 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This research investigated the effects of processing by dry heating, boiling, and steaming on the antioxidant activity and DNA protection against oxidative damage of bambara groundnut seeds (Vigna subterranea (L.) Verdc.). Comparing raw and processed samples of bambara groundnut seeds, dry heating caused a significant (P < 0.05) reduction of ferric-reducing antioxidant power (FRAP), metal chelating activity, DPPH• and ABTS•+ radical scavenging activity. The boiling process did not cause a significant difference in FRAP and metal chelating activity and caused smaller losses in DPPH• and ABTS•+ radical scavenging activity than the dry heating and steaming processes. The steaming process caused a significant (P < 0.05) reduction of FRAP, DPPH• and ABTS•+ radical scavenging activity. For DNA protection against oxidative damage, boiled and steamed bambara groundnut seed samples were more effective with a lower minimum concentration (50 µg/mL) than raw and dry heated samples. These results indicated that the boiling process caused smaller losses antioxidant activity than dry heating and steaming. Therefore, boiling was recommended as processing method for bambara groundnut seeds to preserve antioxidant components and antioxidant activity.
Introduction
Antioxidants are substances that have the ability to protect the body from damage caused by free radical-induced oxidative stress. They are derived from many dietary sources such as fruits, vegetables, and cereals. Properties of antioxidants are important in biological activity. Plant-derived antioxidants have been shown to function as free radical scavengers, singlet and triplet oxygen quenchers, metal chelators, chain-breakers, synergists, and enzyme inhibitors.[Citation1] These antioxidants include vitamins, carotenoids, phenols, flavonoids, and endogenous metabolites.[Citation2] Phenolic compounds are known to respond to metal chelation and offer anti-inflammatory, anti-cancer, and DNA protection.[Citation3,Citation4]
Bambara groundnut (Vigna subterranea (L.) Verdc.) is a legume species of African origin and is widespread south of the Sahara. However, it is also cultivated in Southeast Asian countries such as Malaysia, Indonesia, and Thailand. The seeds of the bambara groundnut must be processed prior to consumption to destroy antinutritional factors such as protease inhibitors, lectins, and phytic acid. Changes in physical and biochemical properties of the food induced during processing could affect overall acceptability by consumers and the nutritional quality of the product.[Citation5] The methods for processing bambara groundnut seeds prior to consumption vary by region. Previous study found that processing by roasting of Zimbabwean bambara groundnut had no effect on the nutrient content, while sifting significantly reduced the phenolic content and antioxidant activity of roasted samples.[Citation6]
However, no information is available about the effects of processing by dry heating, boiling, and steaming on antioxidant activity and DNA protection of bambara groundnut seeds.
Therefore, the objectives of this study were to evaluate the effect of processing methods (dry heating, boiling, and steaming) of bambara groundnut seeds on antioxidant compounds (total phenolic, tannin, and flavonoid content), antioxidant activity (ferric-reducing antioxidant power (FRAP), metal chelating activity, DPPH• and ABTS•+ radical scavenging activity), and DNA protection against oxidative damage.
Materials and methods
Chemicals
Aluminum chloride, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), dimethyl sulfoxide (DMSO), 2,2´-diphenyl-1-picryl-hydrazyl (DPPH•), ethylenediamine tetra acetic acid (EDTA), ferrous chloride (FeCI2), ferrozine, nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT), polyvinyl polyprrolidone (PVPP), rutin, and trolox were purchased from Sigma-Aldrich (Thailand). 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH), 2,2-azinobis (3-ethyl benzothiazoline-6-sulfonic acid) diammonium salt (ABTS•+), acetyl acetone, ammonium acetate, folin and ciocalteu phenol reagent, gallic acid, iron (III) chloride hexahydrate (FeCI3 · 6H2O), and ferrous (II) sulfate (FeSO4 · 7H2O) were purchased from Merck Millipore (Thailand). The pBR 322 vector was purchased from Vivantis Technologies (Thailand).
Seed samples and processing
Bambara groundnut seeds, the Songkhla 1 variety (red seed coat) were purchased from a farmer in Trang Province, Thailand. Immature and damaged seeds were removed. Bambara groundnut seeds were randomly divided into four batches. The first batch of seeds was kept raw without any treatment. The second batch of seeds was dry heated in a hot-air oven at 120oC for 90 min. The third batch of seeds was boiled using the following conditions: water in a ratio of 1:10 (w/v) at 100oC for 30 min. The fourth batch of seeds was steamed at 120oC at 15 pounds of pressure for 10 min. After decanting the water, the boiled and steamed seed samples were rinsed with distilled water and dried at 55°C for 20 min. All seed samples were ground to a fine powder using a coffee grinder and stored in separate screw-cap bottles at −20°C.
Sample extraction
The raw and processed ground seed samples were extracted by stirring with 80% methanol (1:5 w/v) at room temperature for 48 h. After extraction, the mixture was centrifuged at 12000 g for 15 min. The residues were re-extracted with an additional 50 mL of 80% methanol for 5 h. The solvent from both 80% methanol extractions was combined. To remove methanol, the solvent was evaporated under reduced pressure using a rotary vacuum-evaporator at below 40oC. The remaining water was frozen at −80oC and freeze-dried to obtain a powdered extract.
Determination of total phenolic content
The total phenolic content was determined according to the Folin-Ciocalteu method using gallic acid as a standard.[Citation7] Each 50 mg of the powdered extract was placed in a test tube and made up to the volume of 1 mL with distilled water. A 25-µL aliquot of the dilution was transferred to the tube with the addition of 975 µL of distilled water and 0.5 mL of the Folin-Ciocalteu phenol reagent (1:1 with distilled water). The reaction was mixed and incubated at room temperature for 5 min. Next, 2.5 mL of 20% sodium carbonate solution was added to each tube. The reaction was mixed using a vortex mixer and incubated in the dark at room temperature for 40 min. The absorbance was measured at 725 nm with UV-Vis spectrophotometer. The analysis was performed in triplicate. The total phenolic content was expressed in micrograms of gallic acid equivalent per gram of extract (µg GAE/g extract).
Determination of tannin content
The powdered extract of samples was prepared using the same method described for determination of total phenolic content. A 1-mL aliquot of the sample was mixed with 1 mL of 100 mg/mL PVPP and incubated at 4°C for 4 h. The mixture was centrifuged at 5000 g for 20 min at room temperature. The tannins were precipitated along with the PVPP. Then, the supernatant with only phenolics other than tannins was collected. The supernatant was measured for phenolics other than tannins using the same method described for determination of total phenolic content. The analysis was performed in triplicate. The tannin content was expressed in micrograms of gallic acid equivalent per gram of extract (µg GAE/g extract). The tannin content of the sample was calculated as:
Determination of total flavonoid
The total flavonoid content was determined according to the method of Zhishen et al.[Citation8] using rutin as a standard. Each 50 mg of the powdered extract was added to a test tube, and made up to the volume of 1 mL with distilled water. A 100-µL aliquot of the dilution was transferred to the tube and 1.9 mL of distilled water and 0.15 mL of 5% sodium nitrite were added. The reaction was mixed and incubated at room temperature for 5 min. Next, 0.15 mL of 10% aluminum chloride solution was added to each tube. The reaction was mixed and incubated at room temperature for 6 min. Then, 1 mL of 1 M sodium hydroxide was added to the mixture, and the volume was immediately increased to 5 mL with distilled water. The absorbance was measured at 510 nm with a UV-Vis spectrophotometer. The total flavonoid content was expressed in micrograms of rutin equivalent per gram of extract (µg RE/g extract). The analysis was performed in triplicate.
Ferric-reducing antioxidant power (FRAP) assay
FRAP assay was determined according to the method described by Sasipriya and Siddhuraju.[Citation9] This assay was used to determine the ferric-reducing antioxidant power of raw and processed bambara groundnut seed samples. The amount of Fe2+ produced from the reduction of Fe3+ was calculated from the ferrous sulfate standard curve. Each 50 mg of the powdered extract was added to a test tube, and made up to the volume of 1 mL with distilled water. A 5-µL aliquot of the dilution was transferred to the tube with the addition of 95 µL of distilled water and 900 µL of prepared freshly FRAP reagent (2.5 mL of 20 mM of TPTZ in 40 mM of HCl, 2.5 mL of 20 mM of ferric chloride, 25 mL of 0.3 M of acetate buffer, pH 3.6, incubated at 37°C for 30 min) before use. The mixture was incubated in the dark at 37oC for 30 min in a water bath. After incubation, absorbance was measured at 593 nm with a UV-Vis spectrophotometer. The antioxidant activity was expressed as micromole of Fe (II) per gram extract (µmol Fe(II)/g extract). BHA, BHT, gallic acid, and rutin were used as positive controls. The analysis was performed in triplicate.
Metal chelating activity assay
The ability of raw and processed bambara groundnut seed samples to chelate ferrous ions was estimated using the method described by Dinis et al.[Citation10] The 700 µL of the samples was added to 100 µL of 2 mM ferrous chloride solution. The mixture was incubated at room temperature for 30 s. Next, 200 µL of 1 mM ferrozine was added to the mixture, which was incubated for 10 min at room temperature. The absorbance was measured at 562 nm with a UV-Vis spectrophotometer. Chelating activity was calculated using the following formula:
where Acontrol is the absorbance of control reaction and Asample is the absorbance of the sample reaction. The result is expressed in terms of EC50 value, which is the effective concentration at which the chelating activity is 50%. EDTA was used as a positive control.
DPPH• radical scavenging activity assay
The radical scavenging ability of raw and processed bambara groundnut seed samples was determined according to the method described by Blios.[Citation11] The 50 µL of the samples was added to 2.5 mL of 0.1 mM methanolic solution of DPPH•. The mixture was incubated in the dark at 27oC for 20 min. The absorbance was measured at 517 nm with a UV-Vis spectrophotometer. Radical scavenging activity was calculated using the formula:
The result was expressed in terms of EC50 value, which is the effective concentration at which DPPH• radicals are scavenged by 50%. BHA, gallic acid, rutin, and trolox were used as positive controls.
ABTS•+ radical scavenging activity assay
For ABTS•+ assay, the method of Re et al.[Citation12] was followed. ABTS•+ was dissolved in distilled water to make a concentration of 7 mM. ABTS•+ stock solution was produced by mixing the 7 mM ABTS•+ solution with 2.45 mM potassium persulfate (1:1). The mixture was allowed to stand in the dark at room temperature for 12 to 16 h. The ABTS•+ stock solution was diluted with distilled water to an absorbance of 0.70 ± 0.02 at 734 nm before use. To test the samples, 20 µL of the samples was added to 980 µL diluted ABTS•+ solution. The mixture was incubated at 30°C for 6 min before absorbance was read. The absorbance was measured at 734 nm with a UV-Vis spectrophotometer. Radical scavenging activity was calculated using the formula:
The result is expressed in terms of EC50 value, which is the effective concentration at which ABTS•+ radicals are scavenged by 50%. BHA, BHT, gallic acid, rutin, and trolox were used as positive controls.
DNA protection study
Two microliters of 0.1 mg/mL pBR 322 plasmid DNA was mixed with 2 µL of the extracted samples. The reaction was incubated at room temperature for 10 min. After incubation, 1 µL of freshly made 100 mM AAPH was added. Distilled water was added to increase total volume in each tube to 20 µL. The reaction was incubated at 37oC for 10 min. Ten microliters of the reaction were mixed with 1 µL of 4X loading dye and analyzed on 1% agarose gel.
Statistical analysis
The sample concentration providing 50% inhibition (EC50) was analyzed by the Graphpad Prism 7 program. The EC50 results were expressed as mean ± standard deviation (SD) of the responses of three replicates per sample. Statistical significance between the groups was determined by one-way ANOVA followed by Tukey’s post hoc test. Differences were considered to be significant at P < 0.05. Correlation was determined by two- tailed Pearson correlation analysis. All statistical analyses were performed using SPSS 17.0.
Results and discussion
Total phenolic and tannin content
The total phenolic and tannin content of the raw and processed bambara groundnut seed samples was calculated from the calibration curve of gallic acid (R2 = 0.999). The results from show that bambara groundnut seed samples processed by all methods (dry heating, boiling, and steaming) exhibited significant (p < 0.05) reduction in total phenolic and tannin content compared to the raw sample (about 8.47–11.86% reduction for total phenolic and 9.78–11.96% reduction for tannin). These results indicate that processing caused degradation of phenolics and tannins. This could be due to phenolic breakdown during processing.[Citation13]
Table 1. Total phenolic, tannin, and flavonoid content of raw and processed bambara groundnut seeds.
Xu and Chang[Citation14] reported that thermal processing may cause degradation of polyphenols and the release of bound phenolic compositions. In other legumes, processing caused degradation of phenolics, with 30–40% of phenolics potentially removed from common beans (Proteus vulgaris) by cooking and discarding the cooking water.[Citation15] The raw seed extract of Vigna vexillata (L.) A. Rich contains higher levels of total phenolics and tannins than processed seed extracts (i.e., dry heating, soaking followed by steaming with 0.1% sodium bicarbonate, soaking followed by autoclaving, and autoclaving without soaking).[Citation5] Entada scandens seed kernels and Canavalia gladiata seeds have reduced phenolic, tannin, and flavonoid content after autoclaving and soaking followed by autoclaving.[Citation9] According to Spanos and Wrolstad [Citation16] processing and storage of grapes resulted in decrease of phenolic levels as measured by HPLC.
Total flavonoid content
The total flavonoid content of raw and processed bambara groundnut seed samples was calculated from the calibration curve of rutin (R2 = 0.999). Total flavonoid content is presented in . Comparing the raw sample and the processed samples of bambara groundnut seeds, total flavonoid content was not significantly different among the raw, dry heated, and boiled samples. However, steaming caused a significant reduction (p < 0.05) in total flavonoid content of about 20.81% when compared to the raw bambara groundnut seed sample ().
Sasipriya and Siddhuraju[Citation9] reported that leaching of soluble compounds into the cooking medium causes a decrease in flavonoid content. Moreover, the stability of flavonoids during heating may be due to the formation of antioxidant compounds like Maillard reaction products such as hydroxymethylfurfuraldehyde (HMF).[Citation17]
FRAP assay
The FRAP activity of raw and processed bambara groundnut seeds is presented in . The reducing power was no significant differences between raw and boiled samples of bambara groundnut seeds. Dry heated and steamed samples of bambara groundnut seeds had significantly lower (p < 0.05) FRAP values compared to raw samples. There was a 17.58% reduction in FRAP values in the dry heated and steamed samples of bambara groundnut seeds. These results indicate that the dry heating and steaming processes cause reduced FRAP activity in bambara groundnut seeds compared to raw samples.
Table 2. FRAP, metal chelating, DPPH, and ABTS activity of raw and processed bambara groundnut seeds.
The correlation analysis in shows a significant positive correlation between FRAP and total phenolic content (r2 = 0.919, P < 0.05) for bambara groundnut seeds. This indicates that total phenolic content of bambara groundnut seeds contributed to reduced activity. This result is similar to that of Sasipriya and Siddhuraju[Citation9], who found that phenolics in Entada scandens and Canavalia gladiate were the main contributors to their reducing activity. Benzie and Szeto[Citation18] found that FRAP assay results correlate with total phenolic content. Moreover, Rice-Evans et al.[Citation19] reported that phenolic compounds play a key role in antioxidant potential due to their redox properties (reducing agents, hydrogen donators, and singlet oxygen quenchers).
Table 3. The correlation between total phenolic, tannin, and flavonoid content and antioxidant activity (FRAP and 1/EC50 of metal chelating, DPPH, and ABTS) of bambara groundnut seeds.
Metal chelating activity
The metal chelating activity EC50 values of raw and processed bambara groundnut seed samples are presented in . There were no significant differences in metal chelating activity EC50 values between the raw, boiled, and steamed samples of bambara groundnut seeds. The dry heated sample had significantly higher (p < 0.05) metal chelating activity EC50 values than the raw sample. Dry heated sample had a 314.87% increase in metal chelating activity EC50 values. These results are similar to those of Siddhuraju[Citation17], who found that metal chelating capability was higher for raw Vigna aconitifolia seeds than dry heated seeds. From the correlation analysis, total phenolic, tannin and flavonoid content in bambara groundnut seeds was not correlated with metal chelating activity ().
DPPH• radical scavenging activity
The DPPH• radical scavenging activity EC50 values of raw and processed bambara groundnut seeds are presented in . A low EC50 value indicates strong ability of the sample to act as a DPPH• radical scavenger. Dry heating, boiling, and steaming processes caused increased DPPH• EC50 values of bambara groundnut seeds by about 8.51%, 5.67%, and 22.70%, respectively compared to the raw sample. However, there was no significant difference between the DPPH• EC50 values of dry heated and boiled samples. These results indicate that the steamed sample had the highest increase in DPPH• EC50 value. Among the positive controls, gallic acid exhibited the maximum scavenging effect at a very low concentration, with an EC50 value of 0.52 µg/mL. These results indicate that bambara groundnut seeds reduced DPPH• radical scavenging activity for all types of processing.
A significant correlation coefficient (r2 = 0.983, P < 0.01) was found between flavonoid and DPPH• radical scavenging activity for bambara groundnut seeds (). The correlation coefficient for bambara groundnut seeds showed that the loss of DPPH• radical scavenging activity in processed samples was due to the leaching of flavonoids.
ABTS•+ radical scavenging activity
The results are presented in . A low EC50 value indicates high antioxidant activity. The dry heated, boiled, and steamed samples had significantly lower (p < 0.05) ABTS•+ radical scavenging activity, with 24.59%, 14.75% and 22.30% increases in EC50 values, respectively, compared to the raw sample. Among the positive controls, gallic acid showed the maximum scavenging effect at a very low concentration, with an EC50 value of 0.51 µg/mL. The study results show that processing by dry heating, boiling, and steaming reduces ABTS•+ radical scavenging activity in bambara groundnut seeds.
A significant correlation coefficient confirms that phenolic (r2 = 0.997, P < 0.01) and tannin (r2 = 0.984, P < 0.01) content is correlated with ABTS•+ radical scavenging activity in bambara groundnut seeds (). Phenolics can act as free radical scavengers by donating their alcoholic hydrogen or one of their delocalized electrons to radicals. Hagerman et al.[Citation20] reported that tannin has the ability to quench free radicals of ABTS•+. In vitro studies have shown that flavonoids can directly scavenge molecular species of active oxygen such as superoxide, hydrogen peroxide, hydroxyl radical, single oxygen, or peroxyl radical.[Citation21]
DNA protection against oxidative aamage
The results show that all the raw and processed bambara groundnut seed samples have the capacity to protect plasmid DNA against AAPH-induced oxidative damage. The DNA protective ability of raw and dry heated samples against AAPH was effective with minimum concentrations of 100 µg/mL and 50 µg/mL for the boiled and steamed samples (). Cao et al.[Citation22] reported that scavenging of peroxyl radicals is the ability of flavonoid. Moreover, flavonoids are effective inhibitors of lipid peroxidation and display pro-oxidant activity.
Figure 1. DNA protection of raw and processed bambara groundnut seeds. Lane 1 is pBR 322 plasmid DNA. Lane 2 is pBR 322 plasmid DNA + AAPH. Lanes 3–16 are pBR 322 plasmid DNA + AAPH + bambara groundnut seed extract. The bambara groundnut seed extract concentrations of lanes 3–16 are 10,000, 5,000, 2,000, 1,000, 500, 300, 100, 50, 10, 5, 1, 0.5, 0.1, and 0.05 µg/mL, respectively. The arrows indicate the minimum concentration of extract that can protect DNA against oxidative damage.
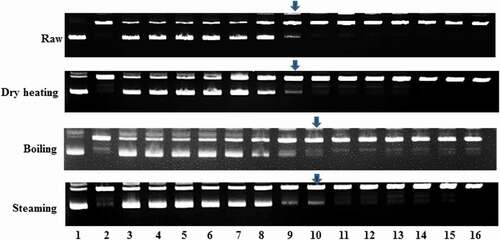
These results indicated that in general, boiling and steaming are the best method for processing bambara groundnut seeds to enhance DNA protection ability against peroxyl radicals. In addition, this suggests that consumption of bambara groundnut seeds may protect humans from peroxyl radical-induced diseases such as neurodegenerative diseases, carcinogenesis, and cytotoxicity.
Conclusion
In conclusion, the results obtained in the present study clearly demonstrate that the seed extracts of raw and processed bambara groundnut seeds contain a number of antioxidant compounds, including phenolics, tannins, and flavonoids, which offer effective antioxidant activity and DNA protection against oxidative damage. Processing by boiling caused smaller losses of antioxidant activity than dry heating and steaming. Therefore, boiling is recommended for processing bambara groundnut seeds to preserve antioxidant components and activity. Furthermore, boiling and steaming are the best methods for processing bambara groundnut seeds to enhance DNA protection against oxidative damage.
Acknowledgments
This research was supported by the Walailak University Fund (Grant number WU59202).
References
- Manach, C.; Morand, C.; Crespy, V.; Demigne, C.; Texier, O.; Regerat, F.; Remesy, C. Quercetin Is Recovered in Human Plasma as Conjugated Derivatives Which Retain Antioxidant Properties. FEBS Letters 1998, 426, 331–336.
- Larson, R. A. The Antioxidants of Higher Plants. Phytochemistry 1988, 27, 969–978.
- Sekhar, S.; Sampath-Kumara, K. K.; Niranjana, S. R.; Prakash, H. S. In Vitro Antioxidant Activity, Lipoxygenase, Cyclooxygenase-2 Inhibition and DNA Protection Properties of Memecylon Species. International Journal of Pharmacy and Pharmaceutical Sciences 2013, 5, 257–262.
- Valdés, S. T.; Coelho, C. M. M.; Michelluti, D. J.; Tramonte, V. L. C. G. Association of Genotype and Preparation Methods on the Antioxidant Activity, and Antinutrients in Common Beans (Phaseolus Vulgaris L.). LWT- Food Science and Technology 2011, 44, 2104–2111.
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S. Antioxidant and Free Radical Scavenging Capacity of the Underutilized Legume, Vigna Vexillata (L.) A. Rich. Journal of Food Composition and Analysis 2011, 24, 160–165.
- Adewumi, T. O.; Kirthee, P.; Samson, T.; Muthulisi, S. Physical, Nutritional and Antioxidant Properties of Zimbabwean Bambara Groundnut and Effects of Processing Methods on Their Chemical Properties. International Journal of Food Science and Technology 2017, 52, 2238–2247.
- Siddhuraju, R.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa Olifera Lam.) Leaves. Journal of Agricultural and Food Chemistry 2003, 51, 2144–2155.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents on Mulberry and Their Scavenging Effects on Superoxide Radical. Food Chemistry 1999, 64, 555–559.
- Sasipriya, G.; Siddhuraju, P. Effect of Different Processing Methods on Antioxidant Activity of Underutilized Legumes, Entada Scandens Seed Kernel and Canavalia Gladiata Seeds. Food and Chemical Toxicology 2012, 50, 2864–2872.
- Dinis, T. C. P.; Madeira, V. M. C.; Almeida, L. M. Action of Phenolic Derivatives (Acetoaminophen, Salycilate, and 5-Aminosalycilate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Archives of Biochemistry and Biophysics 1994, 315, 161–169.
- Blios, M. S.;. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Crozier, A.; Lean, M. E. J.; McDonald, M. S.; Black, C. Quantitative Analysis of the Flavonoid Content of Commercial Tomatoes, Onions, Lettuce, and Celery. Journal of Agricultural and Food Chemistry 1997, 45, 590–595.
- Xu, B.; Chang, S. K. C. Total Phenolics, Phenolic Acids, Isoflavones, and Anthocyanins and Antioxidant Properties of Yellow and Black Soybeans as Affected by Thermal Processing. Journal of Agricultural and Food Chemistry 2008, 56, 7165–7175.
- Bressani, A.; Elias, L. G. The Nutritional Role of Polyphenols in Beans. In Polyphenols in Cereal and Legumes; Hulse, J. H.; Eds.; International Development Research Center: Ottawa, Canada, 1980; 61–68.
- Spanos, G. A.; Wrolstad, R. E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. Journal of Agricultural and Food Chemistry 1990, 38, 1565–1571.
- Siddhuraju, P. The Antioxidant Activity and Free Radical-Scavenging Capacity of Phenolics of Raw and Dry Heated Moth Bean (Vigna Aconitifolia) (Jacq.) Marechal Seed Extracts. Food Chemistry 2006, 99, 149–157.
- Benzie, I. F. F.; Szeto, Y. T. Total Antioxidant Capacity of Teas by the Ferric Reducing Antioxidant Power Assay. Journal of Agricultural and Food Chemistry 1999, 47, 633–636.
- Rice-Evans, C. A.; Miller, N. J.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends in Plant Science 1997, 2, 152–159.
- Hagerman, A. E.; Riedl, K. M.; Jones, G. A.; Sovik, K. N.; Ritchard, N. T.; Hartzfeld, P. W.; Riechel, T. L. High Molecular Weight Plant Polyphenolics (Tannins) as Biological Antioxidants. Journal of Agricultural and Food Chemistry 1998, 46, 1887–1892.
- Arora, A.; Nair, M. G.; Strasburg, G. M. Structure–Activity Relationships for Antioxidant Activities of a Series of Flavonoids in a Liposomal System. Free Radical Biology and Medicine 1998, 24, 1355–1363.
- Cao, G.; Sofic, E.; Prior, R. L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radical Biology and Medicine 1997, 22, 749–760.
