Liquid chromatographic conditions: a 6430 QqQ LC/MS triple quadrupole system (Agilent Technologies, USA) coupled with an Agilent 1260 HPLC system (Agilent Technologies, USA) was used for the quantitative analysis. The LC conditions were the same as 2.7.1. Mass spectrometric conditions: a 6430 QqQ LC/MS system equipped with an ESI source was operated in negative ion multiple reaction monitoring (MRM) mode. The optimum source parameters were as follows: capillary voltage (+4.0 kV); drying gas flow (11.0 L/min); drying gas temperature (300°C); nebulizing gas pressure (15 psi). The fragment voltages and collision energies were optimized individually for each transition and two fragment ions were chosen for the qualitative monitor, and one for the quantitative monitor.
Calibration and quantification of organic acids and phenolics: to prepare a specific calibration plot, all of the 23 standards were accurately weighted by a Mettler Toledo XS105 analytical balance (Mettler Toledo, Inc., Greifensee, Zurich, Switzerland) and dissolved in methanol. The calibration curves were made by injecting each external standard solution in triplicate at concentrations of 0–100 μg/mL.
Statistical analysis
Results were expressed as means ± standard deviations of three independent extractions. The LC-MS data were acquired and analysed by the software MassHunter Acquisition B.03.01, Qualitative Analysis B.03.01 and Quantitative Analysis B.03.02. Other data were analysed by the SPSS statistical software, version 18.0 (SPSS Inc., USA). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests to determine significant differences at the level of P < 0.05. Regression analysis was used to determine the correlation between two datasets.
Results
TPC and TTC
It has been reported that the HDP extracts extracted by different solvents yielded a wide range of phenolic extraction efficiency (18.7% by water, 14.3% by methanol, 4.3% by ethyl acetate, 3.5% by chloroform).[Citation16] In the present study, the highest extraction yield of 4 HAP extracts was conducted by 80% methanol (61.35%), followed by water (40.99%), hexane (8.88%), and ethyl acetate (2.58%). Maieves et al. reported that the TPC of HDP ranged from 4.32 to 17.78 mg GAE/g DW and the total flavonoid content changed from 0.42 to 13.54 mg CAE/g DW at different maturation stages.[Citation6] Hu et al. also reported that the TPC of HDP extracts ranged from 1.42 to 13.21 mg tannic acid equivalents (or mg TAE)/g DW.[Citation16] But there was no analogous report on the HAP until present study. As shown in , ME has the highest TPC value (12.46 mg GAE/g DW), which was significantly higher than that of WE (5.06 mg GAE/g DW), HE (1.24 mg GAE/g DW), and EE (1.12 mg GAE/g DW). And the highest TTC of HAP was found in ME (11.17 mg CAE/g DW), followed by WE (6.78 mg CAE/g DW), HE (5.46 mg CAE/g DW), and EE (1.03 mg CAE/g DW). For both TPC and TTC, there was no significant difference between HE and EE (P > 0.05).
Table 1. The total phenolic, tannin contents, and antioxidant activities of HAP extracted by different solventsa.
According to the “like dissolve like” principle, the extraction efficiency of a certain compound extracted by different solvents is directly related to the compatibility between this compound and the solvent system.[Citation17] In this study, both 80% methanol and water showed great extraction efficiency in extracting phenolics and tannins of HAP. And the former performed the best in all of the four solvents. Therefore, the results of TPC and TTC in the present study agreed with most of the data reported previously, 80% methanol extracted the higher amount of phenolics and tannins from HAP than other solvents.
Antioxidant activities
In the present study, the antioxidant activities of HAP extracts were assessed by three different methods: DPPH, ABTS, and FRAP. As the results showed, ME of HAP had the highest DPPH value (60.95 μmol TE/g DW), followed by WE, HE, and EE (31.24, 5.31, 2.10 μmol TE/g DW, respectively, ). The sequence of the antioxidant ability of different extracts was the same as those of TPC and TTC in HAP (ME > WE > HE > EE). Moreover, the values of ABTS and FRAP were in the degressive order of ME (59.38 μmol TE/g DW, 82.43 μmol FE/g DW), water (22.06 μmol TE/g DW, 20.47 μmol FE/g DW), n-hexone (3.62 μmol TE/g DW, 4.72 μmol FE/g DW), and EE (1.21 μmol TE/g DW, 4.32 μmol FE/g DW, ), which showed an identical order with those of DPPH antioxidant activity.
HDP extracts were reported to have good antioxidant activities (DPPH: 2.88–32.33 μmol TE/g DW; ABTS: 10.92–115.24 μmol TE/g DW; FRAP: 10.55–59.57 μmol TE/g DW), which was considered as the contribution of phenolic compounds, organic acids, and polysaccharides.[Citation6,Citation16] While few studies provided the corresponding data about HAP. In our study, there were significant positive correlations between TPC and antioxidant activities of HAP extracts (DPPH: R2 = 0.9781, P < 0.05; ABTS: R2 = 0.9992, P < 0.01; FRAP: R2 = 0.9783, P < 0.05), which proving that phenolic compounds were the main contributors of excellent antioxidant activities of HAP.
Characterization of chemical constituents of HAP by UPLC-ESI-QTOF-MS
According to previous reports, only a total of 12 compounds (hovenin A-D, kaempferol, 4ʹ,5,7-trihydroxy-3ʹ,5ʹ-dimethoxyflavonoe, vanillic acid, emodin, apigenin, quercetin, myricetin, and taxifolin) were found in the seeds of HA, while other four compounds (saponin C2, hovacerboside A1, coumaroylquinic acid, and 4-hydroxy-N-methylproline) were detected in its leaves.[Citation2] And the full phytochemical data of HAP is still under a shortage. On the other side, although the phenolics in ME have been recognized above as the major contributors to the antioxidant activities of HAP, a few other chemical constituents in low amount are still cannot be ignored for their outstanding bioactivities published before. Therefore, the full profiles of HAP extracted by different solvents were identified rapidly and accurately by UPLC-ESI-QTOF-MS.
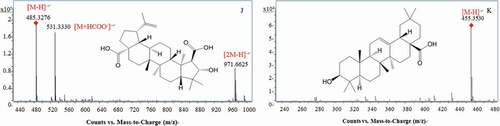
All the identified compounds detected in four HAP extracts were summarized in and S1 (supplemental material). The 67 major antioxidants including phenolics and sapogenins (terpenes and steroids) were found in HAP (), and the other phytochemical ingredients were listed in Table S1. These chemical constituents in HAP were identified based on their retention times (tR), UV spectra, MSn data, fragmentation pattern and compared with published literature and/or standards. A total of 117 compounds, including 7 oligosaccharides, 11 organic acids, 11 phenolic acids, 30 flavonoids, 1 steroids, 25 terpenes, 11 fatty acids, 9 amino acids, and 12 other compounds, were identified or tentatively identified in HAP for the first time. The total ion chromatograms (TIC) and DAD chromatogram at 280 nm of ME were shown in , while those of the other extracts (HE, WE, EE) were placed in supplemental materials (Figure S1-S3). The MS/MS spectra, chemical structures, and fragmentation patterns of several representative compounds were shown in and S4. The details of 117 compounds were described as follows.
Table 2. Characterization of the main phytochemicals in different HAP extracts by UPLC-ESI-QTOF-MS/MS.
Figure 1. Total ion chromatogram (TIC) in negative ion mode (A1, A2) and DAD chromatogram at 280 nm (B1, B2); A1, B1: 0–30 min, A2, B2: 30–70 min.
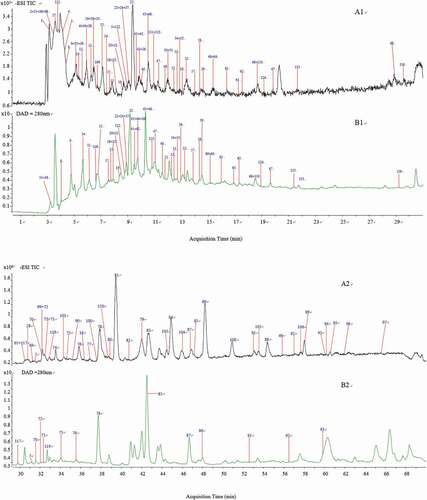
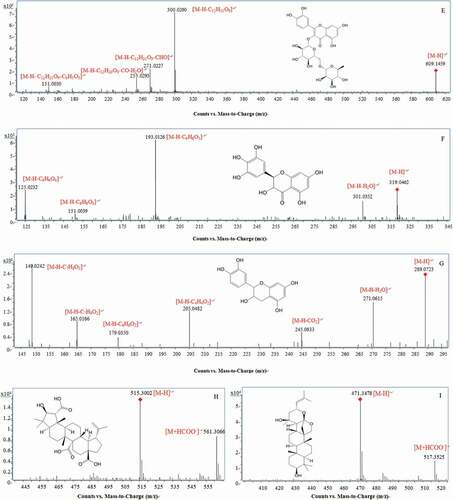
Figure 2. MS/MS spectra and fragmentation patterns of some typical phetochemicals of the peduncles of Hovenia acerba Lindl.: gentiobiose (A); citric acid (B); protocatechuic acid (C); ferulic acid (D); rutin (E); ampelopsin (F); catechin (G); ceanothetric acid (H); jujubogenin (I); ceanothic acid (J); and oleanolic acid (K).
Phenolic acids
A total of 11 phenolic acids, including ferulic acid, hydroxybenzoic acid, protocatechuic acid, and their derivatives, were identified in HAP. Compounds 15 (tR 7.698 min, m/z 153.0193), 43 (tR 9.696 min, m/z 353.0878), 44 (tR 10.348 min, m/z 137.024), 57 (tR 13.716 min, m/z 193.0501), and 63 (tR 17.007 min, m/z 193.0504) were identified as protocatechuic acid (), chlorogenic acid, p-hydroxybenzoic acid, ferulic acid, and isoferulic acid (), respectively, by comparing with the authentic standards. Compounds 18, 19, 20, 54, 62, and 110 were characterized as protocatechuic acid hexoside, woodorien, 2,4-dihydroxy-6-pyruvylbenzoic acid, ethyl gallate, 4-p-coumaroylquinic acid, and 3-(4-methoxyphenyl) propanoic acid vicianoside based on their MSn data and UV absorption. Specifically, compound 18 (tR 7.848 min, m/z 315.0730), with the fragment ions at m/z 153.0125 [M-H-C6H10O5]− and 109.0126 [M-H-C6H10O5-CO2]−, was tentatively assigned to protocatechuic acid hexoside.[Citation18] Furthermore, compound 54 (tR 11.30 min, m/z 197.0455), with the predicted molecular formula of C9H10O5 and fragment ions at m/z 169.0152 [M-H-C2H4]− and 124.0142 [M-H-C3H5O2]−, was identified as ethyl gallate.[Citation19] With the precursor ions [M-H]− at 329.087, compound 19 was tentatively identified as 3-(b-D-glucopyranosyloxy)-4-hydroxy-benzoic acid-methyl ester (woodorien).[Citation20] Compounds 20 (tR 8.581 min, m/z 223.0243) and 62 (tR 2.16 min, m/z 337.0933) were tentatively assigned to 2,4-dihydroxy-6-pyruvylbenzoic acid and 4-p-coumaroylquinic acid according to the predicted molecular formula (C10H8O6 and C16H18O8). With the fragment ions at m/z 341.1234 [M-H-C5H8O4]−, 179.0695 [M-H-C6H10O5-C5H8O4]−, 164.0471 [M-H-C11H18O9-CH3]−, compound 110 (tR 18.695 min, m/z 473.1673) was identified as 3-(4-methoxyphenyl) propanoic acid vicianoside.[Citation21]
Among these 11 phenolic acids, woodorien, 2,4-dihydroxy-6-pyruvylbenzoic acid, chlorogenic acid, ethyl gallate, 4-p-coumaroylquinic acid, and 3-(4-methoxyphenyl) propanoic acid vicianoside were identified in ME and WE, while protocatechuic acid, protocatechuic acid hexoside, p-hydroxybenzoic acid, ferulic acid, and isoferulic acid could be found in ME, EE, and WE, and no phenolic acid was found in HE ().
Flavonoids
The antioxidant, anti-inflammatory, and antiproliferative activities of flavonoids have been reported before.[Citation14] A total of 30 flavonoids, including 14 flavones, 6 flavanones, 7 flavanes and 3 aurones, were found in HAP (). Flavones and derivatives: Compounds 59 (tR 14.709 min, m/z 609.1459, , 70 (tR 32.203 min, m/z 269.0451), 119 (tR 32.741 min, m/z 283.0608), and 124 (tR 14.709 min, m/z 301.0354) were unambiguously identified as rutin, apigenin, genistein, and quercetin by comparing with the authentic standards. And compounds 47, 51, 52, 53, 55, 60, 117, and 118 were identified as quercetin 3-glucoside-7-rutinoside, 3ʹ-O-methylmyricetin, quercetin 3-neohesperidoside-7-rhamnoside, myricetin-rutinoside and myricetin-hexoside, myricetin-rhamnoside, kumatakenin, and 3-hydroxy-2ʹ,4ʹ,7-trimethoxyflavone according to their MS1 and/or MS2 data, as well as UV spectrum.[Citation22–Citation26] Among these 14 flavones, 3ʹ-O-methylmyricetin and myricetin were only found in ME, while chrysin, apigenin, 3-hydroxy-2ʹ,4ʹ,7-trimethoxyflavone, and genistein were only identified in EE. Kumatakenin and quercetin were found in both ME and EE, while other flavones including quercetin 3-glucoside-7-rutinoside, quercetin 3-neohesperidoside-7-rhamnoside, myricetin-rutinoside, myricetin-hexoside, rutin, and myricetin-rhamnoside were observed in either ME or WE.
Flavanones and derivatives: Compounds 46, 56, 115, and 123 were identified as dihydromyricetin (), dihydroquercetin, naringenin, and naringenin-7-O-glucoside compared with the commercial standards. Compounds 24 and 116 were characterized as dihydroquercetin-O-rutinoside and dihydrochrysin.[Citation27] Among these six flavanones, dihydroquercetin-O-rutinoside was only found in ME. Naringenin could only be detected in EE and dihydrochrysin was found in both of ME and EE. Dihydromyricetin and naringenin-7-O-glucoside were found in all the ME, EE, and WE, while dihydroquercetin was found in both ME and WE.
Flavanes and derivatives: Compounds 21, 22, 109, and 122 were identified as epigallocatechin, epicatechin, gallocatechin, and catechin (). Compounds 11 and 13 were identified as epigallocatechin dimer and an unsure epigallocatechin derivative. And Compound 17 was suggested as procyanidin B.[Citation10] Among these 7 flavanes, epigallocatechin dimer was only present in ME, while the unsure epigallocatechin derivatives (compound 13) and epigallocatechin were detected in both of ME and WE. Epicatechin, procyanidin B, gallocatechin, and catechin could be found in all the ME, EE, and WE.
Three aurones and derivatives: Compounds 42, 45, and 64 were identified as maesopsin 4-O-glucoside (hovetrichoside C), maesopsin 4-O-glucoside-4ʹ-O-rhamnoside (hovetrichoside D), and maesopsin, respectively.[Citation28] All these three aurones could be detected in ME of HAP, but only maesopsin 4-O-glucoside-4ʹ-O-rhamnoside was found in EE.
Terpenes and steroids
Terpenes and steroids have higher molecular weights and less polar functional groups, resulting in the lower water solubility and longer retention time (30–60 min) in the reverse elution procedure. A total of 25 terpenes and 1 steroid were tentatively identified in HAP extracts. Compounds 7 (m/z 397.2237) was identified as steroids (stephanol). And compounds 49, 72, 73, 75, 76, 79, 81, 83, 84, 86, 87, 90–94, 100–104, 106, 108, 114, and 120 were characterized as obtusin, hovenic acid, ceanothetric acid (), hovenidulcigenin A, jujubogenin (), fupenzic acid, ceanothic acid (), 3-O-coumaroylarjunolic acid, gypsogenin, ursonic acid, pyracrenic acid, segetalic acid, hovenidulcigenin B, ganoderic acid H, 3-O-protocatechuoylceanothic acid, zizyberenalic acid, ganoderic acid TR, 3-dehydroxy ceanothetric acid, oleanolic acid (), ganoderic acid V, maslinic acid, helicterilic acid, abrisapogenol I, polygalic acid, and platanic acid, respectively.[Citation29]
Terpenes and steroids are distributed throughout various plants as free saponin aglycones and have long been known to have a number of biological effects, such as antioxidant, antitumor, and antimicrobial activities.[Citation7,Citation30,Citation31] Most of these compounds (except polygalic acid and ganoderic acid H) were found in ME and EE. Polygalic acid could only be detected in ethyl acetate, and ganoderic acid H was only found in ME. Fupenzic acid, gypsogenin, ursonic acid, hovenidulcigenin B, 3-O-protocatechuoylceanothic acid, zizyberenalic acid, ganoderic acid TR, oleanolic acid, and ganoderic acid V were monitored in not only ME and EE but also hexane.
Organic acids, oligosaccharides, fatty acids, amino acids, and other compounds
A total of 11 organic acids (malic acid, citric acid/, abscisic acid, quinic acid, gluconic acid, succinic acid, xylonic acid, sanleng acid, adipic acid, benzoic acid, and azelaic acid), 7 hexose derivatives (gentiobiose/, gentianose, gentiobiose/gentianose, yuheinoside, sucrose-6-acetic ester, tetra-O-acetyl-deoxy-D-glucopyranose and sucrose 4,6-methyl orthoester), 10 fatty acids (dihydroxyoctadecadienoic acid, traumatic acid, dihydroxy octadecenoic acid, 9-hydroxy octadecatrienoic acid, 9-hydroxy-10,12-octadecadienoic acid, linolenic acid, linoleic acid, palmitic acid, oleic acid, and stearic acid), 9 amino acids (6-aminohexanoic acid, glycylglycine, fructosyl phenylalanine, fructosyl leucine/isoleucine, tyrosine, pyroglutamic acid, L-glutamic acid, tryptophan, and fructosyl aspartic acid) and 12 other compounds (cordine, cimidahurinine, hovetrichoside G, 6-maleimidocaproic acid, 6,7-dihydroxycoumarin and polydatin, guanosine, uridine, gingerglycolipid B, petrosyne Ia, loganin, and heptaoxatricosanedioic acid) were identified in HAP. The details could be checked in Table S1.
In the present study, 117 compounds were firstly and comprehensively detected in HAP relied on not 1 but 4 solvents with their own characteristics and preferences in extracting bioactive constituents. As the present result of ingredients characterization showed, most of these 11 fatty acids (with the lowest polarity) could be found in all the HE, ME, and EE but not WE. Their higher solubility of fatty acids might be attributed to the same groups (alkyl/hydroxyl/acyl) existing between fatty acids and hexane/methanol/ethyl acetate. Similarly, it is more efficient for low polar compounds such as terpenes extracted by ethyl acetate, hexane and even 80% methanol, mainly due to their long carbon chain (generally 20–40 carbons) along with a small number (3–8) of hydroxyl/carboxyl sides. And water preferred to extract the medium-high polar compounds such as saccharides, organic acids, and amino acids with a high ionization property. These similar groups between compounds and solvents enable these compounds to dissolve. In consideration of the characterization data as well as TPC and TTC, the extraction preference of four solvents for phenolics of HAP was clarified. Firstly, 80% methanol and water were suggested to possess wider ranges of selectivity for phenolic compounds of HAP and available to extract more content from them. On the contrary, ethyl acetate and hexane showed not only a low extract efficiency but also a narrow selectivity for HAP phenolics, thus most of phenolics detected in EE were flavonoid aglycones. Although the results demonstrated that there were extensive tannins exist in HAP (which was the potential cause of astringency of HAP), there was only a small amount of proanthocyanin dimers detected by UPLC-QTOF-MS in the present study. It implied that these tannins might have a relatively high polymerization degree, beyond the detectable range (m/z at 100–1700) of our LC-QTOF-MS.
Many of the edible wild or cultivated plants have both therapeutic and dietary functions. These plants contain a myriad of bioactive phytochemicals that render their potential beneficial health effects via different mechanisms. These functional phytochemicals could be terpenoids including monoterpenoids, sesquiterpenes, and diterpene, polyphenols including stilbenes, phenolic acids, and flavonoids, and other chemical classes of compounds such as carotenoids and glucosinolates. They are known to influence oxidative stress and modulate cell-signalling pathways, hence may be responsible for anti-inflammatory, anti-cancer, and with neuro-, hepato-, and cardioprotective effects. As present results showed, various phenolic acids and flavonoids were found abundantly in HAP. These phenolics can delay or inhibit oxidative damage, thus preventing the onset of oxidative stress-related diseases in the human body.[Citation15,Citation32–Citation35] And the consumption of phenolics-rich food and herbs was inversely associated with the prevalence of chronic diseases including coronary heart disease, type II diabetes mellitus, cardiovascular diseases, cancer, and aging.[Citation36,Citation37] Besides phenolics, other chemical constituents such as saponin aglycones, fatty acids, and oligosaccharides rich in HAP also exerted diverse biological functions. For example, several unsaturated fatty acids such as linoleic acid and linolenic acid, which are two essential fatty acids involved in many important physiological processes including the formation of phospholipids/arachidonic acid and adjustment of immune function in human.[Citation38,Citation39] Elevating oleic acid values in obese hypertensive individuals contributed to the impaired endothelium-dependent vasodilation, and 9-hydroxy-10,12-octadecadienoic acid from rice bran was reported to have antitumor activity.[Citation40,Citation41] Meanwhile, oleanolic acid was also proved to have antiproliferative and antitumor activities in cellular, animal, and clinical studies.[Citation7,Citation42] Sapogenins were also reported with excellent bioactivity. Jujubogenin saponins isolated from Colubrina retusa L. (Rhamnaceae) showed good antifungal activity against Mycobacterium intracellulare, Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus.[Citation31] Saponins with an acyl residue or oxide-ring moiety showed a strong haemolytic activity.[Citation31] Moreover, other terpenes extracted from the plants of Rhamnaceae (the family of Hovenia acerba Lindl.), such as jujuboside A, jujuboside B, ceanothic acid, and maslinic acid, were reported to have the growth inhibitory effects on human hepatoma cells.[Citation30] Various ganoderic acids were reported to have anticancer, anti-adipogenic, and immunostimulatory activities.[Citation3,Citation43] Polydatin has been proved to have pharmacological activities, including anti-inflammatory activities, antioxidative-stress effects, and anti-hyperuricemic effects.[Citation44] Corydine is one of the aporphine alkaloids found in many medical plants, which was reported available to suppress tumour cell growth.[Citation45] Therefore, HAP, with various functional compositions, was proved to be a potential source of functional foods.
The characterization of bioactive constituents and their activity investigation of these three Hovenia plants (HA, HD, and HT) have been conducted. Wang et al. reported the purified polysaccharide from HDP presented excellent immunostimulatory activity.[Citation4] Moreover, six triterpene esters were identified in the roots of HD, and their in vitro antiproliferative activity were verified.[Citation29] The stilbene (pinosylvin) constituent of HD branches and in vitro/in vivo anti-allergy potential of these pinosylvin-rich extracts have also been conducted.[Citation46] The structures of six flavonoids (5,7,4ʹ,5ʹ-tetrahydroxy-3ʹ-methoxydihydroflavonol, 5,7,4ʹ,5ʹ-tetrahydroxy-3ʹ-methoxy-dihydroflavonol, and 5,7,3ʹ,4ʹ,5ʹ-pentahydroxydihydroflavonol ampelopsin, laricetrin, myricetin, and gallocatechin) and five triterpene glycosides (hoduloside III and hovenidulciosides A1/A2/B1/B2) were identified in HDP, and their anti-inflammatory/anti-alcoholic effects were also provided.[Citation47] Furthermore, two organic acids (malic acid and citric acid), three flavonoids (ferulic acid, rutin, and maesopsin) and three saponins (jujubogenin, hovenidulcigenin A, hovenidulcigenin B) have been discovered in the HDP and(or) seeds of HD.[Citation2,Citation6] Apart from HD, HT is another less prevalent cultivation species of Hovenia genus in East Asia. So far, compounds including 13 phenolic glycosides (hovetrichoside A/B/C/D/E/F/G, (+)-lyoniresinol glucoside, (-)-lyoniresinol glucoside, kelampayoside A, shashenoside I, 3,4,5-trimthoxyphenol-1-xylopyranosyl glucoside, and maesopsin), 3 triterpenes (hovenic acid, hovertrichoside H, and ceanothetric acid) and 1 lignan (acanthoside B) have been reported in the composition study of HT barks.[Citation2,Citation28,Citation48] According to Richardson’s report, the genus of Hovenia and Ziziphus is the Paliureae tribe, which belongs to the large family of Rhamnaceae. Thus, they are phylogenetically close and the phytochemical compositions of their roots were proved to be very similar.[Citation29,Citation49] Interestingly, the triterpene compounds, which were originally found in Ziziphus plants with molecular formula at C30H44O6 and C30H44O5, can only be detected in HA (compounds 79 and 101) instead of HD and HT.[Citation29]
For HA, only 1 anthraquinone (emodin), 1 amino acid (4-hydroxyl-N-methylproline), 1 triterpenes (saponins C2), and 11 phenolics (vanillic acid, 3-O-coumaroylquinic acid, myricetin, ampelopsin, apigenin, 4ʹ,5,7-trihydroxy-3ʹ,5ʹ-dimethoxy flavanone, kaempferol, quercetin, kaempferol-3-O-rhamnosyl galactoside, kaempferol-3-O-rutinoside, quercetin-3-O-rhamnosyl galactoside, and rutin) have been detected in the leaves and seeds of HA before. However, there are no reports about the detailed chemical constituent analysis of HAP. In present qualitative study, 117 compounds, including 7 oligosaccharides, 11 organic acids, 41 phenolics, 1 steroid, 25 terpenes, 11 fatty acids, 9 amino acids, and 12 other compounds were firstly found in HAP. Herein, 111 compounds (except quercetin myricetin, ampelopsin, apigenin, coumaroylquinic acid, and rutin) are newly discovered in HA, and 102 compounds (except quercetin, myricetin, ampelopsin, apigenin, coumaroylquinic acid, rutin, ferulic acid, gallocatechin, maesopsin, hovetrichoside C/D, jujubogenin, hovenidulcigenin A, hovenidulcigenin B, ceanothetric acid B) were firstly detected in Hovenia plants overall.
Quantitative analysis
As previously reported, six organic acids (3520.00 μg/g oxalic acid, 1860.00 μg/g tartaric acid, 880.00 μg/g malic acid, 149.00 μg/g ascorbic acid, 13890.00 μg/g citric acid, and 120.90 μg/g fumaric acid), five triterpenes (18.25 μg/g hovenidulcioside A1, 17.38 μg/g hovenidulcioside B1, 6.00 μg/g hovenidulcioside A2, 7.75 μg/g hovenidulcioside B2, 5.13 μg/g hodulcioside III), and two phenolics (42.03 μg/g ampelopsin and 2.75 μg/g gallocatechin) have been quantified in HDP. But no study provided corresponding quantification data for chemical constituents of HAP.
In the present study, the contents of representative organic acids and phenolic compounds in HAP extracts were determined by HPLC-ESI-QqQ-MS/MS. A total of 23 compounds including 4 organic acids, 5 phenolic acids, and 14 flavonoids were quantified by MRM mode and the results were summarized in . Four organic acids in HAP were malic, citric, quinic, and abscisic acids, and they were mainly found in WE and ME. Among them, the highest contents of malic, quinic, and abscisic acids were detected in ME (3525.13, 249.20, and 4.07 μg/g DW), followed by WE (2953.08, 217.44, and 3.62 μg/g DW). However, the highest content of citric acid was found in WE (1117.51 μg/g DW).
Table 3. Calibration curve equatuin and results from UPLC-QqQ-MS/MS for the major chemical constituents in HAPa.
The main phenolic acids, including protocatechuic acid (from 0.12 to 1.16 μg/g DW), chlorogenic acid (from 1.76 μg/g DW to 2.15 μg/g DW), p-hydroxybenzoic acid (from 0.41 to 1.96 μg/g DW), ferulic acid (from 1.04 μg/g DW to 4.59 μg/g DW) and isoferulic acid (from 0.81 μg/g DW to 5.54 μg/g DW), were also mainly found in WE and ME.
Similarly, the highest contents of epigallocatechin, epicatechin, ampelopsin, taxifolin, rutin, myricetin, gallocatechin, catechin, naringenin 7-O-glucoside, and quercetin were found in ME (6.28 μg/g DW, 12.43 μg/g DW, 8.40 μg/g DW, 0.51 μg/g DW, 11.45 μg/g DW, 0.12 μg/g DW, 43.88 μg/g DW, 75.14 μg/g DW, 1.70 μg/g DW, 1.02 μg/g DW, respectively). Moreover, ethyl gallate, myricetin, 3ʹ-O-methylmyricetin, and epigallocatechin derivative were only detected in 80% methanol. It is concluded that 80% methanol could extract more phenolic compounds in both variety and content than the other solvents.
However, several flavonoid aglycones, including apigenin (0.02 μg/g DW), chrysin (3.36 μg/g DW), genistein (0.96 μg/g DW), and naringenin (0.19 μg/g DW), were only detected in the EE, suggesting that ethyl acetate is able to extract higher contents of flavonoid aglycones with medium polarity than other solvents (80% methanol, hexane, and water). Although EE showed a higher percentage of phenolics, such as p-hydroxybenzoic acid, protocatechuic acid, ferulic acid, isoferulic acid, quercetin, and taxifolin, its extraction yield was much lower than those of the other solvents (80% methanol: 61.35%, ethyl acetate: 2.58%, hexane: 8.88%, water: 40.99%). The explanation maybe not only the low quantity of phenolic aglycones existing in HAP but also the relatively high content of phenolic glycosides, organic acids, saccharides, fatty acids, and sapogenins of HAP.
Conclusion
In conclusion, the effects of extraction solvents on the contents of TPC, TTC, various bioactive compounds (phenolic, terpenes, steroids, fatty acids, amino acids, and saccharides) and antioxidant activities of HAP were evaluated. Results suggested that ME exhibited higher TPC, TTC, and greater antioxidant activities than those of EE, HE, and WE. A total of 117 compounds from 4 HAP extracts were identified by LC-QTOF-MS/MS and most of them were characterized in HAP for the first time. Moreover, 4 organic acids and 19 phenolics were quantified by LC-QqQ-MS/MS. This contribution potentially provided important information in the chemical characterization of other plants in the Hovenia genus. The abundant content of phenolics, terpenes, and sterenes with good antioxidant activities enable HAP to be developed as functional foods, beverages, and food supplements besides consumed as fresh fruits.
Supplemental Material
Download ()Supplementary data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Park, J. S.; Kim, I. S.; Rehman, S. U.; Na, C.-S.; Yoo, H. H. Hplc Determination of Bioactive Flavonoids in Hovenia Dulcis Fruit Extracts. Journal of Chromatographic Science 2015, 54, 1–14. DOI: 10.1093/chromsci/bmv097.
- Xu, B.-J.; Deng, Y.-Q.; Lee, J.-H.; Mo, E.-K.; Sung, C.-K. Chemical Compositions of the Genus Hovenia. Natural Product Sciences 2003, 9, 143–153.
- Hyun, T. K.; Eom, S. H.; Yu, C. Y.; Roitsch, T. Hovenia dulcis–An Asian Traditional Herb. Planta Medica 2010, 76, 943–949. DOI: 10.1055/s-0030-1249776.
- Wang, M.; Jiang, C.; Ma, L.; Zhang, Z.; Cao, L.; Liu, J.; Zeng, X. Preparation, Preliminary Characterization and Immunostimulatory Activity of Polysaccharide Fractions from the Peduncles of Hovenia dulcis. Food Chemistry 2013, 138, 41–47. DOI: 10.1016/j.foodchem.2012.09.098.
- Yohsikawa, M.; Murakami, T. Four Methyl-Migrated 16, 17-Seco-Dammarane Triterpene Glycosides from Chines Natural Medicine, Hovenia Semen Seu Fructus, the Seeds and Fruit of Hovenia Dulcis Thunb. Chemical & Pharmaceutical Bulletin 1996, 44, 1736–1743. DOI: 10.1248/cpb.44.1736.
- Maieves, H. A.; López-Froilán, R.; Morales, P.; Pérez-Rodríguez, M. L.; Ribani, R. H.; Cámara, M.; Sánchez-Mata, M. C. Antioxidant Phytochemicals of Hovenia Dulcis Thunb. Peduncles in Different Maturity Stages. Journal of Functional Foods 2015, 18, 1117–1124. DOI: 10.1016/j.jff.2015.01.044.
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic Acid: An Anti‐And Pro‐Inflammatory Triterpenoid. Molecular Nutrition & Food Research 2008, 52, 26–42. DOI: 10.1002/mnfr.200700389.
- Rivas, S.; Conde, E.; Moure, A.; Domínguez, H.; Parajó, J. C. Characterization, Refining and Antioxidant Activity of Saccharides Derived from Hemicelluloses of Wood and Rice Husks. Food Chemistry 2013, 141, 495–502. DOI: 10.1016/j.foodchem.2013.03.008.
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free Amino Acids and Peptides as Related to Antioxidant Properties in Protein Hydrolysates of Mackerel (Scomber Austriasicus). Food Research International 2003, 36, 949–957. DOI: 10.1016/S0963-9969(03)00104-2.
- Peng, H.; Li, W.; Li, H.; Deng, Z.; Zhang, B., Extractable and Non-Extractable Bound Phenolic Compositions and Their Antioxidant Properties in Seed Coat and Cotyledon of Black Soybean (Glycinemax (L.) merr). Journal of Functional Foods 2017, 32, 296–312. DOI: 10.1016/j.jff.2017.03.003.
- Ye, F.; Liang, Q.; Li, H.; Zhao, G. Solvent Effects on Phenolic Content, Composition, and Antioxidant Activity of Extracts from Florets of Sunflower (Helianthus annuus L.). Industrial Crops and Products 2015, 76, 574–581. DOI: 10.1016/j.indcrop.2015.07.063.
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of Extraction Solvent Mixtures on Antioxidant Activity Evaluation and Their Extraction Capacity and Selectivity for Free Phenolic Compounds in Barley (Hordeum vulgare L.). Journal of Agricultural and Food Chemistry 2006, 54, 7277–7286. DOI: 10.1021/jf061087w.
- Metrouh-Amir, H.; Duarte, C. M.; Maiza, F. Solvent Effect on Total Phenolic Contents, Antioxidant, and Antibacterial Activities of Matricaria pubescens. Industrial Crops and Products 2015, 67, 249–256. DOI: 10.1016/j.indcrop.2015.01.049.
- Sun, Y.; Li, H.; Hu, J.; Li, J.; Fan, Y.-W.; Liu, X.-R.; Deng, Z.-Y. Qualitative and Quantitative Analysis of Phenolics in Tetrastigma hemsleyanum and Their Antioxidant and Antiproliferative Activities. Journal of Agricultural and Food Chemistry 2013, 61, 10507–10515. DOI: 10.1021/jf4037547.
- Zhang, B.; Deng, Z.; Ramdath, D. D.; Tang, Y.; Chen, P. X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic Profiles of 20 Canadian Lentil Cultivars and Their Contribution to Antioxidant Activity and Inhibitory Effects on Α-Glucosidase and Pancreatic Lipase. Food Chemistry 2015, 172, 862–872. DOI: 10.1016/j.foodchem.2014.09.144.
- Hu, W.; Lee, K.-Y.; Wang, M.-H. Antioxidant Activities of Various Extracts of Hovenia dulcis Thunb Fruits. Korean Journal of Plant Resources 2010, 23, 207–213.
- Thoo, Y. Y.; Ho, S. K.; Liang, J. Y.; Ho, C. W.; Tan, C. P. Effects of Binary Solvent Extraction System, Extraction Time and Extraction Temperature on Phenolic Antioxidants and Antioxidant Capacity from Mengkudu (Morinda citrifolia). Food Chemistry 2010, 120, 290–295. DOI: 10.1016/j.foodchem.2009.09.064.
- De la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive Characterization by Uhplc-Esi-Q-Tof-Ms from an Eryngium bourgatii Extract and Their Antioxidant and Anti-Inflammatory Activities. Food Research International 2013, 50, 197–204. DOI: 10.1016/j.foodres.2012.09.038.
- Iswaldi, I.; Gómez-Caravaca, A. M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of Phenolic and Other Polar Compounds in Zucchini (Cucurbita pepo L.) By Reverse-Phase High-Performance Liquid Chromatography Coupled to Quadrupole Time-Of-Flight Mass Spectrometry. Food Research International 2013, 50, 77–84. DOI: 10.1016/j.foodres.2012.09.030.
- Ma, X.-Q.; Leung, A. K. M.; Chan, C. L.; Su, T.; Li, W.-D.; Li, S.-M.; Fong, D. W. F.; Yu, Z.-L. UHPLC UHD Q-TOF MS/MS Analysis of the Impact of Sulfur Fumigation on the Chemical Profile of Codonopsis Radix (Dangshen). Analyst 2014, 139, 505–516. DOI: 10.1039/c3an01561k.
- Barkawi, L. S.; Tam, -Y.-Y.; Tillman, J. A.; Pederson, B.; Calio, J.; Al-Amier, H.; Emerick, M.; Normanly, J.; Cohen, J. D. A High-Throughput Method for the Quantitative Analysis of Indole-3-Acetic Acid and Other Auxins from Plant Tissue. Analytical Biochemistry 2008, 372, 177–188. DOI: 10.1016/j.ab.2007.08.009.
- Lin, L.-Z.; Chen, P.; Ozcan, M.; Harnly, J. M. Chromatographic Profiles and Identification of New Phenolic Components of Ginkgo biloba Leaves and Selected Products. Journal of Agricultural and Food Chemistry 2008, 56, 6671–6679. DOI: 10.1021/jf800488x.
- Seyoum, A.; Asres, K.; El-Fiky, F. K. Structure–Radical Scavenging Activity Relationships of Flavonoids. Phytochemistry 2006, 67, 2058–2070. DOI: 10.1016/j.phytochem.2006.07.002.
- Goulas, V.; Gomez-Caravaca, A. M.; Exarchou, V.; Gerothanassis, I. P.; Segura-Carretero, A.; Gutiérrez, A. F. Exploring the Antioxidant Potential of Teucrium polium Extracts by HPLC–SPE–NMR and On-Line Radical-Scavenging Activity Detection. LWT-Food Science and Technology 2012, 46, 104–109. DOI: 10.1016/j.lwt.2011.10.019.
- Ivancheva, S.; Manolova, N.; Serkedjieva, J.; Dimov, V. Ivanovska, N., Polyphenols from Bulgarian Medicinal Plants with Anti-Infectious Activity, Plant Polyphenols, Springer, Boston: 1992, 59, 717-728.
- Chandrasekara, A.; Shahidi, F. Determination of Antioxidant Activity in Free and Hydrolyzed Fractions of Millet Grains and Characterization of Their Phenolic Profiles by HPLC-DAD-ESI-MSn. Journal of Functional Foods 2011, 3, 144–158. DOI: 10.1016/j.jff.2011.03.007.
- Turco, I.; Ferretti, G.; Bacchetti, T. Review of the Health Benefits of Faba Bean (Vicia faba L.) Polyphenols. Journal of Food & Nutrition Research 2016, 55, 283–293.
- Yoshikawa, K.; Eiko, K.; Mimura, N.; Kondo, Y.; Arihara, S. Hovetrichosides Cg, Five New Glycosides of Two Auronols, Two Neolignans, and a Phenylpropanoid from the Bark of Hovenia trichocarea. Journal of Natural Products 1998, 61, 786–790. DOI: 10.1021/np9800396.
- Kang, K. B.; Jun, J. B.; Kim, J. W.; Kim, H. W.; Sung, S. H. Ceanothane-And Lupane-Type Triterpene Esters from the Roots of Hovenia dulcis and Their Antiproliferative Activity on Hsc-T6 Cells. Phytochemistry 2017, 142, 60–67. DOI: 10.1016/j.phytochem.2017.06.014.
- Huang, X. Thesis, Department of Food and Human Health Sciences, Graduate School of Human Life Science, Osaka City University, Japan, 2008.
- Sparg, S.; Light, M.; Van Staden, J. Biological Activities and Distribution of Plant Saponins. Journal of Ethnopharmacology 2004, 94, 219–243. DOI: 10.1016/j.jep.2004.05.016.
- Choi, S.; Woo, J.-K.; Jang, Y.-S.; Kang, J.-H.; Jang, J.-E.; Yi, T.-H.; Park, S.-Y.; Kim, S.-Y.; Yoon, Y.-S.; Oh, S. H. Fermented Pueraria lobata Extract Ameliorates Dextran Sulfate Sodium-Induced Colitis by Reducing Pro-Inflammatory Cytokines and Recovering Intestinal Barrier Function. Laboratory Animal Research 2016, 32, 151–159. DOI: 10.5625/lar.2016.32.3.151.
- Guerra, M.; Speroni, E.; Broccoli, M.; Cangini, M.; Pasini, P.; Minghetti, A.; Crespi-Perellino, N.; Mirasoli, M.; Cantelli-Forti, G.; Paolini, M. Comparison between Chinese Medical Herb Pueraria lobata Crude Extract and Its Main Isoflavone Puerarin: Antioxidant Properties and Effects on Rat Liver Cyp-Catalysed Drug Metabolism. Life Sciences 2000, 67, 2997–3006.
- Lin, R. C.; Guthrie, S.; Xie, C. Y.; Mai, K.; Lee, D. Y.; Lumeng, L.; Li, T. K. Isoflavonoid Compounds Extracted from Pueraria lobata Suppress Alcohol Preference in a Pharmacogenetic Rat Model of Alcoholism. Alcoholism: Clinical and Experimental Research 1996, 20, 659–663. DOI: 10.1111/j.1530-0277.1996.tb01668.x.
- Guajardo-Flores, D.; Serna-Saldívar, S. O.; Gutiérrez-Uribe, J. A. Evaluation of the Antioxidant and Antiproliferative Activities of Extracted Saponins and Flavonols from Germinated Black Beans (Phaseolus vulgaris L.). Food Chemistry 2013, 141, 1497–1503. DOI: 10.1016/j.foodchem.2013.04.010.
- Amarowicz, R.; Pegg, R. B. Legumes as a Source of Natural Antioxidants. European Journal of Lipid Science and Technology 2008, 110, 865–878. DOI: 10.1002/ejlt.v110:10.
- Villegas, R.; Gao, Y.-T.; Yang, G.; Li, H.-L.; Elasy, T. A.; Zheng, W.; Shu, X. O. Legume and Soy Food Intake and the Incidence of Type 2 Diabetes in the Shanghai Women’s Health Study. The American Journal of Clinical Nutrition 2008, 87, 162–167. DOI: 10.1093/ajcn/87.1.162.
- Moran, J. H.; Nowak, G.; Grant, D. F. Analysis of the Toxic Effects of Linoleic Acid, 12, 13-Cis-Epoxyoctadecenoic Acid, and 12, 13-Dihydroxyoctadecenoic Acid in Rabbit Renal Cortical Mitochondria. Toxicology and Applied Pharmacology 2001, 172, 150–161. DOI: 10.1006/taap.2001.9149.
- Albers, R.; Van der Wielen, R.; Brink, E.; Hendriks, H.; Dorovska-Taran, V.; Mohede, I. Effects of Cis-9, Trans-11 and Trans-10, Cis-12 Conjugated Linoleic Acid (Cla) Isomers on Immune Function in Healthy Men. European Journal of Clinical Nutrition 2003, 57, 595. DOI: 10.1038/sj.ejcn.1601596.
- Davda, R. K.; Stepniakowski, K. T.; Lu, G.; Ullian, M. E.; Goodfriend, T. L.; Egan, B. M. Oleic Acid Inhibits Endothelial Nitric Oxide Synthase by a Protein Kinase C–Independent Mechanism. Hypertension 1995, 26, 764–770.
- Hayshi, Y.; Nishikawa, Y.; Mori, H.; Tamura, H.; Matsushita, Y.-I.; Matsui, T. Antitumor Activity of (10e, 12z)-9-Hydroxy-10, 12-Octadecadienoic Acid from Rice Bran. Journal of Fermentation and Bioengineering 1998, 86, 149–153. DOI: 10.1016/S0922-338X(98)80053-6.
- Parikh, N. R.; Mandal, A.; Bhatia, D.; Siveen, K. S.; Sethi, G.; Bishayee, A. Oleanane Triterpenoids in the Prevention and Therapy of Breast Cancer: Current Evidence and Future Perspectives. Phytochemistry Reviews 2014, 13, 793–810. DOI: 10.1007/s11101-014-9337-5.
- Ding, Y.; Liang, C.; Kim, J. H.; Lee, Y.-M.; Hyun, J.-H.; Kang, H.-K.; Kim, J.-A.; Min, B. S.; Kim, Y. H. Triterpene Compounds Isolated from Acer mandshuricum and Their Anti-Inflammatory Activity. Bioorganic & Medicinal Chemistry Letters 2010, 20, 1528–1531. DOI: 10.1016/j.bmcl.2010.01.096.
- Chen, L.; Lan, Z.; Lin, Q.; Mi, X.; He, Y.; Wei, L.; Lin, Y.; Zhang, Y.; Deng, X. Polydatin Ameliorates Renal Injury by Attenuating Oxidative Stress-Related Inflammatory Responses in Fructose-Induced Urate Nephropathic Mice. Food and Chemical Toxicology 2013, 52, 28–35. DOI: 10.1016/j.fct.2012.10.037.
- Konda, Y.; Imai, Y.; Hojo, H.; Endo, T.; Nozoe, S. Suppression of Tumor Cell Growth and Mitogen Response by Aporphine Alkaloids, Dicentrine, Glaucine, Corydine, and Apomorphine. Journal of Pharmacobio-Dynamics 1990, 13, 426–431.
- Lim, S. J.; Kim, M.; Randy, A.; Nho, C. W. Inhibitory Effect of the Branches of Hovenia dulcis Thunb. And Its Constituent Pinosylvin on the Activities of IgE-Mediated Mast Cells and Passive Cutaneous Anaphylaxis in Mice. Food & Function 2015, 6, 1361–1370. DOI: 10.1039/C4FO01203H.
- Yoshikawa, M.; Murakami, T.; Ueda, T.; Matsuda, H.; Yamahara, J.; Murakami, N. Bioactive Saponins and Glycosides. Iv. Four Methyl-Migrated 16, 17-Seco-Dammarane Triterpene Glycosides from Chinese Natural Medicine, Hoveniae Semen Seu Fructus, the Seeds and Fruit of Hovenia dulcis Thunb: Absolute Stereostructures and Inhibitory Activity on Histamine Release of Hovenidulcioseds A1, A2, B1, and B2. Chemical and Pharmaceutical Bulletin 1996, 44, 1736–1743.
- Yoshikawa, K.; Kondo, Y.; Kimura, E.; Arihara, S. A Lupane-Triterpene and A 3 (2→ 1) Abeolupane Glucoside from Hovenia trichocarea. Phytochemistry 1998, 49, 2057–2060. DOI: 10.1016/S0031-9422(98)00409-9.
- Richardson, J. E.; Fay, M. F.; Cronk, Q. C.; Bowman, D.; Chase, M. W. A Phylogenetic Analysis of Rhamnaceae Using Rbcl and Trnl-F Plastid DNA Sequences. American Journal of Botany 2000, 87, 1309–1324.