?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Potato and its products have become indispensable foods and snacks for most people. Steroidal glycoalkaloids (SGAs) occur in all tissues of the potato, and consuming potatoes with a high SGA content harms human health. Therefore, the effects of different cooking methods on the SGA content in potato foods were investigated in this study. The results indicated that adding food-grade acetic acid during the manufacturing process did not affect the SGA content in stir-fried shredded potatoes or fresh mashed potatoes. However, the SGA content in potato food after peeling was significantly lower than that in non-peeled food, and the volume ratio of potato skin to flesh decreased with the increase of the potato tuber volume. Therefore, potato breeders and farmers should make the most hard to increase the proportion of commodity potato via corresponding science and technology. In addition, frying significantly reduced the SGA content in potato chips. Further research indicated that SGAs degraded slowly at 150°C, while they degraded rapidly at 190°C within 30 min. The temperature of rapeseed oil in the frying process can be as high above 200°C. Thus, frying significantly decreased the SGA content in potato chips, which could be attributed to temperature. These results will provide a theoretical basis and practical guidance for potato breeding and cooking.
Introduction
Potato (Solanum tuberosum L.) is the fourth largest crop next to rice, wheat and corn.[Citation1,Citation2] Potatoes are planted in approximately 160 countries and regions all over the world. China is the largest producer of potatoes, and its potato acreage and production account for approximately 20% of that in world.[Citation3] According to the Food and Agriculture Organization (FAO), humans consume approximately 300 million to 330 million tons of potatoes per year, and human consumption is the main form of potato consumption, accounting for approximately 65% of the total potato consumption.
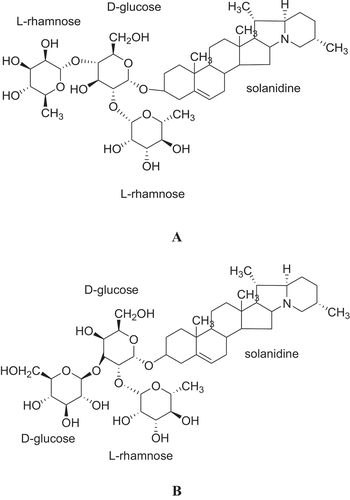
As potatoes are rich in nutrients such as proteins, carbohydrates, vitamins, minerals and phytonutrients[Citation1], and given the implementation of policies and the publication of guides such as the strategy of China’s potato staple in 2015, potato consumption is expected to increase. However, the trouble is that potatoes have natural secondary metabolites, the steroidal glycoalkaloids (SGAs), mainly α-chaconine and α-solanine (), which account for 95% of the total SGAs in potatoes.[Citation4] Although the SGA concentration is positively correlated with the potato’s sensory evaluation at low concentration[Citation5], it can affect the human intestinal tract and nervous system function[Citation6], disrupt biofilm system and inhibit acetylcholinesterase and butylcholinesterase activity at high levels.[Citation7] Excessive consumption can lead to poisoning or even death.[Citation6] Therefore, the safety limit of SGA content in potato tuber is 200 mg/kg. fresh potato weight (mg/kg FW).[Citation8] Given that people consume processed potato products, not fresh potatoes, studies on the effect of cooking methods on the SGA content in processed potato products and the monitoring of SGA content in potato products after processing are of great significance for safe potato consumption.
Potato foods such as potato chips, stir-fried shredded potatoes and fresh mashed potatoes are common in everyday life for farmers and restaurants in China. Although some empirical researches[Citation9,Citation10] have investigated the effects of cooking methods on SGA in potato foods, the SGA level research on stir-fried shredded potatoes and fresh mashed potatoes is rare. In addition, accumulated empirical findings suggest that the majority of SGA content is in the potato skin and that the level in skin, but not in flesh, varies significantly with time.[Citation11–Citation13] Therefore, the purpose of this study was to investigate the effects of different cooking methods on the SGA content of potato foods and fit the volume ratio of potato skin to flesh, and explore degradation temperature of SGA in potato sample to explain possible reasons for SGA content decreased after cooking in potato foods, which will provide a theoretical basis and practical guidance for potato food cooking.
Materials and methods
Reagents and consumables
α-Chaconine and α-solanine standards were purchased from Extrasynthese (Genay, France). HPLC-grade methanol, acetonitrile, formic acid and ammonium acetate were obtained from Sigma (USA). A 0.22 μm PTFE filter and the corresponding filter head were supplied from Supelco (Bellefonte, PA, USA). Purified water was from a water purification system (Sartorius, Germany). Analytical-grade acetic acid and acetonitrile were obtained from China Pharmaceutical Group Chemical Reagent Co. Ltd. Sep-Pak columns were supplied from Waters (USA). Table salt was purchased from Chinese National Salt Industry Corporation. Food-grade acetic acid was obtained from Jiangsu Yourong Trading Co. Ltd. (China). Grade 4 edible rapeseed oil was obtained from Yunnan Dianxue Grain Oil Co. Ltd. (China).
Standard stock solutions (500 and 1,000 μg/mL) of α-chaconine and α-solanine were prepared in methanol. Mixed stock solutions were diluted daily with the mobile phase to concentrations of 2 to 400 μg/L (2, 5, 10, 20, 50, 100, 200 and 400 μg/L) as the working solutions. All standards and stock solutions were stored at −20 °C in the dark, and the working solutions were stored at 4°C in the dark before use.
Experimental subjects
Experimental subjects were Unica potatoes in this study. Potato tubers were washed with tap water and allowed to dry at room temperature, which were the raw materials for the cooking experiments in the present study. The cultivar has a large planting area and a large number of consumers, especially Yunnan province.
Experimental design
To investigate the effect of food-grade acetic acid on SGA content in stir-fried shredded potatoes, the experiment was run with two independent samples and 3 repetitions. The operation included[Citation14]: 1) The potatoes were cut into neat slices. 2) The potato slices were cut into even filaments. 3) We added 10 mL rapeseed oil to the wok and added the chili segments to stir-fry until a fragrance was smelled. 4) We removed the fried prickly ash, and added the scallions, ginger, garlic, pepper and stir-fried them until their fragrance was smelled. 5) We added 50 g potato filaments. 6) We added 5 mL edible acetic acid into the treatment group after stir-frying 1 min, while we added 5 mL pure water into the CK group after stir-frying 1 min, and then we continued to stir fry the potato filaments for 2 min. 7) We added shredded chili to the wok and stir-fried for 2 min. 9) We added scallions and salt to adjust the taste.
To investigate the effect of food-grade acetic acid on SGA content in fresh mashed potatoes, a double-factor design with 3 repetitions was adopted. The factors were whether to peel or not and add food-grade acetic acid or not. The operation included[Citation15]: 1) The about 1000 g potatoes were randomly divided into two groups and numbered. About 2-mm-thick potato skins of the treatment group were peeled and added into a 4 L stockpot, and then 1 L tap water was added. The intact potatoes of the CK group were put into a 4 L stockpot with 1 L tap water. They were boiled for 40 min on an induction cooler under the condition of 1100 W. We drained the cooked potato tubers and tore off the intact potato epidermis. After the potatoes cooled, they were cut into small pieces with a knife and put on a plate for the test. The water-boiled potato was sampled with a 5 mL polyethylene pipe in three replicates and numbered for the test. 2) The dried chili was cut into segments. 3) We added 10 mL rapeseed oil into a heated pot after the oil temperature was high, and then we added the chili segments to it until their fragrance was smelled. 4) We added 50 g potato pieces and stir-fried 3 min. 5) We added 5 mL edible acetic acid to the treatment group, then added 5 mL pure water again. We added 5 mL pure water to the CK group, then added 5 mL pure water again. Both groups simmered for 5 min. 6) We added salt and sesame oil to adjust the taste while the excess water was drying. Meanwhile, we continued to stir-fry over heat. 7) The fresh mashed potatoes were put onto a plate and the excess water was dried with a towel.
To investigate the effect of the frying process on SGA content in potato chips, a paired design was used with 3 repetitions. The operation included[Citation16]: 1) The dried potatoes were cut into the shape of French fries. 2) We added 200 mL rapeseed oil into a pot, and added 50 g French chips after heating. 3) We placed the chips on a paper towel to drain the excess oil until the chips turned golden.
To test the degradation temperature of potato SGA, potato tubers were weighed (fresh weight) and cut into 0.2 mm× 10 mm× 10 mm pieces. They were heated in an oven at 60 °C for at least 24 h to dry[Citation17], then ground into a powder using a Perten 3100 grinding mill (Sweden). The samples were stored at 4°C in the dark before use. In the present study, a single-factor multilevel design with 3 replicates was adopted. Temperature was set to 0 °C, 90°C, 120°C, 150°C, 160°C, 170°C, 180°C or 190°C. The potato powder sample weight was approximately 1 g and the test time was 2 h. To test the effects of 150°C, 160°C, 170°C, 180°C and 190°C for various times on the SGA content in potato samples, single factors with two repeatable measurements were used. The factor was temperature or time (0.5 h, 2 h, 8 h and 24 h). The sample weight was approximately 1 g, with 3 replicates.
Sample collection and preparation
According to our previous results[Citation11], approximately 1 g experiment sample after cooking (or a 0.2 g sample of potato powder) was weighed into a 10 mL centrifuge tube, and 10 mL of 10% acetic acid aqueous solution was added. The mixture was blended in a Whirlpool blender for 2 min, then shaken on an oscillator for 2 h. The homogenized sample was centrifuged at 8,000 r/min for 10 min at 4°C on a Himac CF15RN high-speed centrifuge (Hitachi, Japan), and 0.5 mL of the clear supernatant (or 0.5 mL of water from boiled potatoes) was collected, and brought to 25 mL with the mobile phase, to avoid any solvent effects during analysis. In addition, to fit to the standard curve, the samples whose SGA content exceeded the linear range were diluted with mobile phase. The sample was then filtered through a 0.22 μm nylon membrane filter and transferred into an amber HPLC vial (1 mL, Agilent, Santa Clara, CA, USA).
The purification operation with the Sep-Pak column included 6 steps: 1) The Sep-Pak column was balanced with 6–10 column volume of methanol or acetonitrile, then washed with water or buffer salt (6–10 column volume) and not allowed to dry completely. 2) The sample was dissolved in strong solvent and put into the Sep-Pak column. 3) The target compounds were eluted with strong polar solvent. 4) The non-target compounds were eluted with weak polar solvent. 5) The ingredients that were were retained more strongly were eluted with increasing non-polar solvent. 6) The Sep-Pak column was disposed of appropriately after recycling all the components.
Instrumentation and analytical method
The effects of temperature on potato SGA content were assessed with a PrepASH229 automatic moisture ash analyzer (Precisa, Swiss). The instrument was turned on, and weights were used to adjust the balance. The instrument was more accurate when the display quality value was 0.0000 g. Peel/weight mode was selected to set it to single peeling/single weighing (T/S). The temperature program was set after sampling (). The samples were removed and placed into a dryer to cool when the program finished. The water loss rate of samples was recorded, and the data for all the parameters were corrected for moisture loss.
Table 1. The temperature program of PrepASH229 instrument for moisture and ash.
The SGA content in potato samples was detected by ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry (UHPLC-MS/MS), quantified using an external standard. The UHPLC-MS/MS system consisted of a Dionex UltiMate 3000 RSLCnano system (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled with a Finnigan TSQ Quantum Ultra triple quadrupole tandem mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with an electrospray ionization (ESI) source, whose resolution is 1 Da. The Dionex UltiMate 3000 RSLCnano system included SRD-3600 solvent racks, DGP-3600RS pumps, a WPS-3000TRS autosampler (RS) and a TCC-3000RS column compartment. The compounds were separated using an Agilent ZORBAX RRHD HILIC Plus analytical column (2.1 × 100 mm, 1.8 μm) and the mobile phase consisted of 20% solvent A (10 m mol/L ammonium acetate aqueous solution in - 0.01% formic acid) and 80% solvent B (acetonitrile - in 0.01% formic acid). The flow rate was 0.3 mL/min, the injection volume was 5 μL, and the column temperature was 25°C. The analytical data workstation utilized Thermo_Scientific TraceFinder EFS v3.1 software. Nitrogen was used as the desolation gas and cone gas, and argon was used as the collision gas.
The following parameters for MS were selected: 3 kV spray voltage, 300°C vaporizer temperature, 45 Arb sheath gas pressure, 2.0 Arb ion sweep gas pressure, 15 Arb auxiliary gas pressure and 350°C capillary temperature. For each SGA, the collision energies and cone voltages were optimized in MRM mode using the [M + H]+ ions to determine the precursor ions and the two most intense product ions for quantitation and qualitation, respectively (). The compounds were determined according to retention time, relative kurtosis and characteristic production ion of the standard, and they were quantified with the higher response ion pair (α-chaconine: 852.257 > 398.300; α-solanine: 868.257 > 398.300, ).
Table 2. MS/MS parameters of SGAs.
Statistical analysis
The SGA content data in stir-fried shredded potatoes was hypothesis-tested using the single-factor two-level design (group design) for quantitative data.[Citation18] α-Chaconine, α-solanine and total SGA served as dependent variables and treatment served as a fixed factor.
The SGA content data in fresh mashed potatoes was hypothesis-tested using the double-factor design for quantitative data.[Citation18] α-Chaconine, α-solanine and total SGA served as dependent variables, and whether peeled or not and whether acid was added or not served as fixed factors. The SGA content data in water-boiled potatoes was hypothesis-tested using the single-factor two-level design (group design) for quantitative data.[Citation14] α-Chaconine, α-solanine and total SGA served as dependent variables, and whether peeled or not served as the fixed factor.
The SGA data in potato chips was hypothesis-tested using a matching design for quantitative data.[Citation18] α-Chaconine, α-solanine and total SGA served as dependent variables, and frying process (before and after) served as the fixed factor.
Prior to the data analysis, the data normality test was performed. If the data were skewed, they were log-transformed. The SGA contents are expressed in mg/kg FW. Total SGAs are expressed as the sum of α-chaconine and α-solanine. The data analysis was performed using SAS v. 9.2 (SAS Institute Inc., Cary, NC, USA). Post-hoc comparisons of significant effects were performed using Duncan method at P < 0.05. The data are expressed as the mean ± SE.
In addition, the data from the experiments of SGA degradation temperature and the effects of temporal dynamics at 150°C, 160°C, 170°C, 180°C, 190°C and 200°C are expressed as the mean of the peak area that the instrument detected, these data were not hypothesis-tested.
Results and discussion
Effect of edible acetic acid on the SGA content in stir-fried shredded potatoes
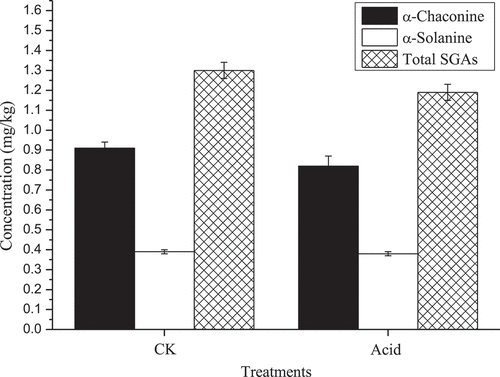
Edible acetic acid was added to the potato food in the process of stir-fried shredded potatoes, and the results showed that the edible acetic acid did not significantly affect the SGA content (α-chaconine: t = 1.59, P = 0.1868; α-solanine: t = 1.28, P = 0.2704; total SGAs: t = 1.83, P = 0.1414). The α-chaconine, α-solanine and the SGA contents in the experimental groups were 0.908 ± 0.032, 0.391 ± 0.006 and 1.299 ± 0.039 mg/kg, respectively, and in the CK groups were 0.816 ± 0.048, 0.377 ± 0.010 and 1.193 ± 0.043 mg/kg. The difference between them was not significant (). However, the SGA levels were much lower than the international safety limit of 200 mg/kg FW.[Citation8]
Effect of edible acid and the cooking process on the SAG content in fresh mashed potatoes
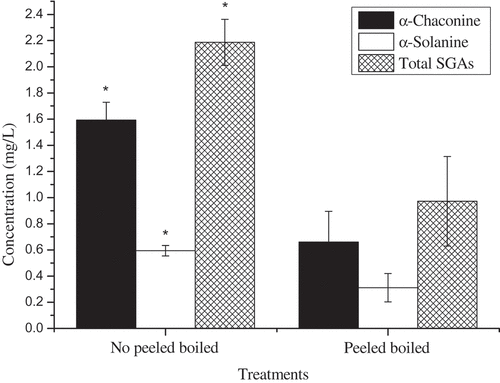
The water-boiled potato was sampled after 40 min, and the results showed that the SGAs were dissolved in water to different degrees, but the effect of peeling on the SGA content in water was significant (α-chaconine: t = 5.96, P = 0.0040; α-solanine: t = 4.24, P = 0.0133; total SGAs: t = 5.46, P = 0.0055). The SGA content in boiled potato water after peeling was lower than that after no peeling (P < 0.05, ). Water is a strongly polar solvent, and SGA is a polar compound. Therefore, potato SGA can be dissolved in water according to the principle of similar solubility. Due to the high content of SGA in potato skins [Citation19] and significant changes with time [Citation11], less SGA was found in boiled potato water after peeling.
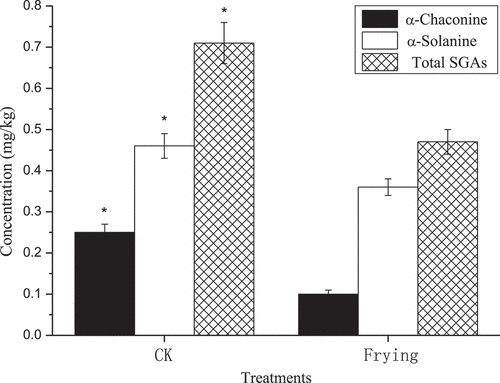
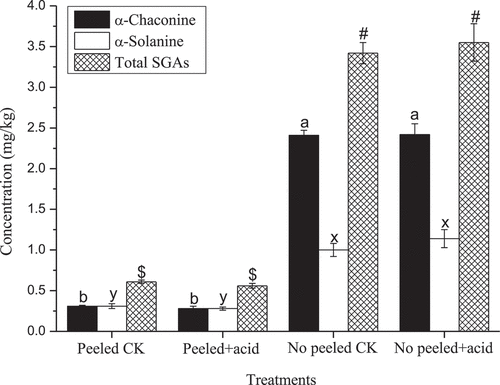
Edible acetic acid was added to fresh mashed potatoes in the cooking process, and the results indicated that this did not affect the SGA content (peeled potatoes: α-chaconinte: t = 0.81, P = 0.4632; α-solanine: t = 0.86, P = 0.4394; total SGAs: t = 1.58, P = 0.1891; non-peeled potatoes: α-chaconine: t = -0.04, P = 0.9674; α-solanine: t = -0.99, P = 0.3790; total SGAs: t = -0.53, P = 0.6244). However, the effect of peeling or not on the SGA content in fresh mashed potatoes was remarkable (α-chaconine: F3,8 = 296.27, P < 0.0001; α-solanine: F3,8 = 42.77, P < 0.0001; total: SGAs: F3,8 = 159.796, P < 0.0001). Multiple comparative analyses showed that SGA content in fresh mashed potatoes after peeling was significantly lower than after not peeling (). According to a previous study, the majority SGA is in the potato skin.[Citation11] Thus, the SGA content can be greatly removed during the preparation stage of raw materials, which can minimize people’s consumption of potato SGA and reduce the harm to human health. These SGA content were much lower than the recommended limit of 200 mg/kg FW[Citation8] ().
Figure 4. The effect of acetic acid on SGAs content from fresh mashed potatoes.
Note: The post-hoc testing was done with Duncan corrections at P < 0.05. P < 0.05 vs. basal level for α-chaconine (a and b), α-solanine (x and y), and total SGAs (# and $).
A previous study indicated that the SGA contents were similar among all process potato foods [Citation20], but peeling can significantly affect it [Citation21–Citation23], possibly reducing the SGA content 20–30%.[Citation22] Other findings showed that peeling potatoes can reduce SGA content by almost half.[Citation24] In addition, the amount of SGA in potatoes can be significantly reduced by rinsing with water.[Citation25]
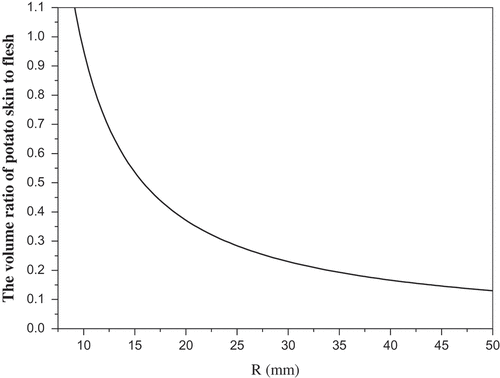
Given that the majority SGA content is in the potato skin and varied significantly with time in skins not in flesh[Citation11], we simulated the volume ratio change of skin to flesh for different size potatoes in the next section. It is expected to provide a theoretical guidance to potato breeders and consumers.
Volume ratio of potato skin to flesh
The experimental subjects, Unica potatoes, are approximately ellipsoid. Therefore, the potato tubers were modeled as ellipsoids ():
Where a ≠ b ≠ c; a, b are equatorial radii; and c is the polar radius. The volume of the potatoes was modeled as follows:
According to a previous study, the thickness of the potato skin was assumed to be 2 mm.[Citation26] Therefore, the volume of potato flesh was modeled as follows:
Volume of potato skin was as follows
It follows that the volume ratio of potato skin to flesh is as follows:
When 5 mm ≤ a = b = c < 50 mm, that is potato tubers are approximately spherical, we have where R is the radius. That is the value of the ratio decreases with increasing potato volume (). Similarly, when 5 mm ≤ a < b < c < 50 mm, that is, potato tubers are approximately ellipsoid, the value of
decreases with increasing potato volume. Therefore, potato breeders and farmers should make an effort to increase the proportion of commodity potatoes via the corresponding science and technology.
Effect of frying process on the SGA content in potato foods
The SGA detection of potato chips before and after frying was showed that the frying process significantly affected the SGA content (α-chaconine: t = 6.06, P = 0.0038; α-solanine: t = 4.05, P = 0.0154; total SGAs: t = 4.20, P = 0.0137). The results from multiple comparisons suggest that the SGA content of potato chips after frying was significantly lower than before frying (). The results are consistent with the finding that, a large amount of potato SGA content can be eliminated in the frying process.[Citation4,Citation22,Citation27] A study on colored potato showed that the SGA content in the food was significantly reduced after potato frying.[Citation9] Relative to raw materials, the frying process can decrease the SGA content by 83% and can reduce the SGA content of potato chips by 92% among their research potato cultivars. Other findings have indicated that the cooking process can reduce the SGA content by approximately 84% [Citation22], and can reduce the SGA content of fried chips by approximately 94%.[Citation27] All in all, the potato frying process can reduce the SGA content of potato food.[Citation10] However, studies investigating main reason for the reductions in the SGA content in fried potato foods are rare. In the present study, we hypothesize that the decrease of SGA in potato chips after frying may be due to temperature, thus, the researches of effects of temperature on the SGA content in potato samples were conducted in the next section.
Effect of temperature on the SGA content in potato samples
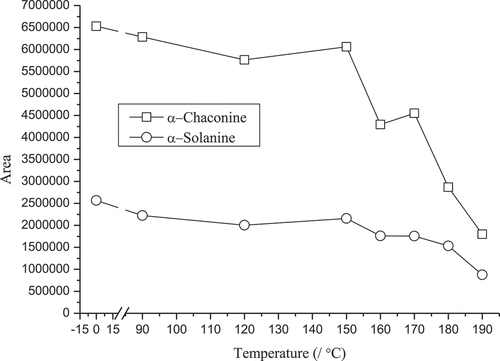
Experiments analyzing the effect of temperature within 2 h on the SGA content in potato samples were carried out by a PrepASH229 automatic moisture ash analyzer, and the results indicated that the SGA content did not vary with time when the temperature was below 150°C. However, the SGA content decreased to various degrees at temperatures higher than 150°C. α-Chaconine content decreased with a constant slope from 150 to 190°C; the slope of the decrease in α-solanine content from 150 to 180°C was lower than that from 180 to 190°C ().
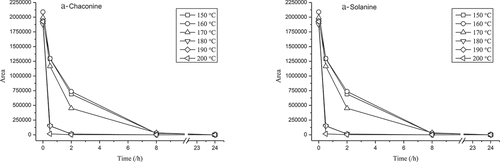
Furthermore, we detected the SGA content in potato samples within various times at different temperatures from 150 to 190°C, and the results indicated that the α-chaconine content decreased quickly within 30 min at 150 to 170°C, and then decreased to 0 within 8 h at a slower speed. However, the α-chaconine content in potato samples decreased quickly to 0 within 2 h at 180 to 190°C. The α-solanine content changed similarly to α-chaconine (). Therefore, the potato SGA degraded at temperatures above 150°C. This could be that the chemical structure of SGA were destroyed, which are needed other empirical evidence for further confirmation. The oil temperature can reach to above 200°C in the process of frying. Therefore, the reason the SGA content decreased could be the temperature of the frying process, which validated the hypothesis proposed in the previous section.
Conclusion
In the process of potato cooking, acetic acid did not affect the SGA content in potato foods. Because of the SGA content is higher in the potato skin, peeling the potato can reduce it significantly during the potato cooking, and the volume ratio of potato skin to flesh decreases with increasing potato tuber volume. Therefore, potato breeders and farmers should make an effort to increase the proportion of commodity potato via corresponding science and technology. The SGA degraded to various degrees at temperature above 150°C and degraded quickly at 190°C. The edible oil brand was Yunnan Dianxue rapeseed cooking oil, which can reach to above 200°C in the process of frying. Therefore, the reason the SGA content decreased could be the temperature of the frying process.
From what has been discussed above, these cooking methods, such as peeling and frying, can significantly reduce the SGA content in the cooking process of potato foods, which is of great significance for helping people to consume potato foods safely.
Acknowledgments
We sincerely thank Zhiling Shao at the Yunnan Provincial Academy of Food and Oil Sciences for his guidance and National Potato Special Fund of the Modern Agricultural Industrial Technological System (CARS-09-P15) and the Youth Project Fund of the Scientific and Technological Departmentof Yunnan province (2017FD025) for their financial support in conducting the experiments.
Additional information
Funding
References
- Burlingame, B.; Charrondière, M.B.; Nutrients, R. Bioactive Non-Nutrients and Anti-Nutrients in Potatoes. Journal of Food Composition & Analysis 2009, 22, 494–502.
- Itkin, M.; Heinig, U.; Tzfadia, O.; Bhide, A.J.; Shinde, B.; Cardenas, P.; Bocobza, S.E.; Unger, T.; Malitsky, S.; Finkers, R.; Tikunov, Y.; Bovy, A.; Chikate, Y.; Singh, P.; Rogachev, I.; Beekwilder, A.P.; Aharoni, G.A. Biosynthesis of Antinutritional Alkaloids in Solanaceous Crops Is Mediated by Clustered Genes. Science 2013, 341, 175–179. DOI: 10.1126/science.1240230.
- Li, Y.; Gao, M.J.; Luo, Q.Y.; Zhang, Q.; He, W.M. Analysis on the Basic Situation and Characteristics of World Potatoes Consumption. Wold Agriculture. 2014. 119–124. In chinese
- Mcdonald, F.M.; Filadelfikeszi, G.M.; Potato Glycoalkaloids:, M.A.; Chemistry, A. Safety, and Plant Physiology. Critical Reviews in Plant Sciences 1997, 16, 55–132.
- Amer, F.S.; Reddivari, L.; Madiwale, G.P.; Stone, M.; Holm, D.G.; Vanamala, J. Effect of Genotype and Storage on Glycoalkaloid and Acrylamide Content and Sensory Attributes of Potato Chips. American Journal of Potato Research 2014, 91, 632–641. DOI: 10.1007/s12230-014-9393-9.
- Morris, S.C.; Lee, T.H. The Toxicity and Teratogenicity of Solanaceae Glycoalkaloids, Particularly Those of the Potato (Solanum Tuberosum): A Review. Food Technology in Australia 1984, 36, 118–124.
- Krasowski, M.D.; McGehee, D.S.; Moss, J. Natural Inhibitors of Cholinesterases: Implications for Adverse Drug Reactions. Canadian Journal of Anaesthesia 1997, 44, 525–534. DOI: 10.1007/BF03011943.
- Clayton, R.; Percival, G. In Glycoalkaloids in Potato Tubers - a Cause for Concern?, World Potato Congress: Proceedings of the Fourth World Potato Congress, Amsterdam, the Netherlands, 4-6 September, 2000, 170–173.
- Tajnerczopek, A.; Rytel, E.; Kita, A.; Pęksa, A.; Hamouz, K. The Influence of Thermal Process of Coloured Potatoes on the Content of Glycoalkaloids in the Potato Products. Food Chemitry 2012, 133, 1117–1122. DOI: 10.1016/j.foodchem.2011.10.015.
- Bushway, R.J.; Ponnampalam, R. alpha-Chaconine and Alpha-Solanine Content of Potato Products and Their Stability during Several Modes of Cooking. Journal of Agricultural & Food Chemistry 1981, 29, 814–817. DOI: 10.1021/jf00106a033.
- Nie, X.H.; Guo, H.C. An Ultra-High-Performance Liquid Chromatography-Triple Quadrupole Mass Spectrometry Method for the Detection of Steroidal Glycoalkaloids in Potato Samples. Analytical Methods 2017, 9, 6613–6621. DOI: 10.1039/C7AY02244A.
- Caprioli, G.; Cahill, M.G.; Vittori, S.; James, K.J. Liquid Chromatography–Hybrid Linear Ion Trap–High-Resolution Mass Spectrometry (Ltq-Orbitrap) Method for the Determination of Glycoalkaloids and Their Aglycons in Potato Samples. Food Analytical Methods 2014, 7, 1367–1372. DOI: 10.1007/s12161-013-9758-6.
- Xu, D.; Liu, H.; Jin, C.Y.; Cao, C.M.; Li, W.G.; Zeng, F.K.; Zhao, Y.C.; Liu, G. A New Potato VarietyGrown in China Suitable forRaw Eating. European FoodResearch & Technology 2017,1-10
- Li, H.H.; Yi, Z.X.; Zhang, Y.; Wang, J.; Sui, H.W. Cooking Process Optimization of “Stir-Fired Crisp Potato Shreds”. Food Science & Technology. 2017. 42, 44–48. In chinese
- Wang, H.P.; Xie, G. Study on the Optimization of Manufacturing Process of Mashed Potato. Food Industry. 2014. 35, 19–21. In chinese
- Liu, S.; Pan, H.D.; Zhao, S.M. Study on the Processing Technology and Flavor Characteristics of Crispy Fries. Science & Technology of Food Industry. 2017. 196–202. In chinese
- Huang, H.P.; Guo, H.C.; Wang, Q.; Shen, C.Z.; Zhou, C. Determination of the Content of Solanine in Potato Tuber in Yunnan. Scientia Agricultura Sinica. 2011. 44, 1512–1518. In chinese
- Hu, L.P.;. Implement Scientific Research Design and Statistical Analysis Correctly; Beijing: The People’s Military Medical Press 2011, 50–150. In chinese
- Knuthsen, P.; Jensen, U.; Schmidt, B.; Larsen, I.K. Glycoalkaloids in Potatoes: Content of Glycoalkaloids in Potatoes for Consumption. Journal of Food Composition & Analysis 2009, 22, 577–581. DOI: 10.1016/j.jfca.2008.10.003.
- Mulinacci, N.; Ieri, F.; Giaccherini, C.; Innocenti, M.; Andrenelli, L.; Canova, G.; Saracchi, M.; Casiraghi, M.C. Effect of Cooking on the Anthocyanins, Phenolic Acids, Glycoalkaloids, and Resistant Starch Content in Two Pigmented Cultivars of Solanum Tuberosum L. Journal of Agricultural & Food Chemistry 2008, 56, 11830–11837. DOI: 10.1021/jf801521e.
- Friedman, M.;. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. Journal of Agricultural & Food Chemistry 2006, 54, 8655–8681. DOI: 10.1021/jf061471t.
- Pęksa, A.; Gołubowska, G.; Aniołowski, K.; Lisińska, G.; Rytel, E. Changes of Glycoalkaloids and Nitrate Contents in Potatoes during Chip Processing. Food Chemistry 2006, 97, 151–156. DOI: 10.1016/j.foodchem.2005.03.035.
- Tajner-Czopek, A.; Jarych-Szyszka, M.; Lisińska, G. Changes in Glycoalkaloids Content of Potatoes Destined for Consumption. Food Chemistry 2008, 106, 706–711. DOI: 10.1016/j.foodchem.2007.06.034.
- Jansen, G.; Flamme, W. Coloured Potatoes (Solanum Tuberosum L.) – Anthocyanin Content and Tuber Quality. Genetic Resources & Crop Evolution 2006, 53, 1321. DOI: 10.1007/s10722-005-3880-2.
- Mäder, J.; Rawel, H.; Kroh, L.W. Composition of Phenolic Compounds and Glycoalkaloids α-Solanine and α-Chaconine during Commercial Potato Processing. Journal of Agricultural & Food Chemistry 2009, 57, 6292. DOI: 10.1021/jf901066k.
- Rizk, A.F.M.;. Poisonous Plant Contamination of Edible Plants; Boca Raton: Crc Press, 1991.
- Rytel, E.; Golubowska, G.; Lisinska, G.; Peksa, A.; Aniolowski, K. Changes in Glycoalkaloid and Nitrate Contents in Potatoes during French Fries Processing. Journal of the Science of Food & Agriculture 2005, 85, 879–882. DOI: 10.1002/jsfa.2048.