 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, different concentrations of aqueous extracts (5%, 10%, 15%, and 20% w/v) from roselle (Hibiscus sabdariffa) calyces were incorporated in formulations of soft-serve ice cream as functional additives. The roselle-flavored ice cream was evaluated for physico-chemical properties (color, viscosity, pH, total solids, total titratable acidity, meltdown rate, overrun) and descriptive sensory properties. High concentration of roselle extracts had an effect on the colour of ice cream as shown by an increase (0–1.50) in redness (+a*-values) whilst the lightness (L*-values) of the ice cream decreased (80.30–64.20). As the concentration of roselle extracts increased in the formulation, the meltdown rate of the ice cream also increased from 0.74 to 2.33 g/min whilst a gradual decrease in viscosity (238.3 to 242.3 cP) was observed compared to the control (246 cP). The overrun progressively increased (14.01–33.49%) as the concentration of roselle extracts increased. The pH and total soluble solids significantly decreased (6.8–6.3 and 35.5–25.6°Brix, respectively) at higher concentrations of roselle extracts whilst total titratable acidity increased (0.16–1.5 g of malic acid per 100 g). The results of this study suggest that the ice cream sample with 5% roselle extract has a potential as a functional ice cream since it exhibited high overrun, low meltdown, desirable viscosity, high total solids and light color intensity. This ice cream was described by sensory panel as cream white, sweet and milky with vanilla flavor. The ice cream could be acceptable as consumers are familiar with its descriptive sensory properties.
Introduction
Health consciousness continues to increase among the snacking cluster of consumers. Snacking has become an important component of consumers’ daily eating and drinking habits and the health-conscious consumers demand snacks that deliver health benefits while giving the consumer a feeling of satisfaction.[Citation1] This demand has also appeared in the ice cream sector. Ice cream is a delicious frozen dairy product consisting of two phases: a continuous phase comprising sugars, proteins, salts, polysaccharides and water, and a disperse phase which consists of ice crystals, air bubbles and partially coalesced fat globules.[Citation2] In the past, it was considered an indulgent category of food items, but ice cream has now developed to a stage where it is largely and gladly perceived as a snacking alternative by consumers.[Citation3] According to Aboulfazli et al.[Citation4] ice cream possess nutritional properties from its major ingredient (milk) even though it does not offer any health benefits. As a result, research interest in the use of ice cream as a vehicle for incorporating health-promoting ingredients, as well as in the reduction of sugar and fat content in ice cream is slowly emerging.
Goraya and Bajwa[Citation3] enhanced the functional properties and nutritional value of ice cream by incorporating a processed Indian gooseberry in ice cream; Fiol et al.[Citation5] replaced dairy products in an ice cream formulation with lactose and sodium casein. In another study, Tekin et al.[Citation6] reduced the fat content of ice cream by 2.8% without compromising its quality using a double emulsion method, and Aboulfazli et al.[Citation4] successfully replaced cow’s milk in a fermented ice cream formulation with vegetable milk. There is a growing interest in using certain plant extracts as additives in ice cream.
Machewad et al.[Citation7] used extracts from Safflower (Carthamus tincttiorius L.) petals as natural colorant in ice cream. In our study, Hibiscus sabdariffa was identified as a potential plant source of color and flavor pigments which can replace synthetic additives in ice cream. H. sabdariffa, popularly known as roselle,[Citation8] is a plant of increasing interest for its applications in health and medicine, beverage and cosmetic products.[Citation9] Roselle is native to Asia and tropical Africa[Citation10,Citation11] and comprises 300 species which are grown in tropical and subtropical regions throughout the world.[Citation12]
In South Africa, roselle is widely grown in Limpopo, Mpumalanga, the Eastern Cape, and KwaZulu-Natal provinces. About 59 species are found in South Africa, but roselle is not widely known as food, especially in native African communities. In Sudan, the leaves of roselle are consumed as either a fresh or dried vegetable that is cooked with onion and groundnuts[Citation13] Roselle leaves are consumed as a vegetable in Malaysia and Nigeria,[Citation13,Citation14] where they are also used for making jam because of their high pectin content.[Citation15] Its brilliant red petals are usually processed into unique and refreshing alcoholic and non-alcoholic drinks.[Citation16] The extracts from roselle calyces are consumed across the world as either a cold beverage or a hot drink.[Citation8,Citation17]
Besides its use as an ingredient in various food applications, roselle has also been used in the treatment of a wide range of health-related problems.[Citation13] According to literature, roselle is effectively used to combat hypertension, inflammation and liver disorders, and its petals have anti-tumor, antioxidant and antihyperlipemic,[Citation16] cardio-protective and antihypertensive properties. In addition, roselle is used effectively to reduce low-density lipoprotein oxidation and hyperlipidemia.[Citation10] Its calyces are traditionally used for their diuretic, choleretic, febrifugal and hypotensive effects, decreasing the viscosity of the blood and stimulating intestinal peristalsis.[Citation13]
The pharmacological properties of roselle are linked to its phytochemical profile and biologically active properties. Among its phytochemicals, polyphenols with anthocyanins are the main constituents.[Citation13,Citation18] The major anthocyanins in roselle are hibiscin and gossypicyanin. These compounds are responsible for the brilliant red color of roselle calyces and therefore make roselle calyces an alternative to synthetic food colorants.[Citation8] Anthocayanins from roselle are safer than most synthetic dyes, which may have negative effects on human health.[Citation19] In this study, crude extracts of roselle calyces were used to flavor and color soft-serve ice cream; their effects on the physicochemical and descriptive sensory characteristics of ice cream were determined.
Materials and methods
Sourcing of raw materials
Fresh roselle calyces were obtained through a community project (Phiphidi Village, Sibasa, Limpopo, South Africa). Milk (full cream milk, Parmalat, SA); sucrose (Selati white sugar, RCL Foods, SA); Orley Whip cream (Kerry Foods, SA); stabiliser (gelatin, Sheridans, SA), emulsifiers (egg yolk, Alpha Farm eggs) and MSNF (milk solids non-fat), in the form of instant skimmed milk powder were purchased from Shoprite and Pick n Pay in Thohoyandou, South Africa. Commercial starch (Stycros) was generously supplied by Tongaat Hulett, South Africa.
Preparation of roselle extracts
The fresh roselle calyces were thoroughly washed and dried at 50°C for 36 hr in a hot-air oven dryer (Ecotherm, Labotec, SA). The dried calyces were milled into powder using a laboratory miller (Polymix, PX-MFC, Switzerland) fitted with a 0.8 mm aperture screen. The extracts were prepared as follows: 5% (w/v, 15 g roselle calyces powder in 300 mL deionized water), 10% (w/v, 30 g roselle calyces powder in 300 mL deionized water), 15% (w/v, 45 g roselle calyces powder in 300 mL deionized water) and 20% (w/v, 60 g roselle calyces powder in 300 mL deionized water). The mixture was allowed to soak in a water bath for 1 h at 75°C. The mixture was filtered using Whatman filter paper 1 (11 µM). The residues were re-extracted with 300 mL of the same solvent as described above, and the extracts combined.[Citation20]
Preparation of ice cream
Four ice cream formulations (5%, 10%, 15%, and 20% w/v, respectively, of roselle extracts) and a control (0% roselle extract) were prepared following a method described by Fiol et al.,[Citation5] Neswati et al.[Citation21] and Sonwane and Hembade.[Citation22] To obtain 1 kg of ice cream, the ingredients were weighed separately and incorporated on w/w basis as follows: milk (50%) and cream (23.5%) were mixed in a saucepan and heated to 40°C. Roselle extract (50 mL), sucrose (15%) with stabilizer (0.1%) were added at 40°C while the mix was stirred manually. The rest of the ingredients (1.3% starch, 5% MSNF, and 0.08% emulsifier) were added and the mix was subsequently heated to 70°C for 30 min while stirring continuously. The mix was further homogenized for 5 min, then cooled to 4–5°C and transferred to the laboratory soft-serve single-flavor ice cream machine model 152 (Taylor United Technologies, Rockton, Illinois) to freeze at −4°C. A control sample (0% roselle extract) was also made following the same procedure as described above.
Quality evaluation of ice cream
Color analysis
The color of ice cream samples was measured using a laboratory Lovibond colorimeter (RM200, USA). The results were expressed using CIELAB parameters (L*, a*, b*); L* (100 = white; 0 = black) is an indication of lightness; a* measures chromaticity, with positive values indicating redness and negative values indicating greenness; and b* measures chromaticity, with positive values indicating yellowness and negative values indicating blueness. The color of the samples was compared using the whiteness index (WI), the hue angle (hab), and Equationequations 1(1)
(1) and Equation2
(2)
(2) respectively, as described by Palavencino et al.[Citation23] The WI indicates how near a sample is to an ideal white (WI = 100), while hab indicates the chromaticity (0°, red; 90°, yellow; 180°, green; 270°, blue).
Overrun
Overrun was measured as described by Daw and Hartel.[Citation24] The measurements were based on the weight of a certain volume of ice cream mix and ice cream. A container was filled with either ice cream mix or ice cream and the weight was recorded. Overrun measurements were taken in triplicate and the calculations were done using the following equation:
Meltdown
Meltdown of the ice cream samples was determined as described by Pon et al.[Citation25] The sample (80.0 g) was placed on a wire mesh that drained into a graduated glass cylinder on top of a weighing balance at controlled temperature (25 ± 1°C). The dripped volume was measured at 10-min intervals for a total of 45 min. The first drop time was measured as the volume drip per minute. The data recorded were used to determine the melting rate (g/min).
Viscosity
Viscosity of the aged ice cream was determined using a Brookfield digital viscometer (RV DV-E, Brookfield Engineering Labs Middleboro, MA, USA). The viscosity of the molten ice cream was taken at 10°C and the viscometer was operated at 30 rpm using spindle number 4. Each result was then recorded in triplicates in cP after 30 s rotation.[Citation26]
pH of ice cream
The pH of the samples was measured directly using a calibrated pH meter (Crison Instruments SA, Barcelona, Spain). About 20 mL of the ice cream sample was poured in a 50 mL beaker and the electrode was inserted while the sample was gently agitated. The final steady pH reading was then recorded from the screen of the pH meter.[Citation27]
Brix of ice cream
The ice cream samples were allowed to melt at room temperature for 1 hr. Then, a drop of ice cream was placed on the prism of a pre-calibrated Atago Smart-1 refractometer (Atago, Japan) and about three readings per sample were recorded.
Total solids in ice cream
To determine the total solids in the ice cream, about 3 g of the melted ice cream sample was weighed in a previously dried and weighed pan. The moisture was evaporated on a steam bath and then dried overnight in a forced air-drying oven at 105°C. On cooling, the pans and their dry contents were weighed and the percentage of total solids was calculated as follows:
Titratable acidity (TA)
TA was determined according to Gbadegesin et al.[Citation28] One g of ice cream sample was weighed and placed in a 100 mL glass beaker. About 40 mL of distilled water was added, heated to reach 70°C and allowed to stand for 1 h. The supernatant was filtered through Whatman filter paper no. 4. The roselle residues were rinsed with two portions of 20 mL of hot distilled water. The filtrate and the washings were transferred to a 100 mL flask, cooled down to room temperature, brought to volume and thoroughly mixed. An aliquot of 25 mL of the extract was titrated with 0.1 N NaOH until it reached pH 8.3. TA was calculated using the following equation and the results were reported as percentage malic acid:
Sensory properties of ice creams
The descriptive sensory profiling of five ice cream samples was performed based on the generic descriptive method as described by Eistein.[Citation29] Twenty judges were recruited from the Department of Food Science and Technology at the University of Venda and screened for acuity. The screening tests included difference methods (triangle test and duo-trio test), odors identification methods and an exercise to describe differences among commercial ice cream flavor (vanilla and chocolate). About eight trained panelists selected from the initial 20 prospective panelists were used. The panel comprised four females and four males aged 22 to 26 years. To prepare for evaluation, the panelists were trained for four days in sessions of 2 h each. They generated 13 descriptors or lexicons with definitions and reference standards to anchor their scores (). During each session, five ice cream samples randomly labeled with three-digits were blind-tasted. These samples were presented in random order to each panelist member who was seated in an individual booth under a white light and tap water was provided as a palate cleanser. Each of the five ice cream samples was rated for appearance, taste, texture, flavor, and mouthfeel, using 13 descriptors on a 10-point scale (0 = not intense; 10 = more intense). The panellists assessed each sample and responses were recorded on evaluation forms. The descriptive sensory analysis of ice cream samples was carried out in duplicates.
Table 1. Descriptors used for the sensory profiling of roselle-flavored ice creams.*
Ethical considerations
Ethical approval to conduct this study was obtained from the University of Venda Research Ethics Committee. The selected judges signed consent forms to indicate their willingness to participate in the study and to confirm that they understood the purpose of the study. Participation by the judges was voluntary, and they were free to withdraw from the study at any stage. The agreement about payment for participation was well explained, and the panel members were told that a panelist could be withdrawn from the study if his or her performance was not satisfactory. Panelists who could no longer participate, would be paid pro rata for their participation. The consent form also clearly stated that the personal information of panelists would be kept confidential.
Statistical analysis
This analysis was done using Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM, New York, USA). A one-way analysis of variance (ANOVA) was performed and the significant differences were defined at p < 0.05. The results obtained were expressed as the mean values ± the standard deviation of three replicates, and the mean comparison was done using Duncan’s multiple range method. The descriptive sensory mean scores were subjected to Principal Component Analysis using XLSTAT software version 2017 (Addinsoft SARL, Paris, France) to evaluate and identify variations between ice cream samples based on their sensory attribute loadings.
Results and discussion
The color of ice cream shifted from white towards the red/yellow chromaticity as shown by decreasing lightness (L* values) from 80.30 to 64.20 (). This change in color corresponded with increasing concentrations of roselle extracts. The control sample exhibited the highest lightness (80.30) compared to the roselle-flavored ice creams (L* = 64.20–72.0). Regarding the a* values, the control sample had an intense greenness, as indicated by a negative a* mean value (−1.43), while the roselle-flavored samples exhibited an increase in redness (+ve a* values). The intense redness of roselle-flavored samples corresponded with the increase in concentrations of roselle extracts. In terms of b* values, the control showed more yellowness (7.13) than roselle-flavored samples [5% Extract (IC5); 10% extract (IC10) and 20% extract (IC20)], which exhibited a shift towards blueness (3.10–0.43), while the ice cream with 15% extract (IC15) was found to be in the blueness chromaticity.
Table 2. Color parameters of roselle-flavored ice cream
The combination of the CIELAB parameters (L*, a*, b*) can be clearly explained by the whiteness index (WI) and the hue angle of the ice cream samples. As expected, the WI of the roselle-flavored ice cream samples was lower than that of the control (79.0). They ranged from 64.2 to 71.8, with IC5 having the highest mean value and IC15 the lowest mean value. This shows that as the concentration of roselle extracts increased in the formulation, the color of ice cream samples shifted away from white towards the red and yellow chromaticity, as shown by the hue angle mean values. IC5 and IC20 were in the red shifting towards the yellow zone since their mean values were close to zero, while IC10 and IC15 were almost in the yellow zone (82.23 and 62.27, respectively). These color changes in roselle-flavored ice cream samples were due to the presence of color pigments in roselle extracts.
The results of selected physical characteristics of roselle-flavored ice cream are presented in . The overrun (incorporation of air) and melting rate increased as the concentration of roselle extracts increased, while viscosity decreased. The ice cream samples with roselle extracts exhibited significantly (p ≤ 0.05) higher overrun values (17.6–33.5%) than the control sample (14.0%), with the ice cream containing 5% roselle extract (IC5) having the lowest overrun value, while the ice cream with 20% roselle extract (IC20) had the highest overrun value.
Table 3. Some physical characteristics of roselle-flavored ice cream
Overrun is a measurement that relates to an increase in the volume of an ice cream product during processing[Citation30] and it defines the structure of the final product, as the presence of air gives ice cream an enjoyable light texture.[Citation31] Air cell structure is one of the main factors that influence melting rate, shape retention during the meltdown and the rheological properties in the molten state, which are correlated to creaminess.[Citation32] In our study, the overrun of ice cream was within the same range as the overrun of premium ice creams. According to literature, the overrun of super premium ice cream can be as low as 20%.[Citation33] The overrun of our ice cream ranged between 14% and 33.5%. Therefore, the overrun values of ice cream samples IC10, IC15, and IC20 fall within the range of desired overrun. As the volume of the ice cream increased, the ice cream became more resistant to melting while the viscosity of the ice cream decreased.
The onset melting rate of ice cream with 5% roselle extract (IC5) was at 1.3 g/min, which was significantly lower (p ≤ 0.05) than the 1.6 g/min for ice cream with 10% roselle extract (IC10), 2.1 g/min for ice cream with 15% roselle extract (IC15), and 2.3 g/min for ice cream with 20% roselle extract (IC20), which was significantly faster than all the samples including the control (0.7 g/min). The melting rate of ice cream is known to be correlated with overrun. According to literature, ice cream with low overrun values tend to melt quicker.[Citation31,Citation34] However, this was not the case in our study. The control sample which had the lowest overrun exhibited the highest resistance to melting (only 0.7 g melted in 1 min), while the sample with the highest overrun (IC20) melted a larger portion in 1 min (2.3 g). The high melting rate of ice cream samples with high overrun was probably due to the poor stability of air cells, air cell size distribution and the network of fat globules formed during freezing.[Citation35] Air, fat globules and ice are the main microstructural components of ice cream and have a significant effect on the meltdown or drip-through behavior of ice cream.[Citation36]
The viscosity of the control sample was significantly (p ≤ 0.05) higher (246.0 cP), while the viscosity of roselle-flavored ice cream samples decreased as the concentration of roselle extracts increased. The viscosity of the roselle-flavored ice cream samples ranged from 238.3 cP to 242.3 cP, with IC5 having the highest value while IC20 exhibited the lowest viscosity. The decrease in viscosity of roselle-flavored ice cream was probably due to the destabilization (partial coalescence) of the lipid layer around air cells by water from roselle extracts. Air in ice cream provides a light texture and influences the physical properties such as melting rate and viscosity.[Citation37] As the overrun of the roselle-flavored ice cream increased, the ice cream became less viscous and the melting rate increased in comparison to the control sample.
The pH of the ice cream decreased as the concentration of roselle extracts increased while the acidity of the ice cream increased (). The control, IC5, and IC10 had significantly (p ≤ 0.05) higher pH values (6.74 and 6.68, respectively) than the IC15 and IC20 samples (6.48 and 6.33, respectively). This decrease in pH of ice cream samples with roselle extract may be attributed to the presence of organic acids in roselle calyces.[Citation38,Citation39]
Table 4. Selected chemical properties of roselle-flavored ice cream
Roselle calyx is high in hibiscus acid (13–24%), citric acid (12–20%), malic acid (2–9%), tartaric acid (8%) and ascorbic acid (0.02–0.05%).[Citation10,Citation13]
The high concentration of these compounds resulted in an increase in the acidity of ice cream samples. The acidity (0.15% malic acid) of IC5 and IC10 samples was significantly lower than that of IC15 (0.19% malic acid) and IC20 (0.22% malic acid) samples, but similar to that of the control sample (0.16% malic acid). The titratable acidity values for IC15 and IC20 were similar to those reported by Choo et al.[Citation40] According to Choo et al.[Citation40] ice cream formulations with 11.7% MSNF has a normal acidity ranging from 0.19% to 0.22%. Interestingly, our formulations only contained 5% of MSNF. The acidity of the roselle-flavored ice cream may have been influenced by the presence of organic acids in roselle extracts.
According to the descriptive sensory panel, roselle-flavored ice cream samples became grayish in color as the concentration of roselle extracts increased in the formulation (). However, IC5 samples also reflected a cream white color, although it had a lower intensity compared to that of the control sample. The perceived gray color may be attributed to changes owing to degradation of anthocyanins. Anthocyanins appear as red pigments in acidic conditions, but undergo color changes as the conditions in the food matrix change.[Citation41] They are more reddish at lower pH, but their color fades as the pH increases to above 4.5.[Citation42] The pH of ice cream samples were substantially higher (6.3–6.7). Other factors that contribute to the instability of anthocyanins include temperature and complexation of anthocyanins with other compounds in the food matrix.[Citation43]
Table 5. Sensory characteristics of roselle-flavored ice cream as described by trained panel
An intense freezing effect (icy mouthfeel), stiffness (hard mouthfeel) and coarse mouthfeel were perceived in all roselle-flavored ice cream samples. A milky flavor comparable to the control sample was perceived in IC5, IC10, and IC15. In addition to a milky flavor, a vanilla flavor was also detected in IC5 at a higher intensity than in other roselle-flavored ice cream samples. These flavors are desirable characteristics of ice cream. The milky flavor was mainly imparted by milk since it is the main ingredient of ice cream, while the perceived vanilla flavor was probably due to vanillic acid.[Citation44] Vanillic acid is widely used in the food industry as a flavoring, preservative, and food additive.[Citation45] Vanilla flavor is one of the most popular choices of ice cream by consumers. The control, IC5, and IC10 were perceived to be sweet with less resistance to scooping and melted unusually quicker (watery) than the control sample.
The watery characteristic is unpopular in ice cream. It may be assumed that this watery characteristic was caused by the liquid phase of water in which other components such as sugar reside.[Citation46] The presence of a significant number of detectable ice crystals was probably caused by the formation of large and unevenly distributed ice crystals, which were probably due to recrystallization.[Citation47] According to Shrivastav and Goswami,[Citation48] the presence of detectable ice crystals is a defect and undesirable as they negatively affect the smooth texture (creaminess) of ice cream. Apparently, the addition of low concentrations of stabilizers (0.5–2%) to ice cream formulations has no significant effect on growth of ice crystals.[Citation49] In our study, the concentration of stabilizers was low (0.1%).
Overall, these results also show that the control and IC5 sample had intense smoothness (creamy) compared to other ice cream samples. It appears that IC5 is almost comparable to the control ice cream in terms of desirable attributes such as flavor (milky, vanilla), sweetness, and texture (creamy). The principal component analysis separated the ice cream samples based on the intensity of their sensory attributes. Principal component 1 (PC1) and principal component 2 (PC2) explained 83.52% and 12.79% of the total variation, respectively (). Thus, the two-dimensional graph in could explain 96.31% of the variability in the experimental data.
Figure 1. Parameter loadings and factor scores for principal component 1 (PC1) and principal component 2 (PC 2).Control = 0% roselle extract; IC5 = 5% roselle extract; IC 10 = 15% roselle extract;IC20 = 20% roselle extract1
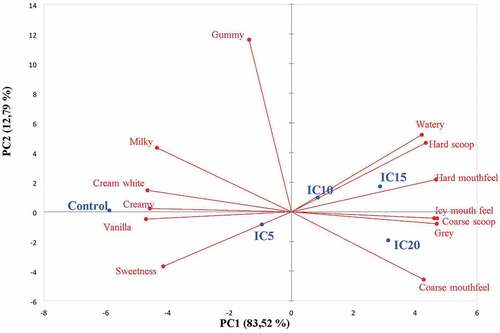
Ice cream samples IC10, IC15, and IC20 were loaded on PC1 and strongly correlated with milky, grey, hard scoop, coarse scoop, icy mouthfeel, coarse mouthfeel, and watery. The intense icy mouthfeel and detectable ice crystals (coarse in scoop) significantly (p ≤ 0.05) reduced the perceived vanilla flavor and smooth texture (creamy) (). The intensity of a milk flavor in the control ice cream significantly (p ≤ 0.05) reduced its coarse mouthfeel, while the opposite was observed in the case of IC20. IC5 was loaded on PC3 and strongly correlated with intense sweetness.
Conclusion
The addition of roselle extracts in ice cream formulation could potentially impart health benefits while flavoring and coloring the ice cream. However, increasing the concentration of roselle extracts above 5% resulted in less viscous ice cream with a high melting rate, an unpopular color and some undesirable sensory attributes. It appears that IC5 is almost comparable to the control ice cream in terms of lower melting rate, overrun, pH, total solids and desirable sensory attributes such as flavor (milky, vanilla), sweetness and texture (creamy). Therefore, IC5 has potential as a functional ice cream and may be accepted by consumers, as they are familiar with these descriptive sensory properties.
Acknowledgments
The authors thank the sensory panelists who participated in this study. Their hard work and dedication are highly appreciated. The technical assistance by Ms. Tabea Mokhele of the University of Venda is also acknowledged. The authors confirm that there are no known conflicts of interest associated with this publication and this research did not receive any specific grant from funding agencies in the public, commercial, or non-profit organizations that could have influenced its outcome.
References
- Beswa, D.; Dlamini, R. D.; Siwela, M.; Amonsou, E. O.; Kolanisi, U. The Effect of Amaranth Addition on the Nutritional Composition and Consumer Acceptability of Extruded Provitamin A-Biofortified Maize Snacks. Food Sci. Technol. Campinas. 2016, 36(1), 30–39. DOI: 10.1590/1678-457X.6813.
- Tsevdou, M.; Gogou, E.; Dermesonluoglu, E.; Taoukis, P. Modelling the Effect of Storage Temperature on the Viscoelastic Properties and Quality of Ice Cream. J. Food Eng. 2015, 148, 35–42. DOI: 10.1016/j.jfoodeng.2014.07.002.
- Goraya, R. K.; Bajwa, U. Enhancing the Functional Properties and Nutritional Quality of Ice Cream with Processed Amla (Indian Gooseberry). J. Food Sci. Technol. 2015, 52(12), 7861–7871. DOI: 10.1007/s13197-015-1877-1.
- Aboulfazli, F.; Shori, A. B.; Baba, A. S. Effects of the Replacement of Cow Milk with Vegetable Milk on Probiotics and Nutritional Profile of Fermented Ice Cream. LWT Food Sci. Technol. 2016, 70, 261–270. DOI: 10.1016/j.lwt.2016.02.056.
- Fiol, C.; Prado, D.; Romero, C.; Laburu, N.; Mora, M.; Alava, J. I. Introduction of a New Family of Ice Creams. Int. J. Gastronomy Food Sci. 2017, 7, 5–10. DOI: 10.1016/j.ijgfs.2016.12.001.
- Tekin, E.; Sahin, S.; Sumnu, G. Physicochemical, Rheological, and Sensory Properties of Low-Fat Ice Cream Designed by Double Emulsions. Eur. J. Lipid Sci. Technol.. 2017, 119(9), 1600505. DOI: 10.1002/ejlt.201600505.
- Machewad, G. M.; Ghatge, P.; Chappalwar, V.; Jadhav, B.; Chappalwar, A. Studies on Extraction of Safflower Pigments and Its Utilization in Ice Cream. J. Food Process. Technol. 2012, 3,(172). DOI: 10.4172/2157-7110.1000172.
- Amor, B. B.; Allaf, K. Impact of Texturing Using Instant Pressure Drop Treatment Prior to Solvent Extraction of Anthocyanins from Malaysian Roselle (Hibiscus sabdariffa). Food Chem. 2009, 115, 820–825. DOI: 10.1016/j.foodchem.2008.12.094.
- Villani, T.; Juliani, H. R.; Simon, J. E.; Wu, Q. Hibiscus sabdariffa: Phytochemistry, Quality Control, and Health Properties. ACS Symp. Ser. 2013, 1127. DOI: 10.1021/bk-2013-1127.ch014.
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P. A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J. F.; Segura-Carretero, A. Characterization of Phenolic Compounds, Anthocyanidin, Antioxidant and Antimicrobial Activity of 25 Varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crops Prod. 2015, 69, 385–394. DOI: 10.1016/j.indcrop.2015.02.053.
- Hamid, A. Z.; Lin, W. H. L.; Abdalla, B. J.; Yuen, O. B.; Latif, E. S.; Mohamed, J.; Rajab, N. F.; Wah, C. P.; Harto, M. K. A. W.; Budin, S. B. The Role of Hibiscus Sabdariffa L. (Roselle) in Maintenance of Ex Vivo Murine Bone Marrow-Derived Hematopoietic Stem Cells. Sci. World J. 2014. DOI: 10.1155/2014/258192.
- Singh, P.; Khan, M.; Hailemariam, H. Nutritional and Health Importance of Hibiscus sabdariffa: A Review and Indication for Research Needs. J. Nutr. Health Food Eng. 2017, 6(5), 00212. DOI: 10.15406/jnhfe.2017.06.00212.
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, H.; Heinrich, M. Hibiscus sabdariffa L. – A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. DOI: 10.1016/j.foodchem.2014.05.002.
- Apeyuan, K. D.; Nwankiti, O. A.; Ekefan, E. J. Effect of Different Sowing Dates on Disease Initiation and Development of Roselle (Hibiscus sabdariffa L.) Leaf Spot Disease Caused by Coniella musaiensis Var. Hibisci in Makurdi, Central Nigeria. J. Geosci. Environ. Prot. 2017, 5, 94–101. DOI: 10.4236/gep.2017.511007.
- Herranz-López, M.; Olivares-Vicente, M.; Encinar, J. A.; Barrajón-Catalán, E.; Segura-Carretero, A.; Joven, J.; Micol, V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients. 2017, 9(8), 907. DOI: 10.3390/nu9080907.
- Misnawi, J.; Oktafiandhika, D. P.; Noor, A. F. Effect of the Roselle (Hibiscus sabdariffa) Extract on Oxidation Stability of Bulk Frying Oil during Open and Deep-Frying: A Response Surface Approach. Int. Food Res. J. 2014, 21(5), 1843–1850.
- Hopkins, A. L.; Lamm, M. G.; Funk, J.; Ritenbaugh, C. Hibiscus sabdariffa L. In the Treatment of Hypertension and Hyperlipidemia: A Comprehensive Review of Animal and Human Studies. Fitoterapia. 2013, 85, 84–94. DOI: 10.1016/j.fitote.2013.01.003.
- Sindi, H. A.; Marshall, L. J.; Morgan, M. R. A. Comparative Chemical and Biochemical Analysis of Extracts of Hibiscus Sabdariffa. Food Chem. 2014, 164, 23–29. DOI: 10.1016/j.foodchem.2014.04.097.
- Abdel-Moemin, A. R.;. Effect of Roselle Calyces Extract on the Chemical and Sensory Properties of Functional Cupcakes. Food Sci. Hum. Wellness. 2016, 5, 230–237. DOI: 10.1016/j.fshw.2016.07.003.
- Yang, L.; Gou, Y.; Zhao, T.; Zhao, J.; Fang, L.; Zhang, B.; Xiangyang, W. Antioxidant Capacity of Extracts from Calyx Fruits of Roselle (Hisbiscuss Sabdariffa L). Afr. J. Biotechnol. 2012, 11(17), 4063–4068. DOI: 10.5897/AJB11.2227.
- Neswati, A. F.;. Ropanti. The Addition of Broccoli (Brassica oleracea var italica) to Increase the Functional Properties of Ice Cream. Pak. J. Nutr. 2014, 13(4), 196–203. DOI: 10.3923/pjn.2014.196.203.
- Sonwane, R. S.; Hembade, A. S. Sensory Quality of Dietetic Soft Serve Ice-Cream Prepared by Using Different Proportions of Maltodextrin. Int. J. Curr. Res. Acad. Rev. 2014, 2(6), 51–55.
- Palavencino, P. M.; Penci, M. C.; Calderon-Dominguez, G.; Ribotta, P. D. Chemical Composition and Physical Properties of Sorghum Flour Prepared from Different Sorghum Hybrids Grown in Argentina. Starch/Starke. 2016, 68, 1055–1064. DOI: 10.1002/star.201600111.
- Daw, E.; Hartel, R. W. Fat Destabilization and Melt-Down of Ice Creams with Increased Protein Content. Int. Dairy J. 2015, 33–41. doi:10.1016/j.idairyj.2014.12.001.
- Pon, S. Y.; Lee, W. J.; Chong, G. H. Textural and Rheological Properties of Stevia Ice Cream. Int. Food Res. J. 2015, 22(4), 1544–1549.
- Akbulut, M.; Çoklar, H. Physicochemical and Rheological Properties of Sesame Pastes (Tahin) Processed from Hulled and Unhulled Roasted Sesame Seeds and Their Blends at Various Levels. J. Food Process Eng. 2008, 31, 488–502. DOI: 10.1111/j.1745-4530.2007.00162.x.
- Umelo, M. C.; Uzoukwu, A. E.; Odimegwu, E. N.; Agunwah, I. M.; Njoku, N. E.; Alagbaoso, S. Proximate, Physicochemical and Sensory Evaluation of Ice Cream from Blends of Cow Milk and Tigernut (Cyperus esculentus) Milk. Int. J. Sci. Res. Innovative Technol. 2014, 1(4), 63–74.
- Gbadegesin, A. R.; Gbadamosi, S. O.; Odunlade, T. V. Physicochemical and Sensory Properties of Pineapple Flavored Roselle Powders. Cogent Food Agric. 2017, 3(1). DOI: 10.1080/23311932.2017.1292833.
- Eistein, M. A.;. Descriptive Techniques and Their Hybridization. In Sensory Science Theory and Application in Foods; Lawless., H. T., Klein, B. P., Eds.; Mercel Decker: New York, 1991; pp 317–338.
- Soukoulis, C.; Tzia, C. Sensory Profiling and Hedonic Judgement of Probiotic Ice Cream as a Function. LWT Food Sci. Technol. 2010, 43(9), 1351–1358. DOI: 10.1016/j.lwt.2010.05.006.
- Cakmakcı, S.; Topdas, E. F.; Cakır, Y.; Kalın, P. Functionality of Kumquat (Fortunella margarita) in the Production of Fruity Ice Cream. J. Sci. Food Agric. 2016, 96, 1451–1458. DOI: 10.1002/jsfa.7241.
- Bahramparvar, M.; Tehrani, M. M. Application and Functions of Stabilizers in Ice Cream. Food Rev. Int. 2011, 27, 389–407. DOI: 10.1080/87559129.2011.563399.
- Marshall, R. T.; Goff, H. D.; Hartel, R. W. Ice Cream, 5th ed.; Springer Science+Business Media: New York, 2003.
- Moeenfard, M.; Mazaheri Tehrani, M. Effect of Some Stabilizers on the Physicochemical and Sensory Properties of Ice Cream Type Frozen Yogurt. Am. Eurasian J. Agric. Environ. Sci. 2008, 4, 584–589.
- Sofjan, R. P.; Hartel, R. W. Effects of Overrun on Structural and Physical Characteristics of Ice Cream. Int. Dairy J. 2004, 14, 255–262. DOI: 10.1016/j.idairyj.2003.08.005.
- Warren, M. M.; Hartel, R. W. Structural, Compositional, and Sensorial Properties of United States Commercial Ice Cream Products. J. Food Sci. 2014, 79(10), E2005–E2013. DOI: 10.1111/1750-3841.12592.
- Park, S. H.; Jo, Y.-J.; Chun, J.-Y.; Hong, G.-P.; Davaasteren, M.; Choi, M.-Y. Effect of Frozen Storage Temperature on the Quality of Premium Ice Cream. Korean J. Food Sci. Animal Resour. 2015, 35(6), 793–799. DOI: 10.5851/kosfa.2015.35.6.793.
- Su, N.; Li, J.; Yang, L.; Hou, G.; Ye, M. Hypoglycemic and Hypolipidemic Effects of Fermented Milks with Added Roselle (Hibiscus sabdariffa L.). Extract. J. Funct. Foods. 2018, 43, 234–241. DOI: 10.1016/j.jff.2018.02.017.
- Ali, B. H.; Al Wabel, N.; Blunden, G. Phytochemical, Pharmacological and Toxicological Aspects of Hibiscus Sabdariffa L: A Review. Phytotherapy Res. 2005, 19, 369–375. DOI: 10.1002/(ISSN)1099-1573.
- Choo, S. Y.; Leong, S. K.; Henna, L.; Physicochemical, F. S. Sensory Properties of Ice-Cream Formulated with Virgin Coconut Oil. Food Sci. Technol. Int. 2010, 16(6), 531–541. DOI: 10.1177/1082013210367546.
- Khoo, H. E.; Azlan, A.; Tang, S. T.; Lim, S. M. Anthocyanidins and Anthocyanins: Coloured Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61(1), 1361779. DOI: 10.1080/16546628.2017.1361779.
- Krahl, T.; Fuhrmann, H.; Dimassi, S. Ice Cream. In Handbook on Natural Pigments in Food and Beverages – Industrial Applications for Improving Food Colour, Carle, R., Schweiggert, R., Eds.; 2016; DOI: 10.1016/B978-0-08-100371-8.00009-9.
- Cortez, R.; Luna-Vital, D. A.; Margulis, D.; de Mejia, E. G. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16(1), 180–198. DOI: 10.1111/1541-4337.12244.
- Ain, Q.; Naveed, M.; Mumtaz, A. S.; Farman, M.; Ahmed, I.; Khalid, N. Phytochemical Analysis of Hibiscus caesius Using High Performance Liquid Chromatography Coupled with Mass Spectrometry. Pak. J. Pharm. Sci. 2015, 28(5), 1625–1629.
- Almeida, I. V.; Cavalcante, F. M. L.; Vicentini, V. E. P. Different Responses of Vanillic Acid, a Phenolic Compound, in HTC Cells: Cytotoxicity, Antiproliferative Activity, and Protection from DNA-induced Damage. Genet. Mol. Res. 2016, 15(4). DOI: 10.4238/gmr15049388.
- Smith, K. W.;. 2015. Specialty Oils and Fats in Ice Cream. In Specialty Oils and Fats in Food and Nutrition, Geoff, T., Ed., 271–284. Woodhead Publishing: doi:10.1016/B978-1-78242-376-8.00011-9.
- Ndoye, F. T.; Alvarez, G. Characterization of Ice Recrystallization in Ice Cream during Storage Using the Focused Beam Reflectance Measurement. J. Food Eng. 2015, 148, 24–34. DOI: 10.1016/j.jfoodeng.2014.09.014.
- Shrivastav, A.; Goswami, T. K. Low Temperature Extrusion of Ice Cream: A Review. J. Food Nutr. Popul. Health. 2017, 1(2), 11.
- Regand, A.; Goff, H. D. Structure and Ice Crystallization in Frozen Stabilized Ice Cream Model Systems. Food Hydrocolloids. 2003, 17, 95–102. DOI: 10.1016/S0268-005X(02)00042-5.
