ABSTRACT
The present research was carried out to observe the comparative effectiveness of different chemical treatments in combination with simultaneous thermal treatment on soluble (SDF) and insoluble dietary fiber (IDF) ratio to improve functional properties of barley. For this purpose, two varieties of barley, i.e., Haider-93 and Jau-87 were assessed for chemical composition, mineral, non-starch polysaccharides, and dietary fiber contents through respective methods. Two varieties were chemically treated through acid, alkaline, and consecutive acid–alkaline treatments in combination with thermal treatment. Results of chemical composition revealed that Jau-87 was higher in moisture (11.4%), crude fat (2.67%), and crude fiber (4.70%), whereas Haider-93 exhibited higher ash (2.56%) and crude protein content (12.7%). Moreover, barley is a rich source of potassium ranging from 4.77 to 5.07 g/kg. Likewise, main non-starch polysaccharides in barley were arabinoxylan (3.60–3.77%) and beta-glucan (3.65–3.67%). Furthermore, barley contains more IDF (12.00–12.40 g/100 g dm) than SDF (4.73–5.70 g/100 g dm). Additionally, modification of SDF (23.68%) and IDF (11.69%) ratio through acid treatment was nonsignificant, while acid–alkaline treatment showed highly significant results, i.e., 771.46% increased in SDF and 53.39% decreased in IDF. It is concluded that chemical treatments alone or in combination with pressure cooking increased SDF. However, simultaneous effect of acid and alkaline treatment most effectively increased the solubility of barley.
Introduction
For the last few years, efforts have been aimed to determine scientific justification for functional properties of dietary fiber.[Citation1] Dietary fiber refers to the nondigestible carbohydrate entity, which is impervious to enzymatic assimilation and absorption in the small intestine of human. It is conventionally classified into two categories according to their water solubility: insoluble dietary fiber (IDF) and soluble dietary fiber (SDF).[Citation2,Citation3] Each fraction has its own physiological effects. Soluble fractions are fermented faster into short- chain fatty acids and are consumed by the beneficial intestinal bacteria than insoluble fractions. From a functional point of view, SDF is more important than IDF. SDF helps to soften the stool, binds to substance like cholesterol and sugar, prevents and slows their absorption into the blood, regulates blood sugar levels and protects against heart disease, and in turns lowers the cholesterol level, possesses significant role in weight management (feel free for longer after its intake), boosts the population of good bacteria in gut, improves immunity, and enhances the mood.[Citation4] Approximately 20–35% of SDFs and 65–80% of IDFs are present in foods (mainly cereals). As far as the recommended dietary intake ratio is concerned, one quarter for SDF and three quarters for IDF (1:3) are recommended. The main sources of dietary fibers are cereals, nuts, fruits, and vegetables.[Citation1] Barley is one of the richest sources among cereals, as it contains about 24% dietary fiber in the ratio of 3:2 as for IDF and SDF, respectively.[Citation5]
Chemical treatment has been considered as the most important approach among all techniques to increase the solubility of dietary fiber because chemical reagents cause less damage to the molecular structure of the dietary fiber and content of SDF is increased more efficiently. Chemical methods use acid and alkali to solubilize the dietary fiber of cereals.[Citation6] It depends upon the amount of acid and alkali, temperature, and reaction time. Englyst and Cumming[Citation7] used sulfuric acid and trifluoroacetic acid to hydrolyze hemicellulose. This treatment hydrolyzes the long polysaccharide chain to smaller fractions to increase their solubility. Feng et al.[Citation5] used the hydrogen peroxide to improve the SDF content of black soybean hull. Park et al.[Citation8] modified the dietary fiber ratio in whole grain barley by carboxymethylation. Extrusion cooking is another approach for dietary fiber modification. Huang and Ma[Citation9] applied high temperature, pressure, and shear force to gasify and extend the moisture content present in the cereals. This mechanism depends upon the processing parameters such as temperature and pressure.[Citation10,Citation11] In general, a combined method may have greater effect on the modification of IDF into SDF in cereal than the use of the single method.[Citation12,Citation13]
Keeping in mind all the aforementioned perspectives, there is a dire need to partially convert this IDF into SDF and to develop the SDF-enriched value-added barley products. The objective of the current study was to evaluate the comparative and synergistic effect of chemical treatment and pressure cooking on the modification of IDF into SDF in two barley varieties.
Materials and methods
Procurement of raw material
Two barley varieties, i.e. Haider-93 and Jau-87 were procured from Ayub Agriculture Research Institute, Faisalabad. Seeds were cleaned to remove any debris or field dirt and sealed in polyethylene bags.
Chemical composition
Chemical composition of barley varieties was assessed to check the contents of moisture, ash, crude fat, crude protein, crude fiber, and nitrogen-free extract (NFE) according to the method of association of official analytical chemists (AOAC).[Citation14]
Mineral content
The mineral contents like Na, K, Ca, Mg, Fe, Cu, Zn, and Mn in both barely varieties were determined by the method described in AOAC.[Citation15]
Non-starch polysaccharides and dietary fiber
Non-starch polysaccharides content like arabinoxylan and beta-glucan in barley varieties was assessed by the method of Pettolino et al.[Citation16] and AOAC Method 995.16. The contents of dietary fiber in both barley varieties were determined according to the method of AOAC 991.43 enzymatic-gravimetric method.[Citation14]
Chemical treatments
Dietary fiber from barley varieties was chemically modified for the partial conversion of IDF into SDF according to the method of Ning et al.[Citation17] In brief, a mixture of barley fiber and water, at a ratio of 1:5 was used. Its pH values were adjusted acidic (pH 2.0–4.0) and alkaline (pH 9.0–11.0) by using 6.0 N HCl (acid treatment) and 6.0 N NaOH (alkaline treatment), respectively. After pH adjustment, the mixtures were then heated at 90ºC for different periods of time ranging from 1 to 4 h. At the end of each treatment, the supernatant was removed, neutralized, and then centrifuged at 700 ×g for 10 min. Pentose and hexose contents were measured for the acid treatment using high-performance liquid chromatography, and total sugar content was determined for the alkaline treatment according to the procedure of Folkes and Taylor[Citation18] and Dubois et al.[Citation19], respectively. The precipitate was washed with water and dried using an air drier (Proctor Schwartz, Philadelphia, PA) at 75ºC for 2 h, followed by grinding and screening through a 2-mm sieve. The acid–alkaline treatment involved consecutive acid and alkaline treatments, according to the aforementioned procedures. For the acidic treatment, the mixture was adjusted to pH 2.0 with 6.0 N HCl, while for the alkaline treatment, the mixture was adjusted to pH 11.0 with 6.0 N NaOH.
Pressure cooking of native and chemically modified barley fiber
The cleaned grains were pulverized using a plate mill to obtain whole flour (WF). A part of the WF was further sieved through a 44-mesh sieve. The ‘+’ fraction was termed as the bran-rich fraction and the ‘–’ fraction was termed as semi-refined flour. Each batch of the two commercially available barley varieties was pressure cooked for 10 min (9.8 × 104 Pa) (Pushparaj and Urooj, 2011).
Statistical analysis
The data obtained for each parameter were subjected for completely randomized design and Latin square design and later on ANOVA to determine the level of significance.[Citation20]
Results and discussion
Chemical composition and mineral profile
Mean values regarding chemical composition have been depicted in . The results indicated that Jau-87 exhibited relatively high moisture (11.4%), crude fat (2.67%), and crude fiber (4.70%) contents than Haider-93 (i.e., 10.2%, 2.47%, 4.53%, respectively). Whereas, Haider-93 was higher in ash (2.56%), crude protein (12.7%), and NFE (67.55%) contents as compared to Jau-87 that contained about 2.35%, 12.13%, 66.64% ash, crude protein, and NFE content, respectively. The results are in accordance with the earlier findings of Labouar et al.[Citation22] who reported 10.8%, 2–4%, 11.37%, and 3.82% moisture, ash, crude protein, and crude fat in barley, respectively. Similarly, Lee et al.[Citation23] probed 10.10–13.10% moisture, 2.02–2.38% ash, 9.46–11.33% crude protein, 3.90–5.81% crude fat, and 1.70–2.18% ether extract in barley, respectively.
Table 1. Mean values of chemical composition, mineral profile, and non-starch polysaccharide content of barley varieties.
showed the mean values for macro- and micro-minerals content of two different varieties of barley. In Jau-87, macro-minerals, i.e., potassium, magnesium, calcium, and phosphorus were 4.77, 1.31, 0.44, and 3.78 g/kg, whereas in Haider-93, 5.07, 1.35, 0.51 and 4.08 g/kg, respectively. However, micro-minerals including copper, manganese, iron, and zinc were 7.93, 13.40, 49.40, and 23.50 g/kg in Jau-87, and 8.70, 14.50, 52.87, and 24.43 g/kg in Haider-93, respectively. All these findings showed that Haider-93 is the best variety of barley rather than Jau-87. The results are in-line with the observation of Boila et al.[Citation24] who reported that barley contained calcium, phosphorus, magnesium, potassium, copper, zinc, manganese, and iron in range of 0.39–0.56, 3.69–4.48, 1.28–1.41, 4.70–5.31, 7.5–9.6, 22.9–32.3, 12.8–16.2, and 48.1–58.3 g/kg, respectively.
Non-starch polysaccharides
Mean values for monosaccharide content of two different varieties of barley are depicted in . Arabinoxylan content (8.35%) is more in Haider-93 than in Jau-87, i.e., 8.21%, whereas the beta-glucan content is 3.67% and 3.65% in Jau-87 and Haider-93, respectively. These studies were similar to the study of Andersson et al.[Citation25] who reported 75–90 and 28–69 g/kg dm of arabinoxylan and beta-glucan in barley. Likewise, in another study, arabinoxylan and beta-glucan content was 8.4% and 4.1%, respectively, in barley.[Citation26,Citation27]
Dietary fiber
The soluble and insoluble fiber contents of the native and all the chemically modified barley of two different varieties were measured. Mean values for dietary fiber content of two barley varieties before treatment were exhibited in . Results showed that SDF content was higher in Haider-93 (5.70 g/100 g dm) than in Jau-87 (4.73 g/100 g dm), whereas IDF was more in Jau-87 (12.00 g/100 g dm) than in Haider-93 (12.40 g/100 g dm). Literature showed similar results as of present study. Beloshapka et al.[Citation28] explicated that barley contained about 8.6%, 4.8%, and 13.4% IDF, SDF, and total dietary fiber, respectively.
Table 2. Mean values of dietary fiber content of native and chemically modified varieties of barley (g/100g).
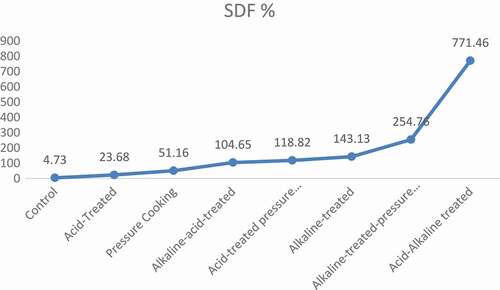
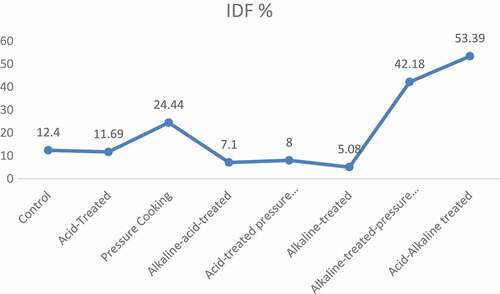
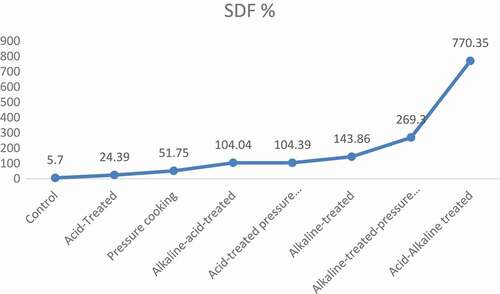
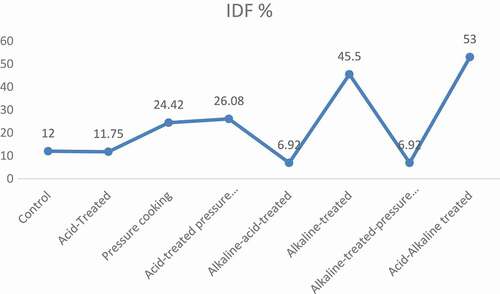
exhibited the mean values of dietary fiber content in chemically modified varieties of barley. Results of pressure cooking revealed that it significantly modified the soluble and IDFratio, i.e., SDF was significantly increased and IDF was significantly decreased in both barley varieties. In Jau-87, SDF was increased from 4.73 to 7.15 g/100 g, and IDF decreased from 12.40 to 9.37 g/100 g, whereas, in Haider-93, increase in SDF was from 5.70 to 8.65 g/100 g, and decrease in IDF was from 12.00 to 9.07 g/100 g. This modification was significant (p < 0.05). Moreover, when the dietary fiber was treated by acid, it slightly modified the SDF and IDFratio, i.e., 23.68% increase in SDF and 11.69% decrease in IDF in Jau-87, while in Haider-93, 24.39% increase in SDF and 11.75% decrease in IDF, respectively. After treatment with acid, dietary fiber was then pressure cooked, and it gave much better results, i.e., in Jau-87, 118.82% increase in SDF and 8.00% decrease in IDF, whereas, 104.39% increase in SDF and 26.08% decrease in IDF in Haider-93 as shown in –.
Furthermore, after acid and acid-pressure cooking treatment, dietary fiber was then treated with alkali (NaOH). This treatment significantly modified the soluble and insoluble ratio. In Jau-87, SDF was significantly increased from 4.73 to 11.50 g/100 g and IDF was significantly decreased from 12.40 to 11.77 g/100 g, while, in Haider-93, SDF was significantly increased from 5.70 to 13.90 g/100 g and IDF was significantly decreased from 12.00 to 11.35 g/100 g. Then, this alkali treated dietary fiber was pressure cooked, and the results were significant (P < 0.05). SDF was increased from 4.73 to 16.78 and from 5.70 to 21.05 g/100 g while IDF was decreased from 12.40 to 7.17 and 12.00 to 11.17 g/100 g in Jau-87 and Haider-973, respectively.
After that, alkaline and acid treatment were applied simultaneously in two sequences (i.e., alkaline–acid treatment, acid–alkaline treatment). The results of acid and alkaline treatments were significant (p < 0.05). Alkaline–acid treatment increased to 104.65% and 104.04% SDF and decreased to 7.10% and 6.92% IDF in Jau-87 and Haider-93, respectively. Whereas, acid–alkaline treatment increased the SDF to 771.46%, whereas decrease in IDF was 53.39% in barley (–).
Among all treatments, the highest results were shown by acid–alkaline treatment. Pressure cooking applied high temperature, pressure and gasified and extended the moisture content present in the both varieties of barley. It led to the modification of intermolecular and intramolecular spatial structure of fiber and barley and formed a porous state. Pressure cooking resulted in disruption of covalent and non-covalent bonds in the carbohydrate and protein moieties leading to smaller and more soluble molecular fragments and less insoluble fractions. Other chemical treatments (acid-treated, acid-treated pressure cooked, alkaline-treated, alkaline-treated-pressure cooked, alkaline–acid-treated, acid-alkaline-treated varieties of barley) followed the same mechanism of polysaccharides chains breakage for the modification of SDF and IDF. Hence, the mobility of the polysaccharide molecules was greatly increased, facilitating their interaction with water molecules and their associated solubility. The mechanism behind the extremely high soluble fiber content and the low insoluble fiber content observed after consecutive acid-alkaline treatments may be due to the fact that acid treatment can open the structure and increase the surface porosity of the fiber particle, making it easier for the hydroxyl groups to penetrate inside and perform hydrolysis during the subsequent alkaline treatment.
Conclusion
Both varieties of barley were found to be a very high-quality reservoir of protein and potassium and a tremendous resource of dietary fiber, mainly IDF. Through chemical and thermal treatments application, barley was modified with respect to SDF and IDF ratio, i.e., SDF was increased while IDF was decreased. Although all treatments had given effective results, acid-alkaline treatment was the most effective. This modification opens the door for the betterment of physiochemical, physiological, and functional properties of dietary fiber by increasing the SDF. As a promising source of SDF, dietary fiber should be exploited for therapeutic and health-enhancing food products.
Acknowledgments
I acknowledge Higher Education Commission, and Institute of Home and Food Sciences, Government College University for providing funds, facilities, and equipment.
References
- Jha, R.; Berrocoso, J.-D. Review: Dietary Fiber Utilization and Its Effects on Physiological Functions and Gut Health of Swine. Animal. 2015, 9(9), 1441–1452. DOI: 10.1017/S1751731115000919.
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.-T. Dietary Fibre in Foods: A Review. Journal of Food Science and Technology. 2012, 49(3), 255–266. DOI: 10.1007/s13197-011-0365-5.
- Lattimer, J.-M.; Haub, M.-D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrition. 2010, 2(12), 1266–1289.
- Anderson, J.-W.; Baird, P.; Davis, R.-H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.-L. Health Benefits of Dietary Fiber. Nutrition Reviews. 2009, 67(4), 188–205. DOI: 10.1111/j.1753-4887.2009.00189.x.
- Feng, Z.; Dou, W.; Alaxi, S.; Niu, Y.; Yu, -L.-L. Modified Soluble Dietary Fiber from Black Bean Coats with Its Rheological and Bile Acid Binding Properties. Food Hydrocolloids. 2017, 62, 94–101. DOI: 10.1016/j.foodhyd.2016.07.032.
- Huang, T.; Xu, M.; Lee, A.; Cho, S.; Qi, L. Consumption of Whole Grains and Cereal Fiber and Total and Cause-Specific Mortality: Prospective Analysis of 367,442 Individuals. BMC Medical. 2015, 13, 59. DOI: 10.1186/s12916-015-0294-7.
- Englyst, H.-N.; Cummings, J.-H. Simplified Method for the Measurement of Total Non-Starch Polysaccharides by Gas-Liquid Chromatography of Constituent Sugars as Alditol Acetates. Analyst. 1984, 109, 937–942. DOI: 10.1039/an9840900937.
- Park, K.-H.; Lee, K.-Y.; Lee, H.-G. Chemical Composition and Physicochemical Properties of Barley Dietary Fiber by Chemical Modification. International Journal of Biological Macromolecules. 2013, 60(6), 360–365. DOI: 10.1016/j.ijbiomac.2013.06.024.
- Huang, Y.-L.; Ma, Y.-S. The Effect of Extrusion Processing on the Physiochemical Properties of Extruded Orange Pomace. Food Chemistry. 2016, 192, 363–369. DOI: 10.1016/j.foodchem.2015.07.039.
- Rashid, S.; Rakha, A.; Anjum, F.-M.; Ahmed, W.; Sohail, M. Effects of Extrusion Cooking on the Dietary Fibre Content and Water Solubility Index of Wheat Bran Extrudates. International Journal of Food Science and Technology. 2015, 50(7), 1533–1537. DOI: 10.1111/ijfs.12798.
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel Blasting Extrusion Processing Improved the Physicochemical Properties of Soluble Dietary Fiber from Soybean Residue and in Vivo Evaluation. Journal of Food Engineering. 2014, 120(1), 1–8. DOI: 10.1016/j.jfoodeng.2013.07.011.
- Ma, M.; Mu, T. Modification of Deoiled Cumin Dietary Fiber with Laccase and Cellulase under High Hydrostatic Pressure. Carbohydrate Polymers. 2016, 136, 87–94. DOI: 10.1016/j.carbpol.2015.09.030.
- Tang, H.-S.; Chen, P.-Y.; Ding, Q.; Liu, L.-Y.; Qi, H.; Li, L. Extraction of Soluble Dietary Fiber from Pomelo Peel by Ultrasonic Assisted Enzymatic Method and Its Antioxidant Activity. In Storage and Process, 2016; pp 6.
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, Washington DC, 18th edition, Method 935.14, 2005.
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington DC, 1990.
- Pettolino, F.-A.; Walsh, C.; Fincher, G.-B.; Bacic, A. Determining the Polysaccharide Composition of Plant Cell Walls. Journal of Natural Products. 2012, 7(9), 1590–1607.
- Ning, L.; Villota, R.; Artz, W.-E. Modification of Corn Fiber through Chemical Treatments in Combination with Twin-Screw Extrusion. Cereal Chemistry. 1991, 68(6), 632–636.
- Folkes, D.-J.; Taylor, P.-W. Determination of Carbohydrates. In HPLC in Food Analysis; Macrae, R.;, Ed.; Academic Press: New York, 1982; pp 149–166.
- Dubois, M.; Gilles, K.-A.; Hamilton, J.-K.; Rebers, P.-A.; Smith, F. Colorimetric Method for Determination of Sugar and Related Substances. Analytical Chemistry. 1956, 28, 350–356. DOI: 10.1021/ac60111a017.
- Steel, R.-G.-D.; Torrie, J.-H.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co. Inc.: New York, 1997.
- Yi, T.; Wang, K.; Zhuang, Z.; Pan, S.; Huang, X. Comparative Analysis of Dietary Fibre Extract Isolated from Citrus Juice By-Products Using Water Extraction, Fermentation and Enzymatic Treatment Methods. Advance Journal of Food Science and Technology. 2014, 6(9), 1058–1066. DOI: 10.19026/ajfst.6.160.
- Labouar, L.; Ghrairi, F.; El Arem, A.; Medimagh, S.; El Felah, M.; Salem, H.-B.; Achour, L. Biochemical Composition and Nutritional Evaluation of Barley Rihane (Hordeum Vulgare L.). African Journal of Traditional, Complementary and Alternative Medicines. 2017, 14(1), 310–317.
- Lee, J.; Nam, D.-S.; Kong, C. Variability in Nutrient Composition of Cereal Grains from Different Origins. Springerplus. 2016, 5, 419. DOI: 10.1186/s40064-016-2046-3.
- Boila, R.-J.; Campbell, L.-D.; Stothers, S.-C.; Crow, G.-H.; Lbrahim, E.-A. Variation in the Mineral Content of Cereal Grains Grown at Selected Locations Throughout Manitoba. Canadian Journal of Animal Science. 1993, 73, 421–429. DOI: 10.4141/cjas93-044.
- Andersson, -A.-A.-M.; Elfverson, C.; Andersson, R.; Regner, S.; Aman, P. Chemical and Physical Characteristics of Different Barley Samples. Journal of the Science of Food and Agriculture. 1999, 79, 979–986. DOI: 10.1002/(SICI)1097-0010(19990515)79:7<979::AID-JSFA313>3.0.CO;2-L.
- Knudsen, B.-K.-E.;. Carbohydrate and Lignin Contents of Plant Materials Used in Animal Feeding. Animal Feed Science Technology. 1997, 67, 319–338. DOI: 10.1016/S0377-8401(97)00009-6.
- McCleary, B.-V.; Glennie-Holmes, M. Enzymic Quantification of (1–3), (1–4)-β-D-glucan in Barley and Malt. Journal of the Institute of Brewing. 1985, 91, 285–295. DOI: 10.1002/j.2050-0416.1985.tb04345.x.
- Beloshapka, A.-N.; Buff, P.-R.; Fahey, G.-C.; Swanson, K.-S. Compositional Analysis of Whole Grains, Processed Grains, Grain Co-Products, and Other Carbohydrate Sources with Applicability to Pet Animal Nutrition. Foods. 2016, 5(2), 23. DOI: 10.3390/foods5020023.