ABSTRACT
Water chestnut peels have good antioxidant activity. The effects of simulated gastric fluid (SGF) and intestinal fluid (SIF) fluid digestion in vitro on the active substance and antioxidant activity of water chestnut peels that were pre-treated using different boiling times (P10 and P30) were investigated. The results showed that the SGF obviously increased both the total phenolic content and total flavonoid content of water chestnut peels. The SGF digestion significantly enhanced both the FRAP and ABTS antioxidant capacity of water chestnut peels only blanched with P10. However, the SIF digestion significantly increased the FRAP and ABTS antioxidant capacity of all water chestnut peels regardless of whether these were pre-treated or not. The HPLC results showed that the simulated digestion in vitro enhanced the flavonoids content of the peels. Water chestnut peels could be used as an inexpensive source of natural functional food ingredients.
Introduction
Water chestnut, which is also known as Eleocharis dulcis, belongs to the sedge family and grows in wet farmlands or pool districts. Furthermore, it is a subterranean bulbous of sedum. [Citation1] Water chestnut, which is native to China and India, is cultivated primarily in the southern Yangtze River provinces of China. [Citation2] It has a long history of cultivation in China and is widely planted. [Citation3] The Chinese water chestnut peels are by-products that constitute approximately 20% (w/w) of the whole fruit, which were often discarded, resulting in wasted resources and environmental pollution. [Citation4] Water chestnut peels are rich in brown pigments, which are an excellent water-soluble natural food coloring, and its main components are flavonoids (such as flavonoids, flavonols, flavonoids). [Citation5] It was reported that the extracts of Eleocharis dulcis peels showed strong bactericidal and antioxidant activities. [Citation5,Citation6]
Organic solvent extraction is usually carried out to determine the content of phenolics and flavonoids of fruit extracts as well as their antioxidant activity. [Citation7,Citation8] However, the digestion fluid is significantly different from organic solvents. The digestion of fruit phenolics in the gastrointestinal tract is quite complex as it is affected by digestive enzymes, pH, inorganic salts and other physiological factors. [Citation9–Citation11] It may interact with pepsin or trypsin to form complexes that can change their molecular structural features, functions and nutritional properties, which can increase or decrease their antioxidant activity. [Citation12] Compared with the method of traditional chemical extraction, the method of gastrointestinal digestion in vitro to evaluate the biological activity of fruit phenolics can better reflect the actual physiological digestion.
A previous study had confirmed that the water chestnut peel has good antioxidant activity. [Citation6] However, there has not been a report on the rules determining the release of phenolic substances in water chestnut peels after gastrointestinal digestion. Herein, the present study aimed to explore the release of phenolic substances from the water chestnut peels that were pre-treated using boiling water after being digested with simulated gastric or intestinal fluid in vitro as well as the changes in the antioxidant activity of FRAP and ABTS.
Materials and methods
Chemicals and reagents
Water chestnut was purchased from local agricultural product markets and washed with deionized water to remove the dirt prior to being peeled by hand. After this, the peels were collected and washed with deionized water again. The water chestnut peels were divided into three groups: the first group was dried with hot air drying (P0+ HAD) (DHG9023A, Shanghai Jinghong Laboratory Instrument Co., Ltd, China); the second group was dried with hot air after a blanching treatment at 95°C (P10+ HAD) for 10 min; and the third group was dried with hot air after a blanching treatment at 95°C (P30+ HAD) for 30 min. All samples were dried at 60°C for 24 h. After this, the dried samples were ground by a universal grinder (FW100, Tianjing Hengrui Science & Teaching Instrument Co., Ltd) and filtered over 80 mesh sieves, before being packed into a sealed bag and stored at –20°C in a refrigerator for further analysis.
Pepsin, trypsin, gallic acid, catechin, and Trolox (6-hydroxy-2,5,7,8-tetramethy lchroman-2-carboxylic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ferrisulfas (GR), Foline-phenol, ABTS (2,2ʹ-Azinobis-(3-ethylbenzthiazoline-6-sulfonate)) and TPTZ (2,4,6-tripyridyl-s-triazine) were obtained from Xiya Reagent Company (Chendu, China). All other reagents were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Simulated digestion in vitro
The simulated gastric fluid treatment and the simulated intestinal fluid digestion followed the method previously described by Fu et al. [Citation13], with slight modifications. For the simulated gastric fluid, pepsin (1.60 g) and sodium chloride (1.00 g) (BR, 3000 USP u/mg activity units, Yuanye biotechnology Co., Ltd, Shanghai, China) were placed into a beaker before 475 mL of double distilled water and 3.5 mL of concentrated hydrochloric acid were added to the beaker. Following this, the pH was adjusted to 1.2 using concentrated hydrochloric acid. After this, the fluid was transferred into a 500-mL volumetric flask and double distilled water was added to reach a volume of 500 mL. For the samples treated by the simulated gastric fluid, three water chestnut peel samples that underwent different pre-treatments all weighed 1.0 g and were placed into three centrifuge tubes. After this, the overnight prepared simulated gastric fluid was added to the three centrifuge tubes at a ratio of 1:15 (g/mL). The samples were subsequently put into a desktop thermostat oscillator (IS-RDD3, Shanghai, China) at 37°C with a speed of 120 rpm/min for 60 min. After that, the samples were centrifuged at 5000 rpm/min for 10 min, before the supernatant was collected into a 1.5-mL centrifuge tube and placed in a refrigerator at –20°C.
For the simulated intestinal fluid, 3.4 g of potassium dihydrogen phosphate was dissolved in 125 mL of deionized water in a beaker. After this, 95 mL of 0.2 mol/L NaOH and 200 mL of deionized water were added in before the mixtures were shaken evenly. A total of 5.00 g of trypsin (BR, 4000 USPu/mg activity units, Yuanye biotechnology Co., Ltd, Shanghai, China) was subsequently added in. The pH of the solution was adjusted to 7.5 ± 0.1 using NaOH or HCl. Finally, the fluid was transferred into a 500-mL volumetric flask and double steamed water was added to reach a volume of 500 mL. For the samples treated by simulated intestinal fluid, the intestinal fluid was added to 1.0 g of three water chestnut peel samples that underwent different pre-treatments in centrifuge tubes at a ratio of 1:15 (g/mL). After this, the sample was mixed thoroughly before being put into a desktop thermostat oscillator at 37°C with a speed of 120 rpm/min for 120 min. After that, the samples were centrifuged at 5000 rpm/min for 10 min, before the supernatant was collected into a 1.5-mL centrifuge tube and placed in a refrigerator at –20°C.
Deionized water extraction
Deionized water was added to 1.0 g of three water chestnut peel samples that underwent different pre-treatments in centrifuge tubes at a ratio of 1:15 (g/mL). After this, the samples were mixed thoroughly before being put into a desktop thermostat oscillator at 37°C at a speed of 120 rpm/min for 120 min. After that, the samples were centrifuged at 5000 rpm/min for 10 min, before the supernatant was collected into a 1.5-mL centrifuge tube and placed in a refrigerator at –20°C.
Organic solvent extraction
Methanol was added to 1.0 g of three water chestnut peel samples that underwent different pre-treatments in centrifuge tubes at a ratio of 1:15 (g/mL). After this, the samples were mixed thoroughly and extracted in an ultrasonic cleaner (KQ100DE, Xinzhi Bioscience Co., Ltd) at 30°C under an ultrasonic power of 600 W for 15 min, before the supernatant was collected. After that, the samples were treated as described above for one more time, before the supernatants were merged with the above, split into a 1.5-mL centrifuge tube and placed in a refrigerator at –20°C.
Total phenolic content
The total phenolic content (TPC) of water chestnut peel samples was determined by the Folin–Ciocalteu (FC) assay following the procedure described by Su et al. [Citation14] with slight modifications. A total of 125 μL of the diluted samples or standards was pipetted into a centrifuge tube, before 0.5 mL of deionized water and 125 μL of Folin–Ciocalteu reagent were added in. The mixtures were thoroughly shaken and left to stand for 6 min, avoiding light. Following this, 1.25 mL of the sodium carbonate solution (m:v = 7%) and 1.00 mL of deionized water were added in sequence, before the samples were shaken and placed in the dark for 90 min. The absorbance was measured at 760 nm using a spectrophotometer (TU-1900, Beijing General Analytical Instruments Co., Ltd.). The total phenolic content was based on the amount of gallic acid and expressed as the gallic acid equivalent (GAE) per 100 g of water chestnut peel.
Total flavonoid content
The total flavonoid content (TFC) in water chestnut peel samples was determined by aluminum chloride–sodium nitrite colorimetry according to the procedures described by Bouayed et al. [Citation15] with slight modifications. A total of 300 μL of diluted samples or standards were pipetted into a centrifuge tube before 1.5 mL of deionized water and 90 μL of 5% sodium nitrite solution were added in. After that, the mixtures were thoroughly shaken and left to stand for 6 min. Following this, 180 μL of the sodium carbonate solution (m:v = 10%) was added in sequence, before the samples were shaken and left to stand for 5 min. After that, 0.6 mL of 1 mol/L sodium hydroxide solution and deionized water were added to make up a volume of 3.00 mL. Finally, the absorbance value of the samples was measured with a UV-visible spectrophotometer at 510 nm. The total flavonoid content was based on the amount of catechin and expressed as catechin equivalent (CE) per 100 g of water chestnut peel.
Antioxidant capacity
The ferric reducing antioxidant power (FRAP) assay was performed according to the method described by Thaipong et al. [Citation16] with slight modifications. For the preparation of the FRAP working fluid, 10 mmol/L TPTZ, 20 mmol/L hexahydrate ferric chloride and 300 mmol/L sodium acetate buffer solution were mixed thoroughly at a ratio of 1:1:10 and placed in a 37°C water bath. After that, 0.3 mL of the samples or standards was added to 2.7 mL of the FRAP working liquid and thoroughly shaken, before the sample was stored in a dark environment for 30 min. Finally, the absorbance value of the samples was measured with a UV-visible spectrophotometer at 593 nm. The FRAP antioxidant capacity value of water chestnut peel samples that underwent different pre-treatments was expressed in terms of ferrous ion equivalent per gram of water chestnut peel (mg FeE/g).
The ABTS+· radical scavenging capacity assay was determined by the method reported by Thaipong, Boonprakob [Citation16] with slight modifications. For the preparation of the ABTS working fluid, 2.6 mmol/L high potassium sulfate solution and 7.4 mmol/L ABTS solution were mixed thoroughly at a ratio of 1:1 and placed in the dark at room temperature for 12 h. The mixture was diluted by deionized water until the absorbance of ABTS working fluid was 0.7 ± 0.02 at 734 nm. After that, 2.4 mL of the above-mentioned working fluid was added to 0.6 mL of water chestnut peel samples or standards (Trolox). The mixtures were shaken evenly and left to stand for 6 min before the absorbance was measured at 734 nm. The ABTS antioxidant activity values were expressed as micromole Trolox equivalents (TE) per g water chestnut peel.
Flavonoid composition by HPLC
The phenolic substances in the water chestnut peels were determined using the HPLC–DAD method previously described by Su, Li. [Citation14] HPLC–DAD analysis was performed on a ZorboxSB-C18 column at 30°C with a flow rate of 1.0 mL/min, the injection volume of 20 uL and detection wavelength of 280 nm. The mobile phases were 10% glacial acetic acid in ultrapure water (A) and acetonitrile (B). The binary gradient elution program was as follows: 0–12 min, 95–75% A; 12–17 min, 75% A; 17–20 min, 75–50% A; 20–30 min, 50–25% A; 30–35 min, 25–5% A; and 35–45 min, 5–95% A, followed by a 5-min equilibration period with 95% A. The chromatographic peak preliminary identities were determined based on the retention time of the standard compounds (catechin, epicatechin, and catechin gallate) and qualitatively analyzed by the peak area. The results were expressed in terms of mg/g of water chestnut peel.
Statistical analysis
All the measurements of each indicator were repeated 3 times and the results were expressed as the mean ± standard deviation. SPSS 24.0 statistical software was used for the single factor analysis of variance, while SNK was used to identify differences. The significance level was p < 0.05, while the significant differences among different treatments were expressed with different letters.
Results
Effects of simulated digestion in vitro on total phenolic content in water chestnut peels that underwent different pre-treatments
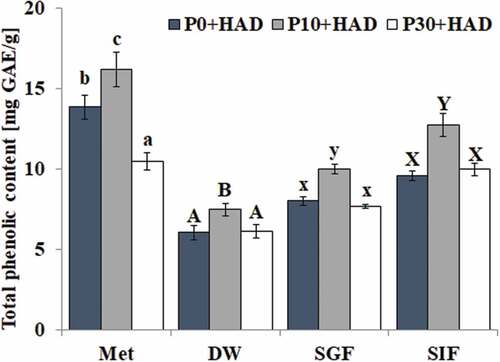
The total phenolic contents in water chestnut peels that underwent different pre-treatments were significantly affected by the simulated digestion treatment, which is shown in . The result of the Met group showed that the total phenolic contents significantly increased (17.01%) after short-time heat treatment (10 min). However, prolonged heat treatment (30 min) would cause the decomposition of heat-sensitive substances and the total phenolic content was significantly (p < 0.05) reduced (24.43%) compared to P0+ HAD. Furthermore, the total phenolic content of the water chestnut peels in the methanol extracts was significantly higher than that in deionized water (DW). The total phenolic contents in the water chestnut peels under different pre-treatment times had a similar change trend in the extraction of deionized water and simulated gastric or intestinal fluid, which were significantly increased only in the shortened pre-heat treatment (10 min) compared to P0+ HAD. The total phenolic content of water chestnut peels could not be increased with increases in the pre-treatment time in all deionized water extractions and simulated gastric or intestinal digestion. The P0+ HAD and P30+ HAD treatments had a similar TPC (p > 0.05) in the above three groups. It has been found that there was a significant difference (p < 0.05) among the simulated gastric fluid group, the simulated intestinal fluid group and the deionized water treatment group after further analysis of the effects of simulated digestion treatment on the total phenolic content of the water chestnut peels under the same treatment. The order of TPC was: SIF > SGF > DW, which showed that the total phenolic content of water chestnut peels was increased after the gastric fluid digestion (increased 33.70% for P10+ HAD) and intestinal digestion treatment (increased 70.72% for P10+ HAD) compared to deionized water treatment. The effect of simulated intestinal fluid digestion was greater than the effect of simulated gastric fluid digestion. These added phenolic substances might be the phenolic compounds that are covalently or noncovalently bound to pepsin or trypsin-hydrolyzed proteins.
Figure 1. Effects of simulated digestion on the total phenolic content of water chestnut peels that underwent different pre-treatments (mean ± SD, n = 3). Bars with no letters in common within one extraction method are significantly different (p < 0.05). DW: distilled water extraction; SGF: simulated gastric fluid extraction; SIF simulated intestinal fluid extraction
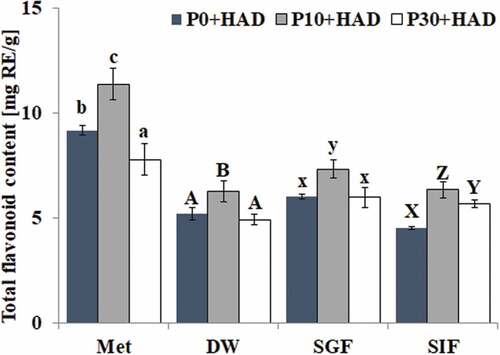
Effects of simulated digestion in vitro on total flavonoid content of water chestnut peels that underwent different pre-treatments
The effect of simulated gastric fluid and simulated intestinal fluid on the total flavonoid content of in water chestnut peels that underwent different pre-treatments is shown in . The results showed that the TFC variation trends in water chestnut peels under different heat treatment times were similar to those of TPC. The total flavonoid content of P10+ HAD was the highest, followed by P0+ HAD and P30+ HAD in all treatments. The TFC of water chestnut peels in the methanol solution was significantly higher than that in the corresponding samples of deionized water, gastric fluid, and intestinal fluid digestion groups (p < 0.05). The effects of simulated gastric fluid digestion and simulated intestinal fluid digestion on the TFC were different to those on TPC. The TFC of P10+ HAD after simulated gastric fluid treatment (7.33 mg RE/g) was significantly higher than that after simulated intestinal fluid digestion (6.35 mg RE/g) and deionized water (6.26 mg RE/g) extraction (p < 0.05). There was no significant difference of the TFC in the water chestnut peels that underwent P0+ HAD and P30+ HAD treatments. The above results indicated that the TFC of water chestnut peels, which underwent different pre-treatments, was increased by SGF treatment, while there was no significant change in TFC after simulated intestinal fluid digestion. This showed that the flavonoids were mainly released after the simulated gastric fluid digestion.
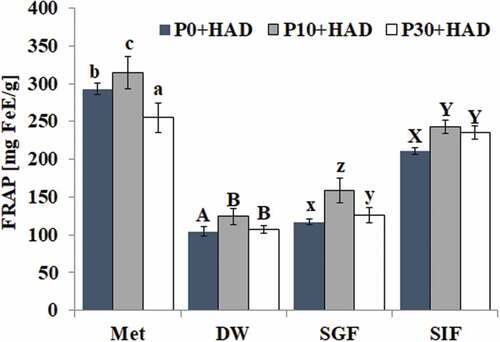
Effects of simulated digestion in vitro on the FRAP antioxidant activity of water chestnut peels that underwent different pre-treatments
The effect of different digestion treatments on the FRAP antioxidant capacity of water chestnut peels that underwent different pre-treatments is shown in . The FRAP antioxidant activity of water chestnut peels treated with P10+ HAD could be significantly improved after solvent extraction and simulated digestion treatment. Among all groups, the FRAP antioxidant activity of water chestnut peels, blanched for different times after organic solvent (methanol) extraction, was higher than other groups. Compared with the deionized water group, there was a significant increase in the FRAP antioxidant activity in the P10+ HAD sample after simulated gastric fluid digestion (increased 28.06%) treatment (p > 0.05) but no significant increase in P0+ HAD. The FRAP antioxidant activities of water chestnut peels of all three samples treated with simulated intestinal fluid were significantly higher than that of the sample treated with deionized water. The FRAP antioxidant activity of water chestnut peels was in the order of SIF > SGF > DW. The above results showed that pepsin and trypsin had different effects on the FRAP antioxidant activity of water chestnut peels. The FRAP antioxidant activity of water chestnut peels blanched for different times was significantly increased after being treated with simulated intestinal fluid.
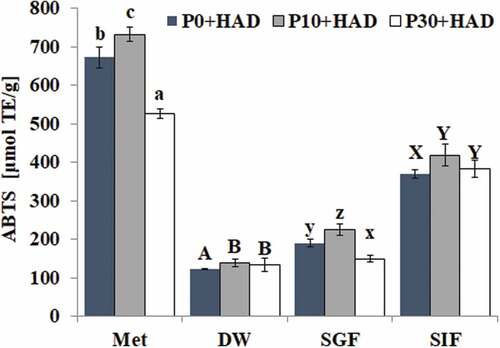
Effects of simulated digestion in vitro on the ABTS antioxidant capacity of water chestnut peels that underwent different pre-treatments
The effect of different digestion treatments on the ABTS antioxidant capacity of water chestnut peels that underwent different pre-treatments is shown in . The ABTS antioxidant capacity of the water chestnut peels treated for different pre-treatment times was similar to that of the FRAP antioxidant capacity. We found that the antioxidant activity of water chestnut peels treated with P10+ HAD was highest after both solvent extraction and simulated digestion compared to P0+ HAD and P10+ HAD. The antioxidant activity of water chestnut peels in the organic solvent methanol extraction group was higher than that of other groups. Furthermore, compared with the deionized water group, the ABTS antioxidant activity of water chestnut peels treated with P10+ HAD was significantly increased after both simulated gastric fluid digestion (increased 60.94%) and simulated intestinal fluid digestion (increased 200.82%). The antioxidant capacity of the water chestnut peel treated with P10+ HAD was higher than that of both the P30+ HAD and P0+ HAD groups. The ABTS antioxidant capacity of water chestnut peels decreased in the order of SIF > SGF > DW. The above results indicated that the ABTS antioxidant capacity of water chestnut peels that underwent different pre-treatments could be significantly improved after simulated intestinal fluid digestion.
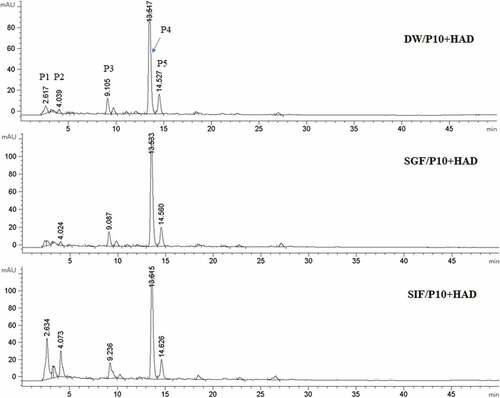
Effects of simulated digestion in vitro on the phenolic composition of water chestnut peels
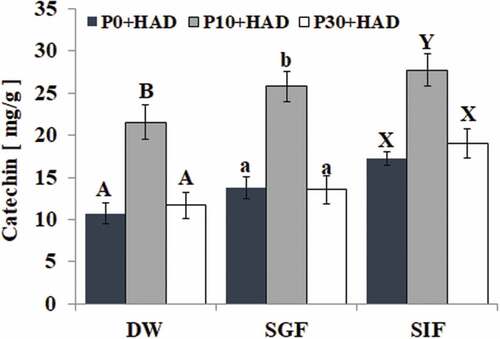
The effect of simulated digestion in vitro on the phenolic composition of the water chestnut peels is shown in . We found that the peak areas of the five peaks (P1, P2, P3, P4, and P5) in the water chestnut peels changed under the wavelength of 280 nm from the HPLC results. There were significant changes in the peak area of the samples treated with P10+ HAD after simulated gastric fluid digestion. We found that P1 peak area was decreased, while the P3, P4, and P5 peak areas were increased. In particular, the P4 peak area was significantly higher than that of the deionized water group. The peak area of the five peaks also changed after simulated intestinal fluid treatment as the peak areas of P1, P2, and P4 were significantly higher than that of the deionized water group. Peak 4 was identified as catechin after comparison to the standard. The effect of simulated digestion on the catechin of water chestnut peels that were pre-treated using different boiling times is shown in . The results show that the catechin content of P10+ HAD water chestnut peels was significantly higher than that of P0+ HAD and P30+ HAD (p < 0.05) in all treatments. The catechin contents of P10+ HAD water chestnut peels were SIF > SGF > DW. The HPLC results were consistent with the results of the total phenolics content of P10+ HAD.
Discussion
Foods, such as fruits, vegetables, and grains, are rich in phenolics, which have been reported to have good antioxidant activity. Positive correlations were found between phenolic contents and antioxidant activities. [Citation17] The digestibility of food could be increased after heat treatment as it makes active substances that are more easily eluted but the active substances might be degraded or polymerized after prolonged heat treatment. [Citation18,Citation19] There would be a great change in the content and composition of the phenolic substances after the foods were digested by the gastrointestinal tract, which directly affects its biological activity. [Citation20] It has been reported that both free phenolics and bound phenolics existed in food. [Citation21] Free phenolics might be polymerized or degraded during heat treatment, while bound phenolics might be released during digestion. The present study found that the total phenolic content of water chestnut peels that underwent different pre-treatments could be increased after simulated gastric fluid digestion or intestinal fluid digestion compared with the extraction of deionized water. Furthermore, the TPC in the SIF group was higher than that in the SGF group, which might be related to the type of protein contained in the sample and pancreatic proteolysis. Pancreatic enzymes hydrolyzed the ester bonds that were formed by the phenolic and proteins inside and outside the cell, releasing bound phenolic. In addition, it has been reported that the release of phenolic could be promoted under an alkaline (higher pH) environment so that the TPC was increased. [Citation22,Citation23] The effects of simulated gastric fluid or simulated intestinal fluid digestion on the TFC of water chestnut peels that were pre-treated using different boiling times were different from that of TPC. The TFC in water chestnut peels that underwent different pre-treatments could be increased after simulated gastric fluid digestion, while the TFC in water chestnut peels that underwent different pre-treatments was not significantly changed after simulated intestinal fluid digestion. We speculated that the flavonoids of water chestnut peels were mainly released in the stomach digestion stage. The simulated digestion of apple [Citation24], grape [Citation25], and blueberry [Citation26] has been previously studied. These studies found that the flavonoids-based polyphenols were released during simulated gastric fluid digestion and most phenolics were stable under simulated gastric fluid conditions in an acidic (lower pH) environment. Based on this speculation, in the present study, the reason for the increase in flavonoids in the simulated gastric fluid may be that the phenolics, which were covalently or non-covalently bound to the protein, are released after the proteins are hydrolyzed by pepsin. This results in a higher TFC compared to that of the deionized water group. However, the TFC in the water chestnut peels cannot be significantly altered after simulated intestinal fluid digestion as flavonoids and proteases could interact to form flavonoid–protease complexes in addition to hydrolysis. It has been reported that phenolic compounds may covalently or non-covalently bind to proteases. [Citation27,Citation28]
This study found that the TPC was also affected by the heat pre-treatment time. The TPC in the water chestnut peels treated with P10+ HAD was significantly higher than that in HAD or P30+ HAD. The TFC after simulated gastric fluid digestion in the water chestnut peels treated with P10+ HAD pre-treatment was significantly higher than that in HAD or P30+ HAD groups, which indicated that the P10+ HAD treatment could increase the amount of released phenolic from the water chestnut peels, while lengthening the preprocessing time of P30+ HAD treatment would reduce the phenolic. Heat treatment time could affect both TPC and TFC. Heating for a short period of time could increase both TPC and TFC, which might be due to the cell wall of the samples being damaged during this short time, facilitating the release of bound phenolic being more easily to be released. It was also possible that the endogenous polyphenol enzyme was inactivated by heating for a short time to prevent the oxidation loss of the polyphenol material. However, TPC and TFC were reduced after a prolonged heat treatment time, which might be due to the prolonged heat treatment causing the oxidation or decomposition of phenolic compounds, which ultimately reduces their content. [Citation29–Citation32] The flavonoids composition and content variation in the water chestnut peels of different simulated digestion and solvent extraction were determined by HPLC. The results showed that the phenolic content of water chestnut peels could be increased after simulated digestion. Compared with the deionized water group, the SGF treatment mainly affected P4, which had the highest content. The P4 with the largest peak area increased, while the P1 and P2 contents increased by SIF digestion. These indicated that simulated digestion could change the content of phenolic compounds although the types of substances produced need to be further analyzed and identified by liquid chromatography.
The active components in the water chestnut peels had better FRAP iron ion reducing ability and ABTS free radical scavenging activity. The antioxidant activity of the extracts from water chestnut peels that had different heat treatment times was different. The antioxidant activity of P10+ HAD after solvent extraction and simulated digestion was higher than that of P30+ HAD and HAD, which might be related to the content of phenolic compounds. This is because the total phenolic content of water chestnut peels treated with P10+ HAD was higher than that of P30+ HAD and HAD after the same treatment. It has been reported that FRAP and ABTS antioxidant activity was positively correlated with the phenolic content. [Citation33,Citation34] There was a series of complex changes in the phenolic substances of water chestnut peels after simulated digestion, such as the release of phenolic compounds and the possible complexes formed by phenolic compounds and proteases. SIF digestion treatment for different periods of time could significantly improve the antioxidant capacity of FRAP and ABTS. The antioxidant capacity of FRAP and ABTS of water chestnut peels that were pre-treated using different times could be significantly improved after SIF digestion. The TPC of the water chestnut peels could also be significantly increased after SIF digestion, indicating that the antioxidant activity of the water chestnut peels was related to the total phenolic content. The above results were consistent with the findings of Koehnlein et al., who discovered that the simulation of intestinal fluid digestion improved the antioxidant activities of 36 popular Brazilian food items. [Citation19]
Conclusion
We found that the TPC and TFC in the water chestnut peels, as well as the antioxidant activity of FRAP and ABTS, were changed after simulated gastric fluid and simulated intestinal fluid digestion. The TPC and TFC of the water chestnut peels with different heat pre-treatment times could be significantly increased after SGF digestion. The TPC of the water chestnut peels could be significantly increased after SIF digestion, but the TFC could not be significantly increased. Compared with the simulated gastric fluid digestion, the TPC of water chestnut peels increased more after SIF digestion. Both the FRAP and ABTS antioxidant capacity of water chestnut peels could be improved after SGF and SIF digestion, while the antioxidant ability improved more after SIF digestion.
Acknowledgments
The project supported by the National Natural Science Foundation of China (31601469; 31601420), Scientific Research Foundation for Hundred-Talent Program of Guangzhou University (69-18ZX10011), Natural Science Foundation of Guangdong Province (2017A030313205), Science and Technology Program of Guangzhou (201604020089). There is no conflict of interest.
Additional information
Funding
References
- Wu, S.; Yu, L. Preparation and Characterisation of the Oligosaccharides Derived from Chinese Water Chestnut Polysaccharides. Food Chem. 2015, 181, 15–18. DOI: 10.1016/j.foodchem.2015.02.066.
- Gao, M.; Jiang, W.; Wei, S.; Lin, Z.; Cai, B.; Yang, L.; Luo, C.; He, X.; Tan, J.; Chen, L. High-Efficiency Propagation of Chinese Water Chestnut [Eleocharis Dulcis (Burm.F.) Trin. Ex Hensch] Using a Temporary Immersion Bioreactor System. Plant Cell Tissue Organ Culture. 2015, 121(3), 761–772. DOI: 10.1007/s11240-015-0732-4.
- Sun, J.; You, Y.; Garciagarcia, E.; Long, X.; Wang, J. Biochemical Properties and Potential Endogenous Substrates of Polyphenoloxidase from Chufa (Eleocharis Tuberosa) Corms. Food Chem. 2010, 118(3), 799–803. DOI: 10.1016/j.foodchem.2009.05.065.
- Zhan, G.; Pan, L.; Tu, K.; Jiao, S. Antitumor, Antioxidant, and Nitrite Scavenging Effects of Chinese Water Chestnut (Eleocharis Dulcis) Peel Flavonoids. J. Food Sci. 2016, 81(10), 2578–2586. DOI: 10.1111/1750-3841.13434.
- Zhan, G.; Pan, L.; Mao, S.; Zhang, W.; Wei, Y.; Tu, K. Study on Antibacterial Properties and Major Bioactive Constituents of Chinese Water Chestnut (Eleocharis Dulcis) Peels Extracts/Fractions. Eur. Food Res. Technol. 2014, 238(5), 789–796. DOI: 10.1007/s00217-013-2151-2.
- Luo, Y.; Li, X.; He, J.; Su, J.; Peng, L.; Wu, X.; Du, R.; Zhao, Q. Isolation, Characterisation, and Antioxidant Activities of Flavonoids from Chufa (Eleocharis Tuberosa) Peels. Food Chem. 2014, 164, 30–35. DOI: 10.1016/j.foodchem.2014.04.103.
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of Antioxidant Activity of Medicinal Plants Containing Polyphenol Compounds. Comparison of Two Extraction Systems. Acta Biochim. Pol. 2010, 57(2), 229–234.
- Abadgarcia, B.; Garmonlobato, S.; Sanchezilarduya, M. B.; Berrueta, L. A.; Gallo, B.; Vicente, F.; Alonsosalces, R. M. Polyphenolic Contents in Citrus Fruit Juices: Authenticity Assessment. Eur. Food Res. Technol. 2014, 238(5), 803–818. DOI: 10.1007/s00217-014-2160-9.
- Majo, D. D.; La Neve, L.; La Guardia, M.; Casuccio, A.; Giammanco, M. The Influence of Two Different pH Levels on the Antioxidant Properties of Flavonols, Flavan-3-Ols, Phenolic Acids and Aldehyde Compounds Analysed in Synthetic Wine and in a Phosphate Buffer. J. Food Compost. Anal. 2011, 24(2), 265–269. DOI: 10.1016/j.jfca.2010.09.013.
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein–Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51(2), 954–970. DOI: 10.1016/j.foodres.2013.02.009.
- Hossain, M. A.; Rahman, S. M. M. Total Phenolics, Flavonoids and Antioxidant Activity of Tropical Fruit Pineapple. Food Res. Int. 2011, 44(3), 672–676. DOI: 10.1016/j.foodres.2010.11.036.
- Lee, K. H.; Cho, J.; Lee, H. J.; Park, K. Y.; Ma, Y.; Lee, S.; Cho, J. A.; Kim, W.; Park, K.; Moon, J. Isolation and Identification of Phenolic Compounds from an Asian Pear (Pyrus Pyrifolia Nakai) Fruit Peel. Food Sci. Biotechnol. 2011, 20(6), 1539–1545. DOI: 10.1007/s10068-011-0213-4.
- Fu, T.-J.; Abbott, U. R.; Hatzos, C. Digestibility of Food Allergens and Nonallergenic Proteins in Simulated Gastric Fluid and Simulated Intestinal FluidA Comparative Study. J. Agric. Food Chem. 2002, 50(24), 7154–7160. DOI: 10.1021/jf020599h.
- Su, D.; Li, N.; Chen, M.; Yuan, Y.; He, S.; Wang, Y.; Wu, Q.; Li, L.; Yang, H.; Zeng, Q. Effects of Invitro Digestion on the Composition of Flavonoids and Antioxidant Activities of the Lotus Leaf at Different Growth Stages. Int. J. Food Sci. Technol. 2018, 53, 1631–1639. DOI: 10.1111/ijfs.13746.
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total Phenolics, Flavonoids, Anthocyanins and Antioxidant Activity following Simulated Gastro-Intestinal Digestion and Dialysis of Apple Varieties: Bioaccessibility and Potential Uptake. Food Chem. 2011, 128(1), 14–21. DOI: 10.1016/j.foodchem.2011.02.052.
- Thaipong, K.; Boonprakob, U.; Crosby, K. M.; Cisneroszevallos, L.; Byrne, D. H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compost. Anal. 2006, 19, 669–675. DOI: 10.1016/j.jfca.2006.01.003.
- Condehernandez, L. A.; Guerrerobeltran, J. A. Total Phenolics and Antioxidant Activity of Piper Auritum and Porophyllum Ruderale. Food Chem. 2014, 142, 455–460. DOI: 10.1016/j.foodchem.2013.07.078.
- Papoutsis, K.; Pristijono, P.; Golding, J. B.; Stathopoulos, C. E.; Bowyer, M. C.; Scarlett, C. J.; Vuong, Q. V. Enhancement of the Total Phenolic Compounds and Antioxidant Activity of Aqueous Citrus Limon L. Pomace Extract Using Microwave Pretreatment on the Dry Powder. J. Food Process. Preserv. 2017, 41(5), e13152. DOI: 10.1111/jfpp.13152.
- Ursache, F. M.; Ghinea, I. O.; Turturică, M.; Aprodu, I.; Râpeanu, G.; Stănciuc, N. Phytochemicals Content and Antioxidant Properties of Sea Buckthorn (Hippophae Rhamnoides L.) As Affected by Heat Treatment - Quantitative Spectroscopic and Kinetic Approaches. Food Chem. 2017, 233, 442–449. DOI: 10.1016/j.foodchem.2017.04.107.
- Koehnlein, E. A.; Koehnlein, E. M.; Correa, R. C. G.; Nishida, V. S.; Correa, V. G.; Bracht, A.; Peralta, R. M. Analysis of a Whole Diet in Terms of Phenolic Content and Antioxidant Capacity: Effects of a Simulated Gastrointestinal Digestion. Int. J. Food Sci. Nutr. 2016, 67(6), 614–623. DOI: 10.1080/09637486.2016.1186156.
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the Free and Bound Phenolic Profiles and Cellular Antioxidant Activities of Litchi Pulp Extracts from Different Solvents. BMC Complementary Altern. Med. 2014, 14(1), 9. DOI: 10.1186/1472-6882-14-9.
- Green, R. J.; Murphy, A. S.; Schulz, B.; Watkins, B. A.; Ferruzzi, M. G. Common Tea Formulations Modulate in Vitro Digestive Recovery of Green Tea Catechins. Mol. Nutr. Food Res. 2007, 51(9), 1152–1162. DOI: 10.1002/mnfr.200700086.
- Tenore, G. C.; Campiglia, P.; Giannetti, D.; Novellino, E. Simulated Gastrointestinal Digestion, Intestinal Permeation and Plasma Protein Interaction of White, Green, and Black Tea Polyphenols. Food Chem. 2015, 169, 320–326. DOI: 10.1016/j.foodchem.2014.08.006.
- Bouayed, J.; Deuser, H.; Hoffmann, L.; Bohn, T. Bioaccessible and Dialysable Polyphenols in Selected Apple Varieties following in Vitro Digestion Vs. Their Native Patterns. Food Chem. 2012, 131(4), 1466–1472. DOI: 10.1016/j.foodchem.2011.10.030.
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120(2), 599–606. DOI: 10.1016/j.foodchem.2009.10.030.
- Correabetanzo, J.; Allenvercoe, E.; Mcdonald, J. A. K.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and Biological Activity of Wild Blueberry (Vaccinium Angustifolium) Polyphenols during Simulated in Vitro Gastrointestinal Digestion. Food Chem. 2014, 165, 522–531. DOI: 10.1016/j.foodchem.2014.05.135.
- Arroyomaya, I. J.; Camposteran, J.; Hernandezarana, A.; Mcclements, D. J. Characterization of Flavonoid-Protein Interactions Using Fluorescence Spectroscopy: Binding of Pelargonidin to Dairy Proteins. Food Chem. 2016, 213, 431–439. DOI: 10.1016/j.foodchem.2016.06.105.
- Silva, F. G. D. E.; Miralles, B.; Hernandezledesma, B.; Amigo, L.; Iglesias, A. H.; Reyes, F. G. R.; Netto, F. M. Influence of Protein-Phenolic Complex on the Antioxidant Capacity of Flaxseed (Linum Usitatissimum L.) Products. J. Agric. Food Chem. 2017, 65(4), 800–809. DOI: 10.1021/acs.jafc.6b04639.
- Choi, Y.; Lee, S.; Chun, J.; Lee, H.; Lee, J. Influence of Heat Treatment on the Antioxidant Activities and Polyphenolic Compounds of Shiitake (Lentinus Edodes) Mushroom. Food Chem. 2006, 99(2), 381–387. DOI: 10.1016/j.foodchem.2005.08.004.
- Jeong, S.; Kim, S.; Kim, D.; Jo, S.; Nam, K. C.; Ahn, D. U.; Lee, S. Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels. J. Agric Food Chemi. 2004, 52(11), 3389–3393. DOI: 10.1021/jf049899k.
- Peng, L.; Jiang, Y. Effects of Heat Treatment on the Quality of Fresh-Cut Chinese Water Chestnut. Int. J. Food Sci. Technol. 2010, 39(2), 143–148. DOI: 10.1046/j.0950-5423.2003.00767.x.
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55(2), 330–335. DOI: 10.1021/jf062517l.
- Islam, T.; Yu, X.; Badwal, T. S.; Xu, B. Comparative Studies on Phenolic Profiles, Antioxidant Capacities and Carotenoid Contents of Red Goji Berry (Lycium Barbarum) and Black Goji Berry (Lycium Ruthenicum). Chem. Cent. J. 2017, 11(1), 59. DOI: 10.1186/s13065-017-0287-z.
- Shumoy, H.; Gabaza, M.; Vandevelde, J.; Raes, K. Soluble and Bound Phenolic Contents and Antioxidant Capacity of Tef Injera as Affected by Traditional Fermentation. J. Food Compost. Anal. 2017, 58, 52–59. DOI: 10.1016/j.jfca.2017.01.004.