?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The physicochemical characteristics, antioxidant properties and botanical discrimination according to the organic acid content in Malaysian stingless bee honey were investigated. The results showed that the botanical origin and bee species significantly influenced the physicochemical characteristics and antioxidant properties of stingless bee honey. Principle component analysis (PCA) revealed that stingless bee honey was differentiable from Apis mellifera honey by acetic, citric, D-malic and tartaric. Starfruit could be distinguished from gelam and acacia honeys by lactic. Whereas the gluconic and succinic acids were confirmed as a marker to discriminate honey samples from Heterotrigona itama and Geniotrigona thoracica species.
Introduction
Over 500 species of stingless bee (Apidae:Hymenoptera:Meliponini) can be found in tropical and subtropical regions throughout the world, like in Australia, South America, Africa and Malaysia. Only two genera, Melipona and Trigona, are commonly domesticated worldwide.[Citation1] In Malaysia, 33 species of stingless bee, known locally as kelulut, have been documented.[Citation2] Only two species have been reared commercially to produced honey, namely, Heterotrigona itama and Geniotrigona thoracica (). Of these, Heterotrigona itama is the most abundant species domesticated by beekeepers since log hives of Heterotrigona itama can be more easily found than those of Geniotrigona thoracica in Malaysian forests. Both species are different in their colour and size. The average body size of Heterotrigona itama was 4.7 ± 1.55 mm whilst Geniotrigona thoracica was 7.44 ± 2.05 mm. Heterotrigona itama are black in colour with grey wings while on the contrary, Geniotrigona thoracica are brown in colour with dark brown wings and white tips at the apex of the wings.[Citation3]
Like honeybee honey, stingless bee honey is formed from the nectar of flowers or plant saps or plant-sucking insect excretions.[Citation4] Honey is a complex substance that contains at least 200 compounds.[Citation5] In general, stingless bee honey has a higher moisture content, water activity, ash content and free acidity than honeybee honey, and its pH and total soluble solid content are typically lower.[Citation6] In addition to those components, proteins, amino acids, enzymes, organic acids, mineral elements and vitamins are also present in honey but only in small amounts.[Citation7] The presence of specific constituents in the honey depends on several factors such as floral source, bee species, geographical origin, weather, harvesting season, processing method and storage conditions.[Citation8,Citation9] Honey produced by different species of stingless bees have different qualities. Generally, several parameters such as moisture content, pH, free acidity, organic acids content and 5-hydroxymethylfurfural (5-HMF) content are used to evaluate the quality of honey.[Citation10] Numerous studies have been done on the physicochemical properties of stingless bee honeys from around the world, such as those from Venezuela [Citation11], Thailand [Citation12] and Mexico.[Citation13] It was reported that the antioxidant activity of stingless bee honey was three-times higher than that of raw honeybee honey and four-times higher than that of processed honeybee honey.[Citation14] Honey comprises various compounds that can act as antioxidants. Thus, the antioxidant capacity in honey is attributed to the synergistic effect of a wide range of compounds including phenolics, flavonoids, products of the Maillard reaction, enzymes, organic acids and other minor compounds.[Citation15] The antioxidant activity of honeys has been reported to have a correlation with phenolics, flavonoids, products of the Maillard reaction, ascorbic acid, organic acids, enzymes, carotenoids, amino acids, proteins and mineral content.[Citation16–Citation18] Honey has often been reported to have potential antioxidant activity, which is beneficial for human health.[Citation19] Antioxidant activity is generally attributed to the ability of antioxidant compounds to donate electrons, scavenge free radicals, and chelate transition metal ions.[Citation20]
There is limited publication available on Malaysian stingless bee honey.[Citation21–Citation23] Most of the publications reported are on the physicochemical and antioxidant properties. However, there is no information on organic acids of Malaysian stingless bee honey. Since stingless bee honey is a new industry in Malaysia, it is important to get as much as information of this honey. Furthermore, to the best of our knowledge, there are only limited publications on the organic acids contents of stingless bee honeys.[Citation24,Citation25] It is reported that organic acids as a useful marker to determine honey botanical and geographical origins.[Citation26,Citation27] For instance, 2-Methoxybutanedioic acid (o-methylmalic acid) and 4-hydroxy-3-methyl-trans-2-pentenedioic acid are reported as floral markers for New Zealand rewarewa honey.[Citation28] Erica sp. honeys could be distinguished by their high quinic acid content, Quercus sp. honeys have low pyruvic acid contents and high malic and succinic acid contents and Thymus sp. honeys have high citric acid contents.[Citation27]
Honey from different botanical and geographical origins can be distinguished based on the compositional data of honey volatile compounds, phenolic acids, flavonoids, carbohydrates, amino acids and some other constituents.[Citation29] Currently, the multivariate analysis used as a method to classify honey from different origins. Therefore, the objectives of this study were (1) to evaluate the influence of botanical origins and bee species on physicochemical and antioxidant properties of stingless bee honey and (2) to discriminate honey samples according to their botanical origins and bee species based on the organic acids content. Discrimination of honey could help to identify the honey origin and quality.
Materials and methods
Standards and chemicals
Sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sodium acetate (C2H3NaO2), iron (II) sulphate (FeSO4 7H2O) and acetic glacial (CH3COOH) were purchased from R&M Chemicals (Essex, UK). 2,4,6-Tri(2-pyridyl)-s-tiazine (TPTZ) and 2,2-diphenyl-1-hydrazyl-hydrate (DPPH), aluminium chloride (AlCl3) and all standards for organic acids (gluconic, formic, tartaric, D-malic, citric, acetic, succinic and lactic acid), 5-hydroxymethylfurfural (HMF), sugars (fructose, glucose, sucrose, maltose) were purchased from Sigma-Aldrich (St. Louis, Missouri, United State). Iron (III) chloride hexahydrate (FeCl3.6H2O), Folin-Ciocaltue’s phenol reagent, sodium sulphate anhydrous (Na2SO4), ethyl acetate (CH3COOC2H5, 99.8%), formic acid (CH2O2, 98%), metaphosphoric acid (EMSURE®), acetonitrile (ACN), methanol (CH3OH), hydrochloric acid fuming (HCl, 37%) were purchased from Merck (Darmstadt, Germany). Sulphuric acid (H2SO4, 96.5%) was purchased from J.T. Baker NEUTRASOB® (USA). The water was purified using an ELGA Pure Lab Classic system (ELGA, Woodridge, IL, USA). All chemicals and solvents used were of analytical grade except for HPLC analysis.
Honey samples
Honey samples were obtained from three stingless bee farms from each of the three-main honey-producing states in Malaysia, namely, Terengganu, Pahang and Johor (). The samples were harvested during the nectar flow season in August 2016. The obtained honey samples were from different floral origins, namely, acacia (Acacia mangium), gelam (Meleleucacajaputi) and starfruit (Averrhoa carambola L) (). The honey samples were harvested from hives placed in the farm of their respective floral origins. Honeybee honey (Apis mellifera) from acacia was used for comparison in this study. All samples were collected using an electric vacuum pump (Rocker 300, Kaohsiung, Taiwan). The samples were stored in airtight plastic containers and kept at 4 ± 2°C until analysis.[Citation30]
Table 1. Description of the source of stingless bee honey used in this study
Analytical procedures
All physicochemical analyses (moisture content, water activity, pH, free acidity, total soluble solids (TSS), ash content, colour characteristics, sugar content, 5-hydroxymethyl furfural (HMF), organic acids and antioxidant properties (total phenolic contents (TPC), total flavonoids content (TFC), antiradical scavenging activity (IC50) and reducing power activity (FRAP)) were conducted for all honey samples. All analyses were done in three replications.
Physicochemical analyses
Moisture content
The moisture content of honey samples was measured according to AOAC official method 969.38.[Citation31] Honey (2 g) was weighted in crucible dish (previously heated in an oven at 110ºC and cooled in a desiccator until it reached room temperature) and placed in a vacuum oven at 60 ± 2ºC under pressure ≤50 mm Hg for 6 h. The weight of crucible dish before and after drying was recorded. The moisture content is calculated using EquationEquation (1)(1)
(1) .
where A and a referred to the weight of crucible dish before and after drying; B and b referred to the weight of honey before and after drying.
Water activity (aw)
Water activity was determined at 25°C using an AQUA LAB Series 3 water activity meter (North East Nelson Court, Pullman, WA).[Citation32] The equipment was calibrated with saturated salt solutions in the aw range of interest. A pure honey sample was used to determine the water activity. Three replicates were performed for each honey sample and the average was reported.
Total soluble solid (TSS)
Total soluble solid was measured at 25°C using digital refractometer (ATAGO, Tokyo, Japan). About 0.3 mL of honey was placed onto the prism surface and the reading was recorded as °Brix.[Citation33]
Ash content
The ash contents were determined by using a method described by Colucci et al. (2016) [Citation33] with a slight modification. A honey sample (2 g) was weighted in a pre-weighed crucible that had been heated in an electric furnace at 550°C for 1 h and cooled in a desiccator until it reaches room temperature. After that, two drops of olive oil were added, and the honey sample was heated (350–400°C) on a hot plate until all the honey had turned black, and then the sample was heated (550°C) in an electric furnace until it turns into white ash. Subsequently, the crucible with white ash was cooled in a desiccator until it reached room temperature and then weighed. The ash content is calculated using EquationEquation (2)(2)
(2) .
where a is the weight of the crucible with a sample, b is the weight of the empty crucible and c is the weight of the sample.
Colour characteristics
Colour characteristics were measured using the HunterLabUltraScan PRO spectrophotometer (Reston, Virginia), according to the CIELAB L*a*b* method [Citation34], with reference to illuminant D65 and a visual angle of 10º.
pH
The pH was determined using pH meter, MP220 Mettler Toledo (Schwarzenburg, Switzerland) according to AOAC official method 962.19.[Citation31] A weight of 10 g of honey was dissolved in 75 mL of distilled water, stirred well using magnetic stirrer, and the reading from the pH meter was recorded.
Free acidity
The free acidity of honey was determined according to AOAC official method 962.19. [Citation31] The pH meter was calibrated with the buffer solution of pH 4.00, 7.00 and 9.00. A weight of 10 g honey was dissolved in 75 mL distilled water (previously titrated with 0.1 M NaOH until the pH reached 8.50) and stirred well using magnetic stirrer. Then, pH of the solution was recorded using pH meter. Afterwards, honey solution was titrated with 0.1 M NaOH and the titration of NaOH was stopped at pH 8.50. The volume of NaOH used was recorded and subtracted with the volume of NaOH used in distilled water. The acidity was expressed as milliequivalents of acid per kg of honey.
Sugar content
The contents of fructose, glucose, sucrose and maltose in the honeys were determined by using high-performance liquid chromatography with a refractive index detector (HPLC-RI) as described by Silvano et al. (2014) [Citation8] with a few modifications. An amino column (Luna® NH2 100 Å (250 mm × 4.6 mm × 5 µm) from Phenomenex Inc. (Torrance, CA, USA) was used to separate the sugars present in the honey sample. The temperature of the column was set at 40°C. An 80:20 mixtures of acetonitrile and water were used as the mobile phase, and the injection volume was 20 µL. The samples for analysis were prepared by mixing 0.5 g of honey with 10 mL of distilled water. The flow rate was 1 mL/min, and the eluate was monitored for 20 min. Each sugar was identified based on the retention time of pure standard. Calibration curves were constructed using five different concentrations of each sugar standard, and the sugar concentrations were calculated based on the equations obtained from the calibration curves. All analyses were performed triplicate. The results are expressed as gram sugar per 100 g of honey.
5-Hydroxymethyl-2-furfural
The method by Harmonised methods of international honey commission, HMIHC.[Citation35] was used to determine the 5-HMF contents in the honey samples. In this method, the 5-HMF contents were analysed by using reversed-phase high-performance liquid chromatography (HPLC-RP). Prior to analysis, the honey samples were extracted using the liquid-liquid extraction technique recommended by Gokmen & Acar (1999).[Citation36] A 1-g honey sample was dissolved in 5 mL of deionized water and mix thoroughly by using a vortex mixer for 5 min. Then, the honey solution was mixed with 10 mL of ethyl acetate and vigorously shaken using a vortex mixer for 1 min. The solution was left to separate, and two phases formed. The organic phase was collected, and this extraction process was repeated twice. The organic phases were combined and mix with 2 mL of 1.5% sodium carbonate solution by shaking for 1 min using a vortex mixer, after which the mixture was left to separate. Then, the aqueous phase was immediately extracted with 5 mL of ethyl acetate by shaking for 1 min using a vortex mixer. All organic phases (a total of 25 mL) were combined and mixed with approximately 3 g of anhydrous sodium sulphate to remove residual water. Subsequently, the dried extract was filtered through No. 1 Whatman filter paper (GE Healthcare, United Kingdom) to remove the remaining anhydrous sodium sulphate particles, and the filter cake was washed by 2 mL of ethyl acetate. The filtrate was evaporated to dryness under nitrogen gas. The HMF residue was promptly dissolved in 500 µL of water at pH 4.0.
The HMF solution was analysed by reversed-phase high-performance liquid chromatography (WATERS, Milford, USA) using a photodiode array detector (WATERS 2996, Milford, USA), and the injection volume was 20 µL. A reversed-phase column (Luna® C18(2) 100 Å (250 mm × 4.6 mm × 5 µm)) from Phenomenex Inc. (Torrance, CA, USA) was used. A combination of water:methanol (90:10) was employed as the mobile phase at a constant flow rate of 0.9 mL/min throughout the analysis (approximately 30 min). HMF was detected by comparing the retention time of the HMF in the sample with that of an HMF standard. The concentration of HMF in the sample was calculated using the equation obtained from a standard curve of HMF prepared from five solutions at different concentrations. All honey samples were injected in triplicate. The results are expressed as mg/kg honey.
Organic acids content
The contents of organic acids were analysed using an HPLC method [Citation37] with some modifications. A honey sample (1 g) was dissolved in 10 mL of ultrapure water. The mixture was homogenized by using a vortex mixer. The pH of the honey solution was adjusted to approximately 10.50 by adding 0.1 M NaOH and then stirred for 15 min using a magnetic stirrer. This procedure was performed to ensure complete decomposition of D-glucano-ᵟ-lactone to D-gluconic acid and to improve the reproducibility of the organic acid migration time. After that, the pH was adjusted to approximately 5.00 using 0.1 M H2SO4. This step was applied to make sure organic acid exist in their non-ionic forms before further used in solid phase extraction (SPE). Then, the mixture was diluted with water up to 20 mL and shaken. Then, 10 mL of the dilute solution was filtered through a 0.45-µL cellulose acetate membrane. A solid-phase extraction (SPE) procedure was used to extract the organic acids from the honey sample. An ion-exchange cartridge (Sep-Pak® Plus Waters Acce IITM Plus QMA, WATERS, Massachusetts, USA) was used, and the cartridge was activated with 10 mL of 0.1 M NaOH (the percolation rate was 3 mL/min). A 10-mL aliquot of honey solution was passed through the cartridge at a flow rate of 1 mL/min. Later, 10 mL of water was passed through the cartridge (3 mL/min). The organic acids were eluted with 4 mL of 0.1 M sulphuric acid (1 mL/min). The solution was kept at −20°C until analysis.
The solution of organic acids was analysed by reversed-phase high-performance liquid chromatography (WATERS, Milford, USA) with a photodiode array detector (WATERS 2996, Milford, USA), and the injection volume was 20 µL. A reversed-phase column (Spherisorb® ODS 2, (4.6 mm × 250 mm × 5 µm), WATERS, Milford, USA) was used. The organic acids were separated using acetonitrile spiked with metaphosphoric acid (pH 2.28) at a flow rate of 1 mL/min. Pure standards of nine organic acids (gluconic, tartaric, formic, acetic, citric, D-malic, succinic, lactic and fumaric) that are common in honey were used for comparison to identify the organic acids in the honey samples. The concentrations of the organic acids in the honey samples were calculated using the equations obtained from the standard curves of each organic acid standard. The LOD and LOQ values were calculated based on the 3.3*standard deviation of blank response/slope and 10*standard deviation of blank response/slope, respectively. [Citation38] The LOD/LOQ values for gluconic acid was 0.06/0.17 g/kg, tartaric acid was 0.003/0.01 g/kg, formic acid was 0.03/0.11 g/kg, D-malic acid was 0.03/0.08 g/kg, lactic acid was 0.01/0.03 g/kg and acetic acid was 0.01/0.03 g/kg, citric acid was 0.01/0.03 g/kg and succinic acid was 0.01/0.03 g/kg.
Analyses of the antioxidant properties
Total phenolic content (TPC)
The concentration of TPC compounds was estimated by using the Folin-Ciocalteu method [Citation18] with slight modifications. The honey solution was prepared by adding 1 mg of honey (dry basis) to deionized water and vortex mixing the solution. Approximately 200 µL of the honey solution was mixed with 1000 µL of Folin-Ciocalteu (FC) reagent (dilution ratio of FC reagent, 1:10 v/v), and the mixture was left in the dark for 6 min. After that, 800 µL of 7.5% sodium carbonate solution was added, and the solution was shaken and left in the dark for 2 h to react. After that time, the absorbance was measured at 740 nm. The total phenolic content of each of the honey samples is expressed as gallic acid equivalent (GAE/100 g honey). A standard curve was constructed using a standard solution of gallic acid.
Total flavonoids content (TFC)
TFC in each of the honey samples was measured using the spectrophotometric method described by Kamboj et al. (2013).[Citation34] Briefly, 1 g of honey (dry basis) was dissolved in deionized water, and 1 mL of the honey solution was mixed with 0.3 mL of 5% NaNO2 solution and left to stand for 5 min before being combined with 0.3 mL of 10% AlCl3. The mixture was stirred for 6 min and then 2 mL of 1 M NaOH was added to neutralize the solution. The absorbance of each solution was read at 517 nm using a UV-VIS spectrophotometer. The total flavonoids contents are expressed in mg quercetin equivalent (QE)/100 g of honey. A standard curve was constructed using a standard solution of quercetin.
Antiradical scavenging activity (IC50)
IC50 was estimated using the method reported by Meda et al. (2005) [Citation39] with a slight modification. Briefly, a series of honey solutions at different concentrations were prepared by dissolving 100 mg of honey (dry basis) in methanol. Afterwards, 0.75 mL of each honey solution was mixed with 1.5 mL (0.02 mg/mL) of a methanolic solution of DPPH. The mixtures were stored protected from light for 15 min at room temperature. The absorbance of each solution was measured at 517 nm using a UV-VIS spectrophotometer. The data collected were used to construct a graph of concentration versus radical scavenging activity, RSA (%). RSA (%) is calculated using EquationEquation (3)(3)
(3) .
where As is the absorbance of the sample solution and ADPPH is the absorbance of the DPPH solution. Each honey sample was analysed in five dilutions. The antiradical activity is expressed as IC50 (concentration of honey solution needed to reduce the concentration of DPPH in the solution to 50% of its initial concentration). The concentration of honey sample requires to scavenge 50% of DPPH (IC50) was determined from the plotted graph of scavenging activity against the honey dilutions.
Reducing antioxidant power activity (FRAP)
FRAP of the honey samples was evaluated by using the method recommended by Khalil et al. (2011) [Citation40] with some modifications. The honey solution was prepared by mixing approximately 100 mg of honey (dry basis) with deionized water, and then, 200 µL of the honey solution was mixed with 1.5 mL of FRAP reagent. After mixing, the solution was incubated in a water bath at 37°C for 4 min. Then, the absorbance was measured at 593 nm by using a UV-VIS spectrophotometer. Distilled water was used as the blank. The FRAP reagent was prepared as follows: 10 mL of 300 mM/L acetate buffer (pH 3.6) was mixed with 1 mL of 10 mmol 2,4,6-tris(1-pyridyl)-1,3,5-triazine (TPTZ) and 1 mL of 40 mM/L HCl containing 20 mM ferric chloride (FeCl3 6H2O). This FRAP reagent solution must be prepared fresh and pre-warmed prior to use. The antioxidant capacity was determined by constructing a standard curve of ferrous sulphate (FeSO4 7H2O) at 20, 40, 60, 80, and 100 µM/L. The FRAP value is expressed as micromoles of ferrous equivalent (µM Fe(II)) per kilogram of honey.
Statistical analysis
All determinations were carried out in three replications. The data were expressed as the mean ± standard deviation. The differences between species and botanical origin of the honey samples were analysed using the two-way ANOVA (MINITAB version 17, Sydney, Australia) followed by Fisher test at a significance level of p < 0.05. Possible correlations among total phenolics and flavonoids contents and the antioxidant capacity of honeys were investigated using Pearson’s correlation coefficient (r) at a significant level of p < 0.05. Discrimination of honey samples from different botanical origins and bee species based on organic acids content were analysed using principle component analysis (PCA) (MINITAB version 17, Sydney, Australia). PCA is a powerful explorative tool for pattern recognition that attempts to explain the variance of a large set of variables.
Results and discussion
Physicochemical analyses
Moisture content
The moisture contents of honeys from Heterotrigona itama ranged from 19.49 to 26.28 g/100 g and from 21.32 to 33.93 g/100 g for honeys of Geniotrigona thoracica species (). Acacia honeys from both species had significantly higher moisture contents than those from gelam and starfruit. For Heterotrigona itama, honeys with different floral origins shown significantly different (p < 0.05) water contents. However, the water content of gelam and starfruit honeys from Geniotrigona thoracica was not significantly different (p > 0.05).
Table 2. Physicochemical parameters of stingless bee honey from different floral origins
The moisture content of honeys in this study was lower than those found for stingless bee honeys from Mexico, Guatemala and Venezuela (31.40 to 38.74 g/100 g) as reported by Patricia et al. (2016).[Citation41] The moisture content is greatly influenced by the floral source used by the stingless bee as well as by the harvesting season, maturation level in the honeypots, weather and environmental conditions.[Citation42]
The mean moisture content for the stingless bee honeys was significantly higher than that of Apis mellifera honey (14.74 g/100 g of honey). The difference in the moisture contents of Apis mellifera and stingless bee honey may be due to the different preferred food sources used by the stingless bee and honeybee to produce the honey. According to Suntiparapap et al. (2012) [Citation43], the honeybee has behaviour to vaporize their honey while stingless bee does not. That action can affect the moisture content of the resulting honey. Additionally, different floral behaviours and floral sources might be contributing to the different moisture content. High water contents will shorten the shelf life of the honey due to fermentation during storage.[Citation44]
Water activity
The water activity ranged from 0.76 to 0.87 (). Acacia honey from both stingless bee species had significantly (p < 0.05) higher water activity than other honey samples. All honey samples exhibited high water activity (greater than 0.6), which means stingless bee honeys are susceptible to fermentation.[Citation45] Water activity is associated with the water content in honey. High water activity can promote microbial growth in the honey and caused unwanted fermentation.[Citation45] According to Abramovic et al. (2008) [Citation46], osmotolerant yeast, at least, requires a water activity of 0.6 to grow. Our results are consistent with the findings of Cavia et al. (2004) [Citation47] and Abramovic et al. (2008) [Citation46], who revealed that water activity is strongly correlated with water content in honey. Water activity in honey can be influenced by several factors such as botanical origin, the temperature in the hive and climate conditions during harvesting.[Citation48]
pH and free acidity
Stingless bee honeys have a sweet and sour taste than Apis mellifera honey due to their low pH and high free acidity values. It was reported that the free acidity of stingless bee honey ranged from 5.9 to 592 meq/kg honey, which is lower than Apis mellifera honey (12.3 to 32.6 meq/kg honey).[Citation49] The pH and free acidity values found in stingless bee honeys ranged from 3.17 to 3.40 and from 64.50 to 207.67 meq/kg of honey, respectively (). The wide variation in free acidity value might be due to the different floral composition and bee species. All honey samples showed low pH values, and among the tested samples, starfruit honeys had the lowest pH and highest free acidity values for both species. These results suggested that these honeys are better in terms of stability against microbial damage due to low pH. However, Apis mellifera honey exhibits significantly higher pH (3.55) and lower free acidity (38.17 meq/kg honey) values than stingless bee honeys. The main factor determining the acidity of the honey is the organic acids present in the sample.[Citation50] The amount of organic acids in the honey is related to the enzymatic action of glucose-oxidase on glucose, which involves the transformation of glucose into gluconic acid.[Citation50] In addition to that, fermentation also decreases the pH and increases the free acidity in the honey.[Citation8] Jimenez et al. (2016) [Citation45] documented higher pH and lower free acidity value in stingless bee honey produced by Scaptotrigona mexicana species from Mexico; the values were 3.50 to 3.96 and 32.90 to 35.10 meq/kg honey, respectively. This might be caused by the different floral sources, locations, weather and storage conditions.[Citation51]
Total soluble solids content (TSS)
The Brix value of the honey samples varied from 60.85 to 72.25 (). Acacia honey of stingless bee had significantly lower Brix values, 60.85 (Geniotrigona thoracica) and 66.25 (Heterotrigona itama), than Apis mellifera honey (76.70). The Brix values of the starfruit and gelam honeys from both species were not significantly different (p > 0.05). These results are consistent with those found for three species of stingless bee from Brazilian Mellipona (62.20–77.00) as reported by Lage et al. (2012). [Citation6] In contrast, Moo-Huchin et al. (2015) [Citation13] found higher TSS contents (72.80–77.30) in 27 stingless bee honey samples from the Yucatan Peninsula, Mexico. The difference might be caused by the different levels of sugar present in the honey. It has been reported that the Brix value is directly related to the level of sugar in the honey.[Citation42] This means our honey samples had lower sugar contents than the honeys tested by.[Citation13] Honeys with higher sugar contents will exhibit higher Brix values. In addition, the acid and mineral contents also contribute to the total soluble solids in honey.[Citation52]
Ash content
The ash contents of the honey samples ranged from 0.07 to 0.24 g/100 g of honey () for different species of stingless bee, only the gelam-derived honeys showed significantly different (p < 0.05) ash contents. The ash content is influenced by the amount of minerals present in the nectar.[Citation53] Variations in the ash contents of the honey samples might be caused by the different floral sources used by the bees during foraging activity.[Citation43] Honeys from Tetragonula laeviceps and 11 stingless bee species from Thailand presented higher ash contents, 0.26 to 0.04 g/100 of honey [Citation43] and 0.22 to 3.1 g/100 g [Citation12], respectively.
Colour characteristics
The colours of all the honey samples are depicted in . Gelam honey produced by Heterotrigona itama had a significantly lower L* value (73.98) than the other honeys. The colour of a honey sample is greatly influenced by the type of nectar used in honey production [Citation54] and the minerals present.[Citation55] Pollen types, phenolic compounds and 5-hydroxymethylfurfural have also been reported to influence the colour of honey.[Citation56] The honey from a stingless bee from Mexico, Scaptotrigona Mexicana, had an L* value of 15.90, which is lower than those of our stingless bee honeys, indicating the Mexican honey is darker in colour.[Citation45] This could be attributed to the different floral sources, species of bee and location of honey production.
Table 3. Colour characteristics of stingless bee honey from different floral origins
5-hydroxymethyl-2-furfural (5-HMF) content
5-HMF was detected in the gelam honey (0.11 mg/kg) and starfruit honey (0.14 mg/kg) from Heterotrigona itama (). None of the honeys from Geniotrigona thoracica contained any 5-HMF. These results suggested that all honey samples were of good quality. However, no significant differences (p > 0.05) were observed between the 5-HMF contents of the gelam and starfruit honey. Oddo et al. (2008) [Citation24] and Suntiparapap et al. (2012) [Citation43] reported 5-HMF contents ranging from 0.4 to 2.1 g/kg and 0.53 to 0.71 mg/kg of honey for stingless bee honeys collected from Australia and Thailand, respectively. The 5-HMF content increases as the age of the honey increase and under poor storage conditions (such as high temperature). [Citation57]
Sugar contents
The total sugar/carbohydrate contents ranged from 44.98 to 61.37 g/100 g of honey (). Acacia honey from both species had a significantly (p < 0.05) lower total sugar content than what was found in the other types of honeys. Kajobe (Citation2007) [Citation58] reported that the nectar composition is affected by the floral origin of the nectar, foraging behaviour and environmental factors. In terms of fructose, all tested stingless bee honeys contained higher fructose than glucose except starfruit and acacia from Geniotrigona thoracica. While, Apis mellifera honey demonstrated lower fructose contents than glucose contents. Almeida-Muradian et al. (2007) [Citation59] reported that honeys from the stingless bee species Melipona compressipes and Melipona seminigra merribae had fructose contents that were higher than their glucose contents; their reported fructose contents were 31.61 g/100 g and 29.33 g/100 g of honey, respectively. Sgariglia et al. (2010) [Citation60] also reported that stingless bee honey of Tetragonisca angustula fiebrigi and Plebeiawitmanni from Argentina had higher fructose contents than glucose contents; 39.98–45.05 g/100 g and 22.00–21.82 g/100 g of honey, respectively. De Sousa et al. (2016) [Citation42] reported findings analogous to those of Sgariglia et al. (2010) [Citation60], in which honeys from different species of stingless bee collected from semiarid regions in north-eastern Brazil had higher fructose contents (50.00–57.60 g/100 g) than glucose contents (38.10–45.70 g/100 g).
Table 4. Sugar content (g/100 g honey), fructose/glucose (F/G) and glucose/water (G/W) of stingless bee honey from different floral origins
Sucrose was not detected in all the stingless bee honey samples tested. Whereas Apis mellifera honey (3.86 g/100 g of honey) showed higher sucrose contents than stingless bee honey. Since honey formation is depending on invertase activity to convert sucrose from flower nectar into simple monosaccharides (fructose and glucose), low sucrose contents indicate that sucrose is completely converted into glucose and fructose, and the honey can be labelled as mature honey.[Citation50] Invertase enzymes are produced by hypopharyngeal glands of foraging bees and the amount dependent to the several factors such as age, diet and physiological stage of the bees, the strength of the colony, temperature and abundance of nectar flow.[Citation61]
The fructose/glucose ratio (F/G) was measured in this study to determine the crystallization ability of the honey.[Citation62] The F/G ratios of all the tested honey samples varied between 0.83 and 1.37, except for gelam honey (1.56) from Heterotrigona itama, which are lower than ratio found by Oddo et al. (2008) [Citation24] for Australian stingless bee honey (1.42). According to Dobre et al. (2012) [Citation63], the crystallization process is slower in honeys with F/G ratios higher than 1.3 and faster if the F/G ratio is less than 1.0. All stingless bee honey except starfruit honey from Geniotrigona thoracica had F/G ratios more than 1, which means they will crystallize slower than the starfruit honey. Other factors that can contribute to honey crystallization include storage temperature, the presence of foreign matter and the viscosity of the honey.[Citation64] Apart from the F/G ratio, glucose/water ratio (G/W) has been reported as an appropriate indicator for the prediction of honey crystallization.[Citation64] Honey crystallization is rapid when the G/W ratio greater than 2.0, and slow or null when the ratio is less 1.70.[Citation65] All stingless bee honey studied showed G/W ratio less than 1.70. This indicted that stingless bee honey will remain in fluid for a longer time than Apis honey which had G/W ratio more than 1.70.
Organic acids
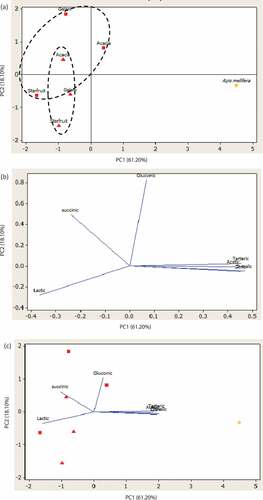
The organic acid contents in the six honey samples are shown in . In this study, only nine organic acids were analysed in each of the honey samples (). However, only four organic acids (gluconic, lactic, acetic and citric acids) were present in all the stingless bee honey samples. Formic and D-malic acids were not detected in the honeys produced by Geniotrigona thoracica from every floral source. Whereas the honeys produced by Heterotrigona itama from every floral source did contain all seven organic acids tested; the
Table 5. Organic acids concentration (g/kg of honey) of stingless bee honey from different floral origins
Figure 3. The HPLC Chromatogram of (a) organic acid standard mixture; (b) Apis mellifera honey; (c) Acacia honey (HT); (d) Acacia honey (HI); (e) Starfruit honey (HT); (f) Starfruit honey (HI); (g) Gelam honey (HT); (h) Gelam honey (HI). HI = Heterotrigona itama; HT = Geniotrigona thoracica. Peak identification: 1 = Gluconic; 2 = Tartaric; 3 = Formic; 4 = D-malic; 5 = Lactic; 6 = Acetic; 7 = Citric; 8 = Succinic; 9 = Fumaric
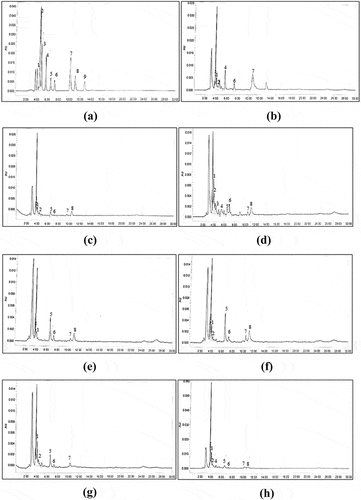
Gluconic acid is the main organic acid in all stingless bee honey samples with the mean content ranging from 0.07 to 1.48 g/kg of honey. Honey produced by Heterotrigona itama showed higher amounts of gluconic acid than those from Geniotrigona thoracica. Significant differences (p < 0.05) were observed in the gluconic acid contents (Heterotrigona itama) between honeys derived from different floral sources. For Geniotrigona thoracica species, starfruit honey displayed a significantly lower gluconic acid content than the acacia and gelam honeys. The differences in the gluconic acid contents might be due to the different amounts of glucose and enzymatic activity in the honey samples. As noted, in honey, gluconic acid is synthesised through glucose oxidase activity during the ripening process and is produced by Gluconobacter spp., which is found in the gut of bees.[Citation66] The contents found in this study are lower than those reported by Oddo et al. (2008) [Citation24] who reported 9.90 g/kg gluconic acid in Australian Trigona carbonaria honey. Sancho et al. (Citation2013) [Citation25] reported that Melipona favosa from Venezuela contained 63.60 g gluconic acid/kg honey, which is 43 times greater than the amount of gluconic acid produced by Heterotrigona itama in gelam honey (1.48 g/kg of honey). The wide variation of gluconic acid content between Melipona favosa and Heterotrigona itama might be due to the different amount of glucose and enzymatic activity in both honeys.
The organic acid is a minor component and present in low concentration in honey. Despite being in small amounts, it has an important contribution to organoleptic, physical and chemical properties of honey.[Citation66] Moreover, organic acid content greatly varies according to the botanical origin.[Citation67] Thus, organic acids profile can be a useful component to identify the honey origin. It was selected as variables for principle component analysis (PCA).
Antioxidant analyses
Total phenolic contents (TPC)
The total phenolic contents () of the six stingless bee honey samples varied between 27.33 and 55.86 mg GAE/100 g of honey. Since phenolic compounds are derived from plants, the phenolic contents in honey are greatly affected by the nectar source chosen by the bees and the bee species.[Citation68] In addition to these, there are several other factors that contribute to the phenolic contents such as harvest season, weather and processing conditions.[Citation16,Citation17] There were significant (p < 0.05) differences in the total phenolic contents among honeys of different floral origins for both species. Acacia honey (55.86 mg GAE/100 g of honey) produced by Geniotrigona thoracica and gelam honey (52.25 mg GAE/100 g of honey) produced by Heterotrigona itama had the highest TPC. When comparing TPC of honeys with the same floral origin produced by different species of stingless bee, only starfruit honey (Geniotrigona thoracica) showed no significant (p > 0.05) differences. Apis mellifera honey had a significantly (p < 0.05) lower TPC than those of acacia and starfruit honeys from Geniotrigona thoracica species and all honey types from Heterotrigona itama species. The differences in TPC content among stingless bee honey from different botanical origins might be related to the nectar composition. The amount and type of phenolic compounds in honey are greatly depend on the botanical origin.[Citation69]
Table 6. Total phenolic acid contents (TPC) (mg GAE/100g), total flavonoids content (TFC) (mg QE/100 g), antiradical scavenging activity (IC50) (mg/mL) and ferric reducing antioxidant power (FRAP) (µmol FeSO4.7H2O/100 g)
The TPC levels in this study are consistent with those reported by Oddo et al. (2008) [Citation24], who reported the phenolic contents of Australian Trigona carbonaria honey averaged 55.74 mg GAE/100 g of honey. Conversely, the TPC found in this study are much lower than those of Tanzanian stingless bee honeys from Melipona species (847.60 mg GAE/100 g) reported by Muruke (Citation2014) [Citation14] and those reported by Da Silva et al. (2013) [Citation70] in nine samples of Melipona subtinida honey (110 to 130 mg GAE/100 g). On the other hand, Mendonca et al. (2007) [Citation71] reported much lower TPCs in Brazilian Melipona subnitida honey (0.60 mg GAE/100 g of honey) than were found in our study. In another study, Duarte et al. (2012) [Citation72] reported a wide range of phenolic contents in honeys from four species of stingless bee in north-eastern Brazil (39.30 to 106.01 mg GAE/100 g). The differences in the phenolic contents of different types of honey are largely related to the floral origin of the honey. [Citation16,Citation34] Moreover, the constituents of the floral source are influenced by the type of the nectar and pollen used by bees, the geographical origins and the species of the honey-producing bee.[Citation30,Citation44,Citation73]
Total flavonoids content (TFC)
The total flavonoids contents () of all the tested stingless bee honey samples were lower than the phenolic contents with values ranging from 2.38 to 9.31 mg QE/100 g of honey. The starfruit honeys from both species had the highest TFC values. Apis mellifera honey (1.40 mg QE/100 g of honey) showed a significantly (p < 0.05) lower TFC value than all the stingless bee honeys from both species. All honey samples collected from different species and with different floral origins showed significantly different (p < 0.05) flavonoids contents. The TFC values found in this study are lower than those of Trigona carbonaria honey (10.02 mg QE/100 g of honey) from Australia reported by Oddo et al. (2008) [Citation24] and higher than that of stingless bee honey from the Brazilian Amazon as revealed by Mendonca et al. (2007) [Citation70] (1.7 mg QE/100 g of honey). On the other hand, Duarte et al. (2012) [Citation71] reported higher levels of flavonoids in honey from M. scutellaris (7.94–29.51 mg QE/100 g of honey) than were found in the honeys tested in this study. Similar to the phenolic contents, the variations in the flavonoids contents of the honey samples may be due to the different botanical and geographical origins of the nectars used by the bees.[Citation74,Citation75] According to Khalil et al. (2012) [Citation76], flavonoids are low-molecular-weight phenolic compounds that are responsible for the aroma and antioxidant activity of honey.
Antiradical scavenging activity (IC50)
The IC50 graph of all honey samples studied is illustrated in . Among the Malaysian stingless bee honeys evaluated, gelam and starfruit honeys produced by Heterotrigona itama had the lowest IC50 value (32.58 mg/mL) and the highest IC50 value (105.53 mg/mL), respectively (). This indicates that gelam honey has high antioxidant activity. The variations in the IC50 values of the honeys might be due to the differences in the phenolic contents and different types of phenolic compounds present [Citation15] since each phenolic acid has different scavenging activity.[Citation73,Citation77] All tested stingless bee honeys showed IC50 values lower than those of Apis mellifera honey (83.28 mg/mL) except for the acacia honey (97.24 mg/mL) from Geniotrigona thoracica and the starfruit honeys from both species. Significant differences (p < 0.05) amongst different floral types were only observed for honeys produced by Heterotrigona itama. When comparing honeys of the same floral origin from different species, significant differences (p < 0.05) were observed for all origins except the starfruit honey samples. Honeys with lower IC50 values had higher antiradical activities than honeys with higher IC50 values.[Citation69] Da Silva et al. (2013) [Citation70] reported low IC50 values for honey from Melipona subtinida from Brazil (10.60 to 12.90 mg/mL). In another study, Muruke (Citation2014) [Citation14] also reported lower IC50 values for honeys from the Tanzanian Melipona species with values ranging from 3.75 to 4.46 mg/mL. However,
Figure 4. The IC50 graph of stingless bee and Apis honeys. HI = Heterotrigona itama; HT = Geniotrigona thoracica; RSA = Radical scavenging activity
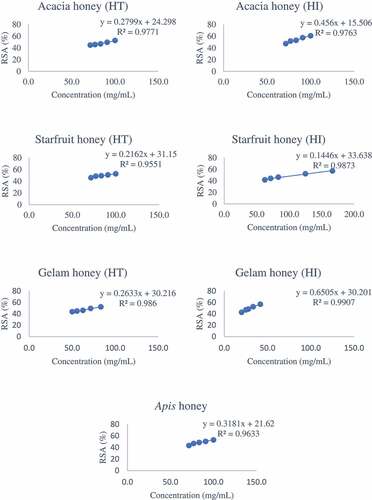
Mendonca et al. (2007) [Citation71] reported that Melipona subtinida honey had much lower IC50 values (0.006 mg/mL) that were found in our study.
Ferric reducing antioxidant power (FRAP assay)
The FRAP values of all tested stingless bee honey samples varied from 53.80 to 163.80 µmol FeSO4 7H2O/100 g of honey (). The highest FRAP value was exhibited by gelam honey from Heterotrigona itama and the lowest FRAP value was shown by acacia honey from Geniotrigona thoracica. Apis mellifera honeys had significantly (p < 0.05) lower FRAP values than all stingless bee honey tested. All stingless bee honey samples from different floral sources and different species had significantly different (p < 0.05) FRAP values. This might be caused by the different concentrations of phenolic compounds and flavonoids in the honey.[Citation78] Moreover, the types of phenolic acids and flavonoids present in the honey can influence the FRAP value, and honeys from different botanical origins have dissimilar profiles of phenolic and flavonoid compounds.[Citation40]
Pearson’s correlation
Significant positive correlation coefficients were observed between the IC50 value and the L* colour parameter and significant negative correlation between the FRAP value and the L* colour parameter (0.754 and −0.749, respectively) (). This indicates that the substances which give colour to honey (phenolics, flavonoids, minerals, 5-HMF and products of Maillard reaction) influence the antioxidant activity. A significant negative correlation was observed between the FRAP value and the IC50 value (−0.800). However, there is no significant correlation between either the TPC (−0.144) or the TFC (0.036) with the colour of the honey. This means the colour is not influenced by the TPC or TFC in Malaysian stingless bee honey as reported in many previous studies on honeybee honey (Apis mellifera) [Citation69,Citation79], which found that dark honeys contained high phenolic and flavonoid contents. No significant correlation was found between the IC50 and either the TPC (−0.154) or the TFC (0.176), indicating that the antioxidant activity is not solely dependent on the total phenolics and flavonoids content. The presence of other compounds such as Maillard reaction products, organic acids, amino acids, protein and ascorbic acid which also have antioxidant properties might contributed to the antioxidant activity via synergistic interaction with phenolic and flavonoid compounds in honey. Ahmed et al. (2014) [Citation51] and Idris et al. (2011) [Citation15] also found results similar to those reported here. Ash and 5-HMF content did not show any significant correlations with the colour L*, TPC, TFC, IC50 and FRAP parameters. There are no data on the correlation of ash content with colour and antioxidant properties available in the literature. Thus, no comparison was performed.
Table 7. Pearson’s Correlation coefficients (r) analysis among total phenolic contents, total flavonoids content, antiradical scavenging activity (IC50), ferric reducing antioxidant power (FRAP), colour, ash and HMF content of stingless bee honey
Multivariate analysis
Principle component analysis (PCA)
The PCA was performed to classify honey based on organic acids concentration (). Two principal components with eigenvalue more than 1 were extracted to determine the best parameters to classify honey samples. The first principal component (PC1) and second principal component (PC2) represented 61.20% and 18.10% of the variance, respectively. The PC1 was strongly correlated with the concentration of acetic, citric, D-malic and tartaric acids. The PC2 was mainly associated with gluconic and succinic acid. The cumulative variance for both principal components was 79.30%. Based on PC1, it can be observed that Apis mellifera honey was located alone at positive score of PC1 and well separated from the stingless bee honey samples. This means Apis mellifera honey had significantly different in concentration of organic acids than stingless bee honeys. This suggested that organic acids content (acetic, citric, tartaric and D-malic) contributed to the separation between stingless bee honey samples and Apis mellifera honey. According to PC2, honey from Heterotrigona itama (HI) clearly separated from Geniotrigona thoracica (HT) honey, regardless of the floral source involved. This can be explained by the higher content of gluconic and succinic acids present in HI honey than HT honey samples (). PC2 also explains the separation of the honey produce from different botanical origins, regardless of the bee species involved. Gelam and acacia honey were well separated from starfruit honey. This separation could be related to the lower values of lactic acid in starfruit honey when compared to the gelam and acacia honeys. This result suggested that organic acid content in stingless bee honey can be a useful parameter in classifying honey based on botanical origin and bee species.
Conclusion
The physicochemical characteristics, antioxidant properties and organic acids content of Malaysian stingless bee honeys from Heterotrigona itama and Geniotrigona thoracica were measured, and the findings indicated that the physicochemical characteristics, organic acids content and antioxidant properties in the honeys are greatly dependent on the botanical origin and bee species. The findings also proved that the physicochemical characteristics, organic acids content and antioxidant properties of stingless bee honey are significantly different than those of Apis mellifera honey (honeybee honey). Based on the IC50 value, gelam honey produced by Heterotrigona itama is a better potential source of antioxidant than honeys from acacia and starfruit from either of the studies species. PCA was able to discriminate among honey samples from different botanical origins and from the same botanical origin but produced by different stingless bee species as well. However, a wider study with a significant number of samples must be carried out to obtain more insights about the effect of botanical origin and bee species on the parameters studied. The results demonstrated that organic acids content can be used as a marker for determination of Malaysian stingless bee honey botanical origin as well as producing bee species.
Additional information
Funding
References
- Michener, C. D.;. The Meliponini. In Pot-Honey: A Legacy of Stingless Bee, 1st ed.; Patricia, V., Silvia, R. M. P., David, R., Eds.; Springer: London, New York, 2013; pp 3–17.
- Norowi, M. H.; Mohd, F.; Sajap, A. S.; Rosliza, J.; Suri, R. Conservation and Sustainable Utilization of Stingless Bees for Pollination Services in Agricultural Ecosystems in Malaysia. In Proceedings of International Seminar on Enhancement of Functional Biodiversity Relevant to Sustainable Food Production, Tsukuba, Japan, 2010; pp 1–11.
- Azmi, W. A.; Ghazi, R.; Nasharuddin, I. S. Morphological, Nest Architecture and Colony Characteristics of Stingless Bees (Hymenoptera; Apidae; Meliponini) from Tasik Kenyir, Terengganu. In Greater Kenyir Landscapes; Springer: Cham, 2019; pp 111–121. DOI:10.1007/978-3-319-92264-5_11.
- Codex Alimentarius Commission. Codex Standard for Honey; FAO: Rome, Italy, 2001.
- Ramanauskiene, K.; Stelmakiene, A.; Briedis, V.; Ivanauskas, L.; Valdas Jakštas, V. The Quantitative Analysis of Biologically Active Compounds in Lithuanian Honey. Food Chem. 2012, 132, 1544–1548. DOI: 10.1016/j.foodchem.2011.12.007.
- Lage, L. G. A.; Coelho, L. L.; Resend, H. C.; Tavares, M. G.; Campos, L. A. O.; Fernandes-Salomao, T. M. Honey Physichochemical Properties of Three Species of the Brazilian Melipona. Anais da Academia Brasileirade Ciencias. 2012, 84, 605–608. DOI: 10.1590/S0001-37652012005000051.
- Da Silva, P. M.; Gauche, C.; Gonzaga, L. V.; Costa, A. C. O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. DOI: 10.1016/j.foodchem.2015.09.051.
- Silvano, M. F.; Varela, M. S.; Palacio, M. A.; Ruffinengo, S.; Yamul, D. K. Physicochemical Parameters and Sensory Properties of Honeys from Buenos Aires Region. Food Chem. 2014, 152, 500–507. DOI: 10.1016/j.foodchem.2013.12.011.
- Subramanian, R.; Umesh Hebbar, H.; Rastogi, N. K. Processing of Honey: A Review. Int. J. Food Prop. 2007, 10, 127–143. DOI: 10.1080/10942910600981708.
- Bonveh´I, J. S.; Manzanares, A. B.; Vilar, J. M. S. Quality Evaluation of Broom Honey (Spartocytisussupranubius L) Produced in Tenerife (The Canary Islands). J. Sci. Food Agric. 2004, 84, 1097–1104. DOI: 10.1002/jsfa.1792.
- Vit, P.; Bogdanov, S. Composition of Venezuelan Honeys from Stingless Bees (Apidae: Meliponinae) and Apis Mellifera L. Apidologie. 1994, 25, 278–288. DOI: 10.1051/apido:19940302.
- Chuttong, B.; Chanbanga, Y.; Sringarmd, K.; Burgette, M. Physicochemical Profiles of Stingless Bee (Apidae: Meliponini) Honey from South East Asia (Thailand). Food Chem. 2016, 129, 149–155. DOI: 10.1016/j.foodchem.2015.06.089.
- Moo-Huchin, V. M.; González-Aguilar, G. A.; Lira-Maas, J. D.; Pérez-Pacheco, E.; Estrada-León, R.; Moo-Huchin, M. I.; Sauri-Duch, E. Physicochemical Properties of Meliponabeecheii Honey of the Yucatan Peninsula. J. Food Res. 2015, 4, 1–8. DOI: 10.5539/jfr.v4n5p25.
- Muruke, M. H. Assessment of Quality of Tanzanian Honey Based on Physicochemical Properties. J. Biol. Agric. Healthcare. 2014, 4, 22–32.
- Idris, Y. M. A.; Mariod, A. A.; Hamad, S. I. Physicochemical Properties, Phenolic Contents and Antioxidant Activity of Sudanese Honey. Int. J. Food Prop. 2011, 14, 450–458. DOI: 10.1080/10942910903243673.
- Baek, Y.; Kim, J. Y.; Baik, M.-Y.; Kim, D.-O.; Lee, H. Total Phenolic Contents and Antioxidant Activities of Korean Domestic Honey from Different Floral Sources. Food Sci. Biotechnol. 2015, 24, 1453–1457. DOI: 10.1007/s10068-015-0187-8.
- Shubharani, R.; Anita, M.; Mahesh, M.; Sivaram, V. Antioxidant and Antibacterial Activity of Different Floral Honeys from Western Ghats of Karnataka. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 104–108.
- Garjanovic, S. Z.; Alvarez-Suarez, J. M.; Novakovic, M. M.; Pastor, F. T.; Pezo, L.; Battino, M.; Suznjevic, D. Z. Comparative Analysis of Antioxidant Activity of Honey of Different Floral Sources Using Recently Developed Polarographic and Various Spectrophotometric Assays. J. Food Compost. Anal. 2013, 30, 13–18. DOI: 10.1016/j.jfca.2012.12.004.
- Erejuwa, O. O.; Siti, A. S.; Mohd, S. A. W. Honey: A Novel Antioxidant. Molecules. 2012, 17, 4400–4423. DOI: 10.3390/molecules17044400.
- Zhao, H. X.; Zhang, H. S.; Yang, S. F. Phenolic Compounds and Its Antioxidant Activities in Ethanolic Extracts from Seven Cultivars of Chinese Jujube. Food Sci. Hum. Wellness. 2014, 3, 183–190. DOI: 10.1016/j.fshw.2014.12.005.
- Abu Bakar, M. F.; Sanusi, S. B.; Bakar, A.; Ong, F. I.; Cong, O. J.; Mian, Z. Physicochemical and Antioxidant Potential of Raw Unprocessed Honey from Malaysian Stingless Bees. Pak. J. Nutr. 2017, 16, 888–894. DOI: 10.3923/pjn.2017.888.894.
- Kek, S. P.; Chin, N. L.; Yusof, Y. A.; Tan, S. W.; Chua, L. S. Classification of Entomological Origin of Honey Based on Its Physicochemical and Antioxidant Properties. Int. J. Food Prop. 2017a, 20(sup3), S2723–S2738. DOI: 10.1080/10942912.2017.1359185.
- Kek, S. P.; Chin, N. L.; Tan, S. W.; Yusof, Y. A.; Chua, L. S. Classification of Honey from Its Bee Origin via Chemical Profiles and Mineral Content. Food Anal. Methods. 2017b, 10(1), 19–30. DOI: 10.1007/s12161-016-0544-0.
- Oddo, L. P.; Heard, T. A.; Rodríguez-Malaver, A.; Pérez, R. A.; Fernández-Muiño, M.; Sancho, M. T.; Vit, P. Composition and Antioxidant Activity of Trigona Carbonaria Honey from Australia. J. med. food. 2008, 11, 789–794. DOI: 10.1089/jmf.2007.0724.
- Sancho, M. T.; Mato, I.; Huidobro, J. F.; Fernandez-Muino, M. A.; Pascual-Mate, A. Nonaromatic Organic Acids in Honeys. In Pot-Honey: A Legacy of Stingless Bee, 1st ed.; Patricia, V., Silvia, R. M. P., David, R., Eds.; Springer: London, New York, 2013; pp 447–457.
- Del Nozal, M. J.; Bernal, J. L.; Marinero, P.; Diego, J. C.; Frechilla, J. I.; Higes, M.; Llorente, J. High Performance Liquid Chromatographic Determination of Organic Acids in Honeys from Different Botanical Origin. J. Liq. Chromatogr. Relat. Technol. 1998, 21(20), 3197–3214. DOI: 10.1080/10826079808001268.
- Nozal, M. J.; Bernal, J. L.; Diego, J. C.; Gómez, L. A.; Higes, M. HPLC Determination of Low Molecular Weight Organic Acids in Honey with Series‐Coupled Ion‐Exclusion Columns. J. Liq. Chromatogr. Relat. Technol. 2003, 26(8), 1231–1253. DOI: 10.1081/JLC-120020107.
- Wilkins, A. L.; Lu, Y. Extractives from New Zealand Honeys. 5. Aliphatic Dicarboxylic Acids in New Zealand Rewarewa (Knightea Excelsa) Honey. J. Agric. Food Chem. 1995, 43(12), 3021–3025. DOI: 10.1021/jf00060a006.
- Kaškonienė, V.; Venskutonis, P. R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9(6), 620–634. DOI: 10.1111/j.1541-4337.2010.00130.x.
- Silva, T. M. S.; Dos Santos, F. P.; Evangelista-Rodrigues, A.; Da Silva, E. M. S.; Da Silva, G. S.; de Novais, J. S.; Camara, C. A. Phenolic Compounds, Melissopalynological, Physicochemical Analysis and Antioxidant Activity of Jandaíra (Meliponasubnitida) Honey. J. Food Compost. Anal. 2013, 29, 10–18. DOI: 10.1016/j.jfca.2012.08.010.
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 19th ed.; AOAC: Washington DC, 2012.
- Chirife, J.; Zamora, M. C.; Motto, A. The Correlation between Water Activity And% Moisture in Honey: Fundamental Aspects and Application to Argentine Honeys. J. Food Eng. 2006, 72(3), 287–292. DOI: 10.1016/j.jfoodeng.2004.12.009.
- Colucci, G.; De Vito, V.; Varricchio, E.; De Cunzo, F.; Coccia, E. Identification of Traceability Markers in Italian Unifloral Honeys of Different Botanical Origin. J. Nutr. Food Sci. 2016, 6, 462. DOI: 10.4172/2155-9600.1000462.
- Kamboj, R.; Bera, M. B.; Nanda, V. Evaluation of Physicochemical Properties, Trace Metal Content and Antioxidant Activity of Indian Honeys. Int. J. Food Sci. Technol. 2013, 48, 578–587. DOI: 10.1111/ijfs.12002.
- Bogdanov, S. Harmonised Methods of the International Honey Commission; IHC, 2009; http://www.ihc-platform.net/ihcmethods2009.pdf
- Gökmen, V.; Acar, J. Simultaneous Determination of 5-Hydroxymethylfurfural and Patulin in Apple Juice by Reversed-Phase Liquid Chromatography. J. Chromatogr. A. 1999, 847, 69–74. DOI: 10.1016/S0021-9673(99)00133-8.
- Sua´rez-Luquea, S.; Matoa, I.; Huidobroa, J. F.; Simal-Lozanoa, J.; Sancho, T. Rapid Determination of Minority Organic Acids in Honey by High-Performance Liquid Chromatography. J. Chromatogr. A. 2002, 955(2), 207–214. DOI: 10.1016/S0021-9673(02)00248-0.
- Shrivastava, A.; Gupta, V. B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chronicles Young Sci. 2011, 2(1), 21. DOI: 10.4103/2229-5186.79345.
- Meda, A.; Lamien, C. E.; Romito, M.; Millogo, J.; Nacoulma, O. G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. DOI: 10.1016/j.foodchem.2004.10.006.
- Khalil, M. I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S. A.; Gan, S. H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, C921–C928. DOI: 10.1111/j.1750-3841.2011.02282.x.
- Patricia, V.; Silvia, R. M. P.; César Vargas, J.; Andino, M.; Maza, F. Chemical Composition of Ecuadorian Commercial Pot-Honeys: Trigona Fuscipennis “Abeja De Tierra”, Melipona Mimetica “Bermejo” and Scaptotrigona Ederi “Catiana”. Mellifera. 2016, 16(2), 13–24.
- De Sousa, J. M. B.; de Souza, E. L.; Marques, G.; de Toledo Benassi, M.; Gullón, B.; Pintado, M. M.; Magnani, M. Sugar Profile, Physicochemical and Sensory Aspects of Monofloral Honeys Produced by Different Stingless Bee Species in Brazilian Semi-Arid Region. LWT Food Sci. Technol. 2016, 65, 645–651. DOI: 10.1016/j.lwt.2015.08.058.
- Suntiparapop, K.; Prapaipong, P.; Chantawannakul, P. Chemical and Biological Properties of Honey from Thai Stingless Bee (Tetragonula Leaviceps). J. Apic. Res.. 2012, 51, 45–52. DOI: 10.3896/IBRA.1.51.1.06.
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Rossi, D.; Paganetto, G.; Acchetti, G. Ecuadorian Stingless Bee (Meliponinae) Honey: A Chemical and Functional Profile of an Ancient Health Product. Food Chem. 2009, 114, 1413–1420. DOI: 10.1016/j.foodchem.2008.11.023.
- Jimenez, M.; Beristain, C. I.; Azuara, E.; Mendoza, M. R.; Pascual, L. A. Physicochemical and Antioxidant Properties of Honey from Scaptotrigonamexicana Bee. J. Apic. Res. 2016, 55, 151–160. DOI: 10.1080/00218839.2016.1205294.
- Abramovič, H.; Jamnik, M.; Burkan, L.; Kač, M. Water Activity and Water Content in Slovenian Honeys. Food Control. 2008, 19, 1086–1090. DOI: 10.1016/j.foodcont.2007.11.008.
- Cavia, M. M.; Fernáez‐Muiño, M. A.; Huidobro, J. F.; Sancho, M. T. Correlation between Moisture and Water Activity of Honeys Harvested in Different Years. J. Food Sci. 2004, 69(5), C368–C370. DOI: 10.1111/j.1365-2621.2004.tb10699.x.
- Wilczyńska, A.; Ruszkowska, M. Water Activity and Colour Parameters Changes during Storage of Linden and Buckwheat Honeys. ZeszytyNaukoweAkademiiMorskiej w Gdyni. 2014, 84, 174–181.
- Nordin, A.; Sainik, N. Q. A. V.; Chowdhury, S. R.; Saim, A. B.; Idrus, R. B. H. Physicochemical Properties of Stingless Bee Honey from around the Globe: A Comprehensive Review. J. Food Compost. Anal. 2018, 91–102. DOI: 10.1016/j.jfca.2018.06.002.
- Dardon, J. M.; Maldonado-Aguilera, C.; Enriquez, E. The Pot-Honey of Guatemala Bees. In Pot-Honey: A Legacy of Stingless Bee, 1st ed.; Patricia, V., Silvia, R. M. P., David, R., Eds.; Springer: London, New York, 2013; pp 3–17.
- Ahmed, M.; Khiati, B.; Meslem, A.; Aissat, S.; Djebli, N. Evaluation of Physicochemical and Antioxidant Properties of Raw Honey from Algeria. J. Microbial. Biochem. Technol. 2014, 2–6. DOI: 10.4172/1948-5948.S4-006.
- A-Rahaman, N. L.; Chua, L. S.; Sarmidi, M. R.; Aziz, R. Physicochemical and Radical Scavenging Activities of Honey Samples from Malaysia. Agric. Sci. 2013, 4, 46–51. DOI: 10.4236/as.2013.45B009.
- Belay, A.; Solomon, W. K.; Bultossa, G.; Adgaba, N.; Melaku, S. Physicochemical Properties of the Harenna Forest Honey, Bale, Ethiopia. Food Chem. 2013, 141, 3386–3392. DOI: 10.1016/j.foodchem.2013.06.035.
- Olga, E.; María, F. G.; Carmen, S. M. Differentiation of Blossom Honey and Honeydew Honey from Northwest Spain. Agriculture. 2012, 2, 25–37. DOI: 10.3390/agriculture2010025.
- Alqarni, A. S.; Owayss, A. A.; Mahmoud, A. A.; Hannan, M. A. Mineral Content and Physical Properties of Local and Imported Honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014, 18, 618–625. DOI: 10.1016/j.jscs.2012.11.009.
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E. A.; Silici, S.; Kolayli, S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015, 180, 133–141. DOI: 10.1016/j.foodchem.2015.02.024.
- Ahmed, M.; Imtiaz Shafiq, M.; Khaleeq, A.; Huma, R.; Abdul Qadir, M.; Khalid, A.; Samad, A. Physiochemical, Biochemical, Minerals Content Analysis, and Antioxidant Potential of National and International Honeys in Pakistan. J. Chem. 2016, 1–10. DOI: 10.1155/2016/8072305.
- Kajobe, R.;. Botanical Sources and Sugar Concentration of the Nectar Collected by Two Stingless Bee Species in a Tropical African Rain Forest. Apidologie. 2007, 38, 110–121. DOI: 10.1051/apido:2006051.
- Almeida-Muradian, L. B. D.; Matsuda, A. H.; Bastos, D. H. M. Physicochemical Parameters of Amazon Melipona Honey. Quím. Nova. 2007, 30, 707–708. DOI: 10.1590/S0100-40422007000300033.
- Sgariglia, M. A.; Vattuone, M. A.; Vattuone, M. M. S.; Soberón, J. R.; Sampietro, D. A. Properties of Honey from Tetragoniscaangustulafiebrigi and Plebeiawittmanni of Argentina. Apidologie. 2010, 41, 667–675. DOI: 10.1051/apido/2010028.
- Oddo, L. P.; Piazza, M. G.; Pulcini, P. Invertase Activity in Honey. Apidologie. 1999, 30(1), 57–65. DOI: 10.1051/apido:19990107.
- Nayik, G. A.; Dar, B. N.; Nanda, V. Physico-Chemical, Rheological and Sugar Profile of Different Unifloral Honeys from Kashmir Valley of India. Arabian J. Chem. 2015. DOI: 10.1016/j.arabjc.2015.08.017.
- Dobre, I.; Georgescu, L. A.; Alexe, P.; Escuredo, O.; Seijo, M. C. Rheological Behavior of Different Honey Types from Romania. Food Res. Int. 2012, 49, 126–132. DOI: 10.1016/j.foodres.2012.08.009.
- El Sohaimy, S. A.; Masry, S. H. D.; Shehata, M. G. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. DOI: 10.1016/j.aoas.2015.10.015.
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M. C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. DOI: 10.1016/j.foodchem.2013.10.097.
- Mato, I.; Huidobro, J. F.; Simal-Lozano, J.; Sancho, M. T. Analytical Methods for the Determination of Organic Acids in Honey. Crit. Rev. Anal. Chem. 2006, 36, 3–11. DOI: 10.1080/10408340500451957.
- Mohandes, E.; Sawsan, S. Organic Acids in Different Types of Egyptian Honey. J. Plant Prot. Path. 2011, 2(10), 865–872.
- Truchado, P.; Ferreres, F.; Bortolotti, L.; Sabatini, A. G.; Tomás-Barberán, F. A. Nectar Flavonolrhamnosides are Floral Markers of Acacia (Robiniapseudacacia) Honey. J. Agric. Food Chem. 2008, 56, 8815–8824. DOI: 10.1021/jf801625t.
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and Bioactive Properties of Six Honey Samples from Various Floral Origins from Tunisia. Arabian J. Chem. 2014, 11, 265–274. DOI: 10.1016/j.arabjc.2014.08.011.
- Da Silva, I. A. A.; Da Silva, T. M. S.; Camara, C. A.; Queiroz, N.; Magnani, M.; de Novais, J. S.; de Souza, A. G. Phenolic Profile, Antioxidant Activity and Palynological Analysis of Stingless Bee Honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. DOI: 10.1016/j.foodchem.2013.06.072.
- Mendonça, S.; Torres, E. A. F. S.; Bastos, D. H. M.; Dos Santos, M. C. M. Antioxidant Capacity and Phenolic Content of Stingless Bee Honey from Amazon in Comparison to Apis Bee Honey. In II International Symposium on Human Health Effects of Fruits and Vegetables: FAVHEALTH, Houston, TX (United State of America), 2007; Vol. 841, pp 483–486.
- Duarte, A. W. F.; Dos Santos Vasconcelos, M. R.; de Menezes, A. P. D.; Da Silva, S. C.; Oda-Souza, M.; López, A. M. Q. Composition and Antioxidant Activity of Honey from Africanized and Stingless Bees in Alagoas (Brazil): A Multivariate Analysis. J. Apic. Res. 2012, 51, 23–35. DOI: 10.3896/IBRA.1.51.1.04.
- Aljadi, A. M.; Kamaruddin, M. Y. Evaluation of the Phenolic Contents and Antioxidant Capacities of Two Malaysian Floral Honeys. Food Chem. 2004, 85, 513–518. DOI: 10.1016/S0308-8146(02)00596-4.
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant Activities and Total Phenolics of Different Types of Honey. Nutr. Res. 2002, 22, 1041–1047. DOI: 10.1016/S0271-5317(02)00406-2.
- Alzahrani, H. A.; Boukraa, L.; Bellik, Y.; Abdellah, F.; Bakhotmah, B. A.; Kolayli, S.; Sahin, H. Evaluation of the Antioxidant Activity of Three Varieties of Honey from Different Botanical and Geographical Origins. Global J. Health Sci. 2012, 4, 191–196. DOI: 10.5539/gjhs.v4n6p191.
- Khalil, M. I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M. A.; Islam, M. N.; Gan, S. H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules. 2012, 17, 11199–11215. DOI: 10.3390/molecules170911199.
- Kucuk, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia. Food Chem. 2007, 100, 526–534. DOI: 10.1016/j.foodchem.2005.10.010.
- Moniruzzaman, M.; Sulaiman, S. A.; Azlan, S. A. M.; Gan, S. H. Two-Year Variations of Phenolics, Flavonoids and Antioxidant Contents in Acacia Honey. Molecules. 2013, 18, 14694–14710. DOI: 10.3390/molecules181214694.
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M. G. Physicochemical Characterization and Antioxidant Activity of Commercial Portuguese Honeys. J. Food Sci. 2013, 78, C1159–C1165. DOI: 10.1111/1750-3841.12201.