ABSTRACT
The aim of this study was to investigate the quality changes of raw ground pork with the addition of allspice, bay leaf, black seed, caraway, cardamom, cloves or nutmeg extract and stored at 4°C. Lipid oxidation was evaluated by the peroxide value (POV), conjugated diene (CD) content, oxidation induction period (IP) by differential scanning calorimetry (DSC), thiobarbituric acid reactive substances (TBARS), and hexanal content; whereas protein oxidation by the thiol group content. Moreover, total viable aerobic bacteria count (TVC), Pseudomonas, Enterobacteriaceae, and lactic acid bacteria growths, pH and finally color of all samples were determined. POV, CD, TBARS and thiol group content were found to be highly correlated. Clove extract showed the highest antioxidant activity (1443 μM/g) and total phenolic content (TPC = 167 mg/g) and was the most effective antioxidant and antimicrobial agent in raw ground pork (TBARS = 0.31 mg/kg, POV = 5.1 meq O2/kg, thiol group content = 49 nmol/mg, IP = 68 min, TVC = 6.74). Cardamom and caraway also increased the oxidative stability of raw pork significantly (TBARS were equal to 0.3 and 0.28 mg/kg, POVs to 4.9 and 4.5 and thiol group content to 48 and 49 nmol/mg, respectively), despite their low antioxidant activities (72 and 300-fold lower than for cloves, respectively) and TPCs (1.2 and 2.4 mg/g). The results suggested that the application of natural antioxidants like spice extracts could enhance the stability and safety of raw ground pork, thus increasing its shelf-life.
Introduction
Meat quality is defined by nutritional value, organoleptic characteristics, technological properties, and its safety. Ensuring and maintaining the high quality of meat is a key element in its production, distribution, storage, and processing. The most deleterious effects on the meat quality result from the oxidation process and microbiological growth.[Citation1–Citation3] Lipid oxidation is the major cause of the deteriorations and reduced shelf life of meat products. The process is initiated in the unsaturated fatty acids fraction in subcellular membranes, and leads to the production of primary and secondary products such as hydroperoxides or aldehydes and ketones, affecting sensory and physicochemical properties, nutritional value and finally food safety.[Citation4] Since meat is a very complex matrix, other components, such as proteins, pigments, carbohydrates, and vitamins, undergo oxidative changes induced by the direct reactions with reactive oxygen species (ROS) or indirect reactions with the lipid oxidation products.[Citation2,Citation5] Among all amino acids, cysteine and methionine are especially susceptible to oxidation due to presence of the sulfur groups in their structures. Oxidative modifications of proteins (such as protein fragmentation and aggregation or protein–protein cross-linkage) can change their physical and chemical properties, including conformation, structure, solubility, susceptibility to proteolysis, and enzyme activities. Finally, protein modifications can affect the meat quality by lowering its nutritional value and technological properties.[Citation2,Citation6,Citation7] Other aspects of the oxidation process is discoloration as an effect of the pigment, mainly myoglobin, oxidative modifications.[Citation5]
The rate of oxidation reaction depends on many factors, like ratio of unsaturated fatty acids to saturated ones, which is strictly correlated with a type of tissue and breed, storage conditions (time and temperature) and/or technological procedures meat is subjected to (mincing, cooking, roasting, etc.).[Citation8–Citation10] Chicken meat tends to oxidize more rapidly than pork as it contains a relatively higher content of unsaturated fatty acids. Moreover, minced meat is more prone to oxidation than the whole muscle retail cuts since during grinding the surface of the product increases, which makes it more exposed to ROS.[Citation11,Citation12]
In addition, meat is susceptible to the undesired microorganism growth during processing and storage. The most common spoilage-related bacteria in meat are lactic acid bacteria.[Citation1,Citation3,Citation4] Various methods are applied to protect meat-derived products from oxidation and microbiological changes. The most commonly used are the application of chilled temperatures in the production and storage, and the use of vacuum during packaging.[Citation13] Addition of synthetic antioxidants as TBHQ, BHT, and BHA to meat products is also applied to extend their shelf-life. However, some concerns about their safety have led producers to search for the naturally occurring antioxidants.[Citation14] To meet consumer’s demand for healthier food, plant extracts have been applied for meat preservation during storage.[Citation15]
The addition of herbs and spices to meat products in order to counteract their spoilage has been used since ages. Nowadays, a great number of studies has shown the positive effect of plant extracts on meat quality which is the result of their high antioxidant and antimicrobial activities.[Citation4,Citation6,Citation10,Citation16–Citation21] Allspice, bay leaf, black seed, cardamom, caraway, cloves, and nutmeg are important aromatic spices used for food seasoning. Not only they give a special flavor to meat but also, by possessing high antioxidant and/or antimicrobial activities, they could maintain its oxidative and microbiological stability. Pork meat is the most commonly consumed meat type worldwide.[Citation22] Furthermore, to the authors’ best knowledge, there is a lack of comprehensive data on the influence of these spices on raw meat quality changes during refrigerated storage.
Thus, the aim of this study was to investigate the antioxidant and antimicrobial effects of these spices in raw ground pork meat stored at 4°C. The oxidative stability was determined by the TBARS, CD concentration, POVs, hexanal content, the oxidation IP by DSC and thiol group content. The latter defined protein oxidation. Such an approach, i.e., measurements of many oxidation parameters enables to give more insight into meat quality changes during storage. Moreover, microbiological activity, pH, and color of meat samples were determined.
Materials and methods
Chemicals
2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-tripyridyl-s-triazine (TPTZ), Folin-Ciocalteu’s reagent, 2-thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane, Ellman’s reagent – 5,5ʹ-dithiobis(2-nitrobenzoic acid) (DTNB), and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (Steinheim, Germany). Sodium carbonate (Na2CO3), aluminium chloride (AlCl3), sodium acetate trihydrate (C2H3NaO2•3H2O), acetic acid (C2H4O2), iron chloride hexahydrate (FeCl3•6H2O), hydrochloric acid (HCl), trichloroacetic acid (TCA), ethanol, methanol, trichloromethan, n-hexane, isooctane, and Tris buffer were obtained from POCh (Gliwice, Poland). Organic solvents of HPLC grade were also purchased from Sigma-Aldrich (Steinheim, Germany). Plate Count Agar, Violet Red Bile Glucose Agar, Buffered Peptone Water, de Man Rogosa & Sharpe Agar, and Glutamate Starch Phenol Red Agar were purchased from Yongxin Biological Technology Co., Ltd. (Yixing, Jiangsu, China).
Materials
Dried allspice, bay leaf, black seed, cardamom, caraway, cloves, and nutmeg were purchased from the local distributor of herbs and spices (Ciecierzyn, Polska). Pork neck (64.7 ± 3.2%moisture, 19.6 ± 0.5% protein, 13.6 ± 2 .3% fat) were provided by a local producer of meat (Swarzędz, Poland). Each type of meat was cut, deboned and minced through a 5 mm plate on the place. Then, it was put into insulated, iceboxes and transported to the laboratory in chilled condition (4–8°C) within half an hour.
Preparation of spice extracts
Powdered spices (15 g) were mixed with 225 mL of 50% aqueous ethanol in a closed container for 24 h on the magnetic stirrer in the dark. After filtration through 3HW Filtrak filter paper (Filtrak, Niederschlag Bärenstein, Germany) the antioxidant activity and phenolic content of the extract were analyzed. For meat sample preparation the spice extracts were freeze-dried.
Antioxidant properties of spice extracts
The antioxidant activities of spice extracts were determined by methods, which reflected the various mechanisms of antioxidant action including the scavenging of free radicals (DPPH• – diphenyl picrylhydrazyl radicals) or chelation of transition metal ions (ferric-reducing antioxidant power – FRAP method). A combination of these methods was implemented for assessing the antioxidant activity to get more insight into the antioxidative potential of the extracts studied.
The radical scavenging activity of the spice extracts was evaluated by the DPPH method according to the procedure described by Sánchez-Moreno et al.[Citation23] with some modifications.[Citation10] The DPPH• radical scavenging activities of the plant extracts were expressed as Trolox Equivalents Antioxidant Capacity – TEAC (DPPH) values in μM of Trolox equivalent (TE) per g of dry sample. TEAC (DPPH) values were calculated as the ratio of the slope of the linear plot for the scavenging of DPPH• radicals by the extract tested to the slope of the plot for DPPH• radicals scavenging by the antioxidant standard – the water-soluble vitamin E analog Trolox. The FRAP assay directly measures the ability of antioxidants to reduce a ferric tripyridyltriazine complex (Fe+3-TPTZ) to a ferrous complex (Fe+2-TPTZ) at low pH. The results were expressed as μM Trolox equivalent (TE) per g of dry weight.[Citation24]
Phenolic compound content of spice extract
TPC was determined according to the spectrophotometric method of Singleton and Rossi[Citation25] with Folin-Ciocalteu reagent and expressed as mg of gallic acid equivalents (GAE) per 1 g of dry weight. Total flavonoid content (TFC) was measured by the aluminum chloride method.[Citation21] An aliquot of 100 μL of spice extract was mixed with 2% aluminum chloride in methanol and allowed to stand for 15 min. Then, the absorbance was monitored at 415 nm. The results were read from the calibration curve for quercetin and expressed in mg of quercetin equivalent (QE) per 1 g of extract.
Preparation of meat samples
Freeze dried extract (prepared as above) was dissolved in water (60 mL) on the day of the application to meat (3 kg). Thus, the concentration of the spice extract expressed in the g of powdered spices used for the extraction per 100 g of meat was 0.5% (m/m). The following eight samples were prepared from raw ground pork: one control (C) (meat without any extract addition, only mixed with 60 mL of water) and seven treatments, namely, with allspice, bay leaf, black seed, cardamom, caraway, cloves, and nutmeg 0.5% (m/m). Then, each sample was mixed separately for 3 min, placed in a low-density polyethylene bag and stored at 4°C for 13 days. In order to avoid microbial contamination, a strict sanitation procedure was followed during the preparation of the meat samples. The following analyses were performed on 0, 3, 5, 7, 10 and 12 day of storage: TBARS, hexanal content, thiol group content, microbial count, pH, and color.
Extraction of lipid fraction from meat samples
Fat was extracted from the samples with chloroform: methanol mixture (2:1 v/v) according to the method by Folch et al.[Citation26] The fat samples were dried under a nitrogen flow and stored in a freezer at −20°C until the analysis. The rate of oxidation on DSC, CD concentration and POVs were determined from the samples.
Analysis of POVs
POVs were determined according to AOAC method (1999)[Citation27] and expressed as milliequivalents of active oxygen per kilogram of meat (meq O2/kg), as given in the formula: POV = (V*N)/m*1000, where V is milliliters of sodium thiosulfate solution (corrected to take into account the blank test); N is the normality of the sodium thiosulfate solution, and m is the mass, in grams, of the test portion.
Analysis of CDs
CD concentration was measured spectrophotometrically according to the Ti 1a-64 methods (AOCS, 2003)[Citation28] CDs were calculated using the formula: CD = 0.84* [(A/bc)- K0] where A is the absorbance at 233 nm, b is the cuvette length (cm), c is the sample concentration in isooctane (g/L), and K0 represents the absorptivity by acid or ester groups (0.07 for esters, 0.03 for acids).
DSC analysis
A Perkin Elmer differential scanning calorimeter 7 with a normal-pressure cell (Perkin Elmer Corp., Norwalk, CT, USA) equipped with an Intracooler II and running under Pyris software was used to examine oxidative stability of extracted fat samples. The calorimeter was calibrated using indium (m.p. 156.6°C, ∆Hf = 28.45 Jg-1) and n-dodecane (m.p. −9.65°C, ∆Hf = 216.73 J/g). Purifed nitrogen (99.99% purity) was the purge gas for the dry box and flowed under constant oxygen flow (20 ml/min). Fat samples of 8–10 mg were weighed into open aluminum pans (Perkin Elmer, No. 02190041) and placed in the equipment’s sample chamber. The reference was the same open and empty aluminum pan. Determination of oxidation induction time (isothermal) was carried out accordingly to the method described for oxidative stability measurements.[Citation29] The IP of fat samples was analyzed in at least triplicate repeats by isothermal heat flux at 140°C under a constant oxygen flow of 20 ml/min. The IP was determined by the tangent method as the intersection of the extrapolated baseline and the tangent line (leading edge) of the recorded exotherm.
TBARS determination
TBARS were determined by the method of Mielnik, Olsen, Vogt, Adeline, & Skrede (2006) [Citation30] with some modification.[Citation21] The TBARS values were calculated from the standard curve of MDA (malondialdehyde) which was prepared from 1,1,3,3-tetraethoxypropane and expressed in mg of MDA per kg of meat.
Hexanal content
Hexanal was determined by the method of Schieberle and Grosch[Citation31] with some modification. For isolation of volatile compounds, solid phase microextraction (SPME) was used. Briefly, 5 g of meat sample was placed in 20 ml headspace vials and spiked with internal standard [2H12]-hexanal (Sigma-Aldrich; Poznań, Poland) to reach 1 mg/kg concentration and capped with PTFE/silicon septa caps. Additionally, in the raw meat samples, 5 ml of saturated NaCl solution has been added. Extraction of volatiles was performed with CAR/PDMS/DVB fiber (Supelco) at 45°C during 30 min using CTC combipal autosampler (Agilent Technologies).
The chemical compounds were identified using gas chromatography and mass spectrometry with Agilent Technologies 7890A GC coupled to a 5975C MSD with a Supelcowax-10 column (30 m x 0.25 mm x 0.5 µm). Operating conditions for GC/MS were as follows: helium flow, 32.2 cm/s; oven conditions were as follows: initial oven temperature 40°C (1 min), raised at 9°C/min to 240°C and kept for 3 min. Mass spectra were recorded in an electron impact mode (70 eV) in a scan range of m/z 33–350. The transfer line was heated up to 260°C, and the ion source was kept at 220°C. For SPME fiber desorption, 260°C temperature was used with splitless injection. The identification of hexanal was performed by a comparison of mass spectra and retention indices (RI) with the NIST library and respective standard (Sigma-Aldrich; Poznań, Poland). The concentration in the sample was calculated from the peak area of the hexanal and its corresponding internal labeled standard [2H12]-hexanal obtained for selected ions: 56 and 64, respectively, and corrected with the response factor (Rf = 1.2)1. The Rf was calculated in the standard mixture of labeled and unlabeled compound in a known concentration of 1 mg/kg.
Thiol group content
Thiol group content was determined using Ellman’s method.[Citation32] Thiol group content was expressed based on the cysteine standard curve and expressed in nmol per mg of protein. Protein content was analyzed spectrophotometrically by the absorbance measurements of the filtrate at 280 nm using bovine serum albumin as a standard curve.
Microbiological analysis
TVCs were conducted as an indicator of microbial spoilage in the pork meat samples. The samples (10 g) were homogenized with 90 mL with sterile peptone water (1g/l) using an Ultra-Turrax T25 homogenizer (IKA, Germany). Serial decimal dilution was performed and plated onto a Standard Plate Count Agar (CM 463, Oxoid, Basingstoke, England). Incubation was performed at 30°C for 72 h. Bacterial counts were enumerated and expressed as log10 cfu/g.
For the Enterobacteriaceae counts, a 1 mL sample was inoculated into 15 mL of a molten selective VRBG medium (P-0256, BTL, Poland). After setting, a 10 mL overlay of the molten medium was added and incubation was conducted at 37°C for 24–48 h. The counts of Pseudomonas were determined on a solid Pseudomonas Agar medium (CM 0559, Oxoid, England) supplemented with Pseudomonas CFC Selective Agar Supplement (SR 0103, Oxoid, England) after incubation at 30°C for 48 h. De Man, Rogosa and Sharpe (MRS) Agar (CM 0361, Oxoid, England) was used for determining lactic acid bacteria (LAB) counts. MRS agar was also overlaid with a molten medium and incubation was conducted at 30°C for 48–72 h. An oxidase test was used to confirm lactic acid bacteria (MBO 266, Oxoid, England).
pH determination
pH values were measured using pH-meter Elmetron CP-551 (Elmetron, Zabrze, Poland) with the electrode ERH- 12–6 after calibration. The sample preparation was as follows: 5 g of meat sample was homogenized with 5 mL of distilled water using an Ultra-Turrax T25 homogenizer (16,000 rpm) (IKA, Germany).
Color measurements
Color measurements were carried out on the spectrophotometer CM-5 Konica Minolta in Specular Component Included (SCI) mode, using D65 as a source of light and 10° standard observer. The instrument was automatically calibrated (100% calibration) every start-up using the internal white plate. The zero calibration was performed manually before each analysis. Color was described in terms of the L* (lightness), a* (redness), and b* (yellowness) color space values. Three automatic measurements were made through polystyrene plate on the 30 mm diameter surface of each sample, and mean values of the color parameters were obtained. Each sample was prepared in triplicate.
Statistical analysis
Microbiological analysis was run in eight replicates. All other tests were run in triplicate. The statistical package Statistic 13.1 was used for statistical analysis. The influence of plant additions and time of storage on oxidative and microbial quality of raw pork was assessed using two-way-analysis of variance. Moreover, an analysis of covariance was performed. This analysis procedure is applied when looking at group effects on a continuous outcome (such as TBARS values) when another continuous explanatory variable (storage time) may also have an effect on the outcome. Comparison of the treatment means was based on Duncan’s T-test. In addition, Dunnett’s T-test was performed for a comparison of the treatments to the control. r-Pearson’s correlation coefficients were calculated for the results obtained. Differences were considered significant at the p ≤ 0.05 level. In all figures, vertical bars denote 0.95 confidence intervals, and means with the same superscript within the same day are not significantly different (p > 0.05).
Results and discussion
Antioxidant activity and phenolic content of spice extracts
The antioxidant activity of aqueous ethanol (50%) extracts of spices is shown in . Among tested spices, cloves were characterized by the highest antioxidant activity expressed as DPPH• radical scavenging activity and the ability to reduce metal ions (FRAP), which were equal 1443 μM TE/g and 1311 μM TE/g, respectively. Allspice and bay leaf showed also high antioxidant activity, but their DPPH• radical scavenging capacities were about 2.5 and 6-fold lower, respectively, and the FRAP values were around 4- and 8-fold lower comparing to cloves. Cardamom, black seed, caraway, and nutmeg were characterized by the lowest TEAC (DPPH) values (5.5–22 μM TE/g) as well as FRAP values (5.5–20 μM TE/g) among tested extracts. A high correlation was observed between the results of the DPPH and FRAP method (r = 0.99, p = 0). The results are generally in agreement with the studies of Przygodzka et al.[Citation33] who showed that extracts from cloves and allspice were highly antioxidant active in the ABTS method while cardamom had one of the lowest ABTS radical scavenging capacity. High antioxidant activity and ferric reducing power of clove extract (in 80% ethanol) which corresponds to its high phenolic content had been also shown by El-Maati et al.[Citation34].
Table 1. Antioxidant activity and phenolic compound content of ethanol in water (1/1v/v/) extracts
Phenolic compound content of spice extracts was expressed as TPC and TFC (). The values of TPC and TFC were positively correlated with the DPPH• radical scavenging capacity (r = 0.98 and r = 0.95, respectively) as well as with the FRAP values (r = 0.99 and r = 0.98, respectively). The highest TPC and TFC were reported for the clove extract (167 mg GAE/g and 26 mg QE/g, respectively), while the lowest for cardamom, caraway, black seed and nutmeg (1.2–3.9 mg GAE/g DW and 0.6–1.9 mg QE/g). The spice extracts could be listed by descending order of TPC values: cloves > allspice > bay leaf > nutmeg ≥ black seed ≈ caraway ≈ cardamom which is in agreement with the results reported by others.[Citation33,Citation35] Moreover, in this study, TFC values were highly correlated with TPC values with the r = 0.99. However, comparing to the results of Przygodzka et al.[Citation33] the contribution of TFC to TPC was various and ranged from 9% for allspice, through 23% for nutmeg and 52% for cardamom to 80% for caraway.
Lipid oxidation
Lipid oxidation is a very complex process leading to the formation of a wide variety of products. Hydroperoxides are the most commonly determined primary oxidation products, formed from the polyunsaturated fatty acids.[Citation7] This process is accompanied by the rearrangement of double bonds in order to stabilize the radical state resulting in the formation of the conjugated structures.[Citation36,Citation37] Therefore, CDs are also the primary lipid oxidation products.
includes POVs and CD concentration as the parameters of the first stage of lipid oxidation in raw ground pork with or without spice extracts stored at 4°C. Time and the treatments influenced significantly the POVs (p < 0.05). For all tested samples POV increased with time of storage. This increase was the most pronounced in the control samples (without any treatment) and on the 10th day of storage, POV reached the highest value (15.8 meq active O2/kg). The results are consistent with the study of Choe et al.[Citation7] who reported the maximum of POV in raw ground pork after 10 days of storage at 3°C. Balzan et al.[Citation38] also reported that the POV of raw pork sausages increased during storage at 2°C, but this increase was observed up to the 14th day of studies. The addition of spice extracts decreased significantly the extent of lipid oxidation in raw ground pork comparing to control, which resulted in the lower POV of treated samples. The most potent spice extracts were caraway, nutmeg, cardamom, and cloves. According to the analysis of covariance (Duncan’s T test), there were no statistically significant differences between these four samples and their POVs ranged between 1.98 and 7.68 meq active O2/kg during storage, while for the control sample POVs ranged from 3.83 to 15.7 meq active O2/kg. Surprisingly, the effectiveness of the extracts in meat matrix did not correspond to their antioxidant activities (), since among these extracts only clove extract was characterized by very high antioxidant activity. Bay leaf which also showed high antioxidant activity in DPPH and FRAP methods and high TPC value () inhibited lipid oxidation in pork meat, expressed as POV, in the smallest extent when compared to other treatments (). Juntachote et al.[Citation18] also showed that the antioxidant capacities and TPCs of plant extracts were not correlated with their efficacy in the meat system. In their study, samples treated with holy basil extract had higher POV than those treated with galangal extract despite the fact that holy basil extract showed two-fold higher TPC value than galangal.
Table 2. Effect of various treatments on peroxide values (POVs) and conjugated dienes (CDs) in raw ground pork during refrigerated storage (4°C) for 12 days
According to an analysis of covariance, all treated samples, apart from allspice addition, were characterized by the decreased CD content comparing to control during 12 days of storage at the chilled temperature (). Black seed, nutmeg, and cardamom reduced the most effectively the formation of CD in raw pork among all tested extracts (CD values ranged from 0.07% to 0.16%). The inhibitory effects of spice extracts on lipid oxidation, measured as CD parameter were in the following decreasing order: black seed ≈ nutmeg ≥ cardamom ≥ cloves ≈ bay leaf ≈ caraway > control > allspice. Significant effect of time on the CD formation was also observed (p < 0.05). In all samples, the CD concentration increased with increasing time of storage. In the studies of Choe et al.[Citation7] the increase of CD formation in raw pork was observed up to 10 days of storage and decreased thereafter for all treatments, whereas Lee et al.[Citation19] reported the CD values of raw ground pork peaked on the 4th day of storage at 4°C. Since both parameters, POV and CDs, reflects the formation of primary lipid oxidation products, there was a significant positive correlation observed between them with r-Pearson’s correlation coefficient equal to 0.76 (p < 0.05).
Lipid oxidation could be also investigated by the DSC analysis, since lipid oxidation is an exothermic reaction during which heat is released. The method does not require any chemicals for the analysis and is relatively time-saving. DSC is one of the methods of assessing the lipid stability, allowing the antioxidant activity of extracts under real conditions of the oxidation process to be investigated, as the measurement is carried out in an atmosphere of oxygen at a temperature of 140°C. Determined IP of the oxidation process reflect the behavior of fat and extracts under conditions similar to the thermal treatment of meat products used in practice. The DSC analyses were carried out also at comparable temperatures at which the Rancimat measurements are usually performed.
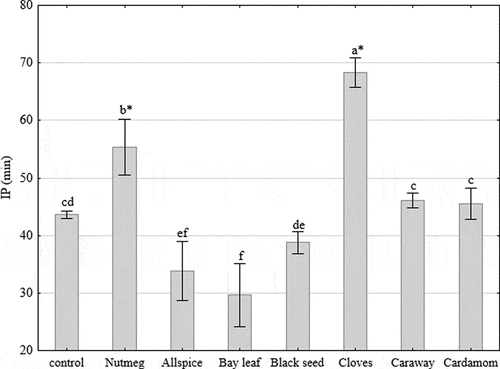
The results of DSC analysis were expressed as oxidation IP, measured in minutes (). The later the oxidation of lipid occurred the higher the IP values were. Thus, it could be observed that the most potent antioxidants among tested extracts, were cloves (IP = 68 min) and nutmeg (IP = 55 min) since their IPs were higher compared to control (IP = 44 min) about 57% and 27%, respectively. Bay leaf, allspice, and black seed showed lower IP values than the control samples which indicated that oxidation of lipids began faster in those samples than in the control one. These findings are consistent with the results listed in , which also indicate the nutmeg, cloves, caraway, and cardamom as the most potent antioxidants. Because the DSC technique is one of the most rarely used methods of assessing oxidative stability, especially in the case of animal fats, the comparison of the results with other authors is quite difficult.
Figure 1. Oxidation induction period (IP) of fat from raw ground pork samples treated with spice extracts determined by differential scanning calorimetry (DSC). Vertical bars denote 0.95 confidence intervals, and means with the same letter in superscript are not different (p > 0.05). Asterisk in superscript refers to a paired comparison (control compared with other natural additives is significant at the p < 0.05 level using Dunnett’s T-test, null hypothesis: treatment > Control)
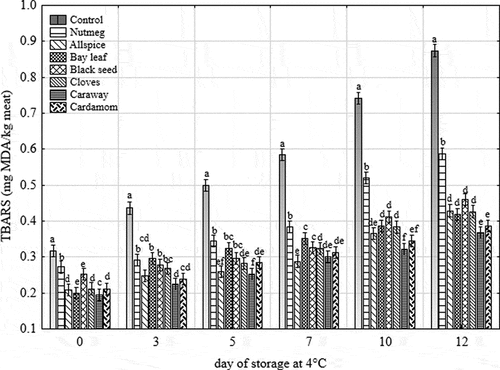
In the further stage of lipid oxidation secondary lipid oxidation products, such as aldehydes and ketones, are produced. These products react with the thiobarbituric acid and thus could be easily measured by the spectrophotometer at the visible region.[Citation21,Citation39] Analysis of covariance indicated a statistically significant effect of storage time and treatment on TBARS values (). Protective effect of plant and their extracts on the oxidative stability of lipids were presented by others.[Citation7,Citation10,Citation40,Citation41] In this study, TBARS values of all tested samples increased with time of storage. Oxidation stability was significantly higher (lower TBARS values) for all treated samples compared to control one at each day of the analysis (). As shown in the most potent inhibitors of lipid oxidation were caraway, cardamom, allspice, cloves, and bay leaf. Apart from nutmeg on 10th and 12th day of storage, all treated samples characterized by TBARS values lower than 0.5 mg MDA/kg meat, which is the threshold value for rancidity perception by consumers.[Citation19] The control sample would be therefore perceived as rancid after 5days of storage. Apart from some differences in the order of antioxidant effectiveness of tested extracts in the methods measuring lipid oxidation, r-Pearson’s correlation coefficient between POV and TBARS values was high and equal to 0.85 (p < 0.05), whereas between CD concentration and TBARS values was equal to 0.63 (p < 0.05).
Figure 2. Changes in TBARS of raw ground pork with different treatments during 12 days of chilled storage at 4°C. Vertical bars denote 0.95 confidence intervals, and means with the same superscript within the same day are not different (p > 0.05)
Hexanal – a volatile carbonyl compound – is also a secondary product of lipid oxidation which is formed from the 6-fatty acids in an oxidizing system.[Citation18,Citation37,Citation42] Hexanal content in raw ground pork is shown in . Addition of extracts and time of storage had an impact on the final content of that carbonyl compound. At the “0” time of storage, the highest hexanal concentration was observed in the control sample, whereas the lowest in allspice-treated sample. The content of hexanal increased sharply during storage and decreased thereafter. In the control sample as well as in nutmeg- and cardamom-treated samples hexanal concentration peaked on the 3rd day of chilled storage. The maximum level of hexanal was observed in allspice, bay leaf, and caraway samples after 5 days whereas in black seed-treated raw pork after 7 days of storage. In raw ground pork, with the addition of cloves, the decrease of that parameter was observed between 0 and 3rd day of storage. The effect of plant extract addition on the hexanal content in cooked ground pork was previously shown by Juntachote et al.[Citation18,Citation42] who noticed that the hexanal level increased as storage progress (up to 14th day of storage at 5°C). Because the authors studied oxidation changes in cooked meat the values of the parameter were lower than in our study.
Table 3. Effect of various treatments on hexanal content (mg/100 g meat) in raw ground pork during refrigerated storage (4°C) for 12 days
Protein oxidation
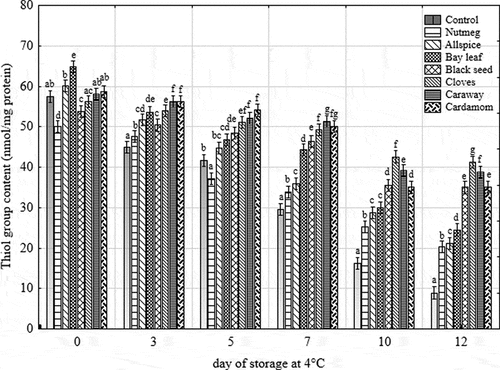
Protein oxidation as lipid oxidation also causes meat quality deterioration and has gained increasing attention in recent year.[Citation2,Citation40] The extent of protein oxidation was assessed in this study by measuring the concentration of thiol groups (). The loss of thiol groups is the most pronounced in the sample, which easily oxidized. Based on the results, it could be stated that both time and the addition of plant extract affected the content of thiol groups. With the increasing time of storage the loss of these species increased (the amount of thiol groups decreased). The lowest content of free thiol groups was observed for the control sample (from 57.4 to 8.9 nmol/mg protein during storage), whereas the highest for the caraway, cloves and cardamom-treated samples (58.6–35 nmol/mg protein). The samples could be ranged in the following order of increasing free thiol group content: control = nutmeg < allspice < bay leaf = black seed ≤ cardamom = cloves = caraway (according to Duncan’s test in the analysis of covariance). However, the results of Dunnett’s test showed that between 7th and 12th day of storage all treated samples were characterized by statistically lower protein oxidation than the control one. Choe et al.[Citation7] and Jia et al.[Citation40] also noticed that in the samples without any natural additives the loss of thiol groups was the most pronounced.
Figure 3. Changes in thiol group content of raw ground pork with different treatments during 12 days of chilled storage at 4°C. Vertical bars denote 0.95 confidence intervals, and means with the same superscript within the same day are not different (p > 0.05)
The results of protein oxidation were highly correlated with the results presenting lipid oxidation. Correlation coefficients were equal to −0.76 for the correlation with CD concentration, −0.85 for the correlation with POV and −0.86 for the correlation with TBARS.
Microbiological analysis
Microbial counts of all samples are shown in . Significant differences were observed in TVC during storage and among treatments (in the analysis of variance). The initial TVC was around 4 log cfu/g for all samples with the highest value for the control one (4.53 log cfu/g). Then, a statistically significant increase of TVC was observed. Similar results were previously shown by others.[Citation43]
Table 4. Effect of various treatments on microbiological quality of raw ground pork during refrigerated storage (4°C) for 12 days
The TVCs of all samples had exceeded the acceptable limit of 6.0 log cfu/g reported in other studies after 5 days of storage.[Citation39,Citation43] Thus the addition of plant extracts did not extend the shelf life of raw ground pork stored at 4°C. Nevertheless, the mean TVC for each treated sample was lower than for the control one with the cloves and cardamom being the most potent antimicrobial extracts.
Pseudomonas spp. counts were also affected by time and treatment. Pseudomonas are the Gram-negative bacteria responsible for the spoilage of aerobically stored fresh meat. Pseudomonas counts increased with increasing time which was consistent with the results reported by others.[Citation4,Citation39,Citation43] The lowest mean count was observed for raw ground pork with clove extract addition (6.89 cfu/g).
Enterobateriaceae counts of all samples increased over time as in the studies of others.[Citation4,Citation39,Citation43] Treatments had also a significant effect on Enterobacteriaceae counts with cloves being the most effective antimicrobial extract. LAB is a substantial part of the natural microflora of meat.[Citation4,Citation43] For allspice- and black seed-treated samples, the LAB count decreased during the first 3 days of storage and then increased. For other samples, including control, the LAB counts increased during the whole storage period reaching the lowest mean value in clove (4.10 cfu/g) and caraway (4.14 cfu/g) samples.
pH and color
The pH value can indicate the amount of microbial and chemical reactions influencing food deterioration.[Citation7] In the present study, pH values were affected by the time and treatments (). The value of pH of all tested samples increased with time (from 5.82 for bay leaf sample at 0 day to 6.85 for the nutmeg-treated one at 12 days). This increase was the most pronounced in the bay leaf-, nutmeg-, caraway, and cardamom-treated samples, respectively, about 16%, 15.4%, 15%, and 14%. Demeyer et al.[Citation44] indicated that pH values may increase because of reactions between protein and ions, resulting in the production of ammonia as storage progresses. The pH values of raw ground pork with the addition of clove extract were the most stable (only 3% change during 12 days). These results were consistent with the microbiological analysis showing cloves as the most potent antimicrobial spice. The level of pH in the control samples was similar to that reported by Hu et al.[Citation39]
Table 5. Effect of various treatments on pH value in raw ground pork during refrigerated storage (4°C) for 12 days
Meat color is the first criterion which consumers use to assess meat quality and acceptability.[Citation10] The results of the instrumental color measurements using L*a*b* color space () showed significant effects (p < 0.05) of treatments and storage time on the color parameters (L*, a*, b*). L* value increased in raw ground pork with allspice, bay leaf and nutmeg within the whole storage period, whereas in the control sample during first 7 days. This increase was observed in black seed, cloves, caraway, and cardamom up to 10th day of storage and, then L* values of the samples slightly decreased. The lightness of treated samples, apart from cardamom, was lower than the control one. Increase of pork lightness stored at 4°C was observed previously by Hu et al.[Citation39] during 15 days and by Jia et al.[Citation40] during 9 days of storage. However, Biswas et al.[Citation20] reported the L* value increased in raw ground pork during the first 3 days of storage at 4°C and thereafter the decrease of the lightness was noticed. Muzolf-Panek et al.[Citation10] indicated that the value of the L* parameter decreased over time in pork meatloaf during chilled storage.
Table 6. Effect of various treatments on color parameters in raw ground pork during refrigerated storage (4°C) for 12 days
The addition of spices caused a generally significant reduction in redness (a*) of raw pork, comparing to the control sample (). This was especially observed in the black seed- and bay leaf-treated samples. During the first days of storage, the value of a* parameter increased slightly and peaked on the 5th day (for cloves and nutmeg on the 3rd day) and thereafter the redness of pork decreased with time in all tested samples. This decrease was the most pronounced in black seed sample and on the 12th day of storage its a* value was negative (green color). The results of our study supported the findings of other authors[Citation20,Citation45] who reported an increase of a* value up to, respectively, 6th day and 8th of storage and indicated that the further changes could be due to the formation of metmyoglobin. Nevertheless, the effect of natural extract addition on the color parameters depends strongly on the type of the treatment.
The changes in yellowness (b* values) showed no obvious trend () as in the study of Carpenter et al.[Citation41] Nevertheless, time and type of the extract added were statistically significant. Black seed, caraway, and cardamom caused a decrease of the parameter, other treatments increased the yellowness of raw ground pork comparing to the control sample.
Conclusion
The results of this study indicated clearly that the addition of spice extracts increased oxidative and microbiological stabilities of raw ground pork stored at 4°C. However, the effectiveness of the particular extracts differed among the method applied. Thus, choosing only one parameter for the assessment of oxidative stability of meat could cause incomplete conclusions on the extent of the process. It is better to combine different methods for measuring lipid oxidation.[Citation36,Citation37] Nevertheless, based on our results it could be concluded that clove extract addition to raw pork provided the most potent antimicrobial and antioxidant effects to meat investigated by almost all methods (apart from hexanal content) and could be used to extend the shelf-life of the product. Clove beneficial effect was a result of high antioxidant activity and high polyphenolic compound content. Moreover, based on the results presented, it could be stated that also cardamom and caraway exhibited high antioxidant benefits to raw ground pork, which was supported neither by the antioxidant potentials nor by the phenolic contents of their extracts. Thus, further research in this area is needed to elucidate the source of their beneficial effects. Concluding, to meet consumer’s demand of healthier and safer food, natural antioxidants could be applied for meat preservation during storage.
Data Availability Statement
The data that support the findings of this study are available (privately) in Mendeley dataset at https://data.mendeley.com/datasets/p496hmyy44/draft?a=e6ba017a-11fc-4af6-9d40-41724b04aafa
Additional information
Funding
References
- Doulgeraki, A. I.; Ercolini, D.; Villani, F.; Nychas, G. J. E. Spoilage Microbiota Associated to the Storage of Raw Meat in Different Conditions. Int. J. Food Microbiol. 2012, 157(2), 130–141. DOI: 10.1016/j.ijfoodmicro.2012.05.020.
- Falowo, A. B.; Fayemi, P. O.; Muchenje, V. Natural Antioxidants against Lipid-Protein Oxidative Deterioration in Meat and Meat Products: A Review. Food Res. Int. 2014, 64, 171–181. DOI: 10.1016/j.foodres.2014.06.022.
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic Acid Bacteria and Their Controversial Role in Fresh Meat Spoilage. Meat Sci. 2015, 109, 66–74. DOI: 10.1016/j.meatsci.2015.04.014.
- Zhang, H.; Wu, J.; Guo, X. Effects of Antimicrobial and Antioxidant Activities of Spice Extracts on Raw Chicken Meat Quality. Food Sci. Hum. Wellness. 2016, 5(1), 39–48. DOI: 10.1016/j.fshw.2015.11.003.
- Estévez, M. Protein Carbonyls in Meat Systems: A Review. Meat Sci. 2011, 89(3), 259–279. DOI: 10.1016/j.meatsci.2011.04.025.
- Jongberg, S.; Skov, S. H.; Tørngren, M. A.; Skibsted, L. H.; Lund, M. N. Effect of White Grape Extract and Modified Atmosphere Packaging on Lipid and Protein Oxidation in Chill Stored Beef Patties. Food Chem. 2011, 128(2), 276–283. DOI: 10.1016/j.foodchem.2011.03.015.
- Choe, J.; Kim, H.; Kim, C. Effect of Persimmon Peel (Diospyros Kaki Thumb .) Extracts on Lipid and Protein Oxidation of Raw Ground Pork during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2017, 37(2), 254–263. DOI: 10.5851/kosfa.2017.37.2.254.
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of Freezing Temperature and Duration of Frozen Storage on Lipid and Protein Oxidation in Chicken Meat. Food Chem. 2010, 120(4), 1025–1030. DOI: 10.1016/j.foodchem.2009.11.042.
- Serpen, A.; Gökmen, V.; Fogliano, V. Total Antioxidant Capacities of Raw and Cooked Meats. Meat Sci. 2012, 90(1), 60–65. DOI: 10.1016/j.meatsci.2011.05.027.
- Muzolf-Panek, M.; Waśkiewicz, A.; Kowalski, R.; Konieczny, P. The Effect of Blueberries on the Oxidative Stability of Pork Meatloaf during Chilled Storage. J. Food Process. Preserv. 2016, 40, 899–909. DOI: 10.1111/jfpp.12668.
- O’Grady, M. N.; Monahan, F. J.; Burke, R. M.; Allen, P. The Effect of Oxygen Level and Exogenous α-Tocopherol on the Oxidative Stability of Minced Beef in Modified Atmosphere Packs. Meat Sci. 2000, 55(1), 39–45. DOI: 10.1016/S0309-1740(99)00123-0.
- Lee, M. A.; Choi, J. H.; Choi, Y. S.; Kim, H. Y.; Kim, H. W.; Hwang, K. E.; Chung, H. K.; Kim, C. J. Effects of Kimchi Ethanolic Extracts on Oxidative Stability of Refrigerated Cooked Pork. Meat Sci. 2011, 89(4), 405–411. DOI: 10.1016/j.meatsci.2011.05.004.
- Rojas, M. C.; Brewer, M. S. Effect of Natural Antioxidants on Oxidative Stability of Frozen, Vacuum-Packaged Beef and Pork. J. Food Qual. 2008, 31(2), 173–188. DOI: 10.1111/j.1745-4557.2008.00196.x.
- Shah, M. A.; Bosco, S. J. D.; Mir, S. A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Sci. 2014, 98(1), 21–33. DOI: 10.1016/j.meatsci.2014.03.020.
- Haugaard, P.; Hansen, F.; Jensen, M.; Grunert, K. G. Consumer Attitudes toward New Technique for Preserving Organic Meat Using Herbs and Berries. Meat Sci. 2014, 96(1), 126–135. DOI: 10.1016/j.meatsci.2013.06.010.
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The Effect of Essential Oil and Extract from Sage (Salvia Officinalis L.) Herbal Dust (Food Industry by-Product) on the Oxidative and Microbiological Stability of Fresh Pork Sausages. LWT. 2018, 89, 749–755. DOI: 10.1016/j.lwt.2017.11.055.
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Škaljac, S.; Ikonić, P.; Džinić, N.; Živković, N.; Jokanović, M.; Tasić, T.; Kravić, S. Effect of Nutmeg (Myristica Fragrans) Essential Oil on the Oxidative and Microbial Stability of Cooked Sausage during Refrigerated Storage. Food Control. 2015, 54, 282–286. DOI: 10.1016/j.foodcont.2015.02.007.
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The Antioxidative Properties of Holy Basil and Galangal in Cooked Ground Pork. Meat Sci. 2006, 72(3), 446–456. DOI: 10.1016/j.meatsci.2005.08.009.
- Lee, M. A.; Choi, J. H.; Choi, Y. S.; Han, D. J.; Kim, H. Y.; Shim, S. Y.; Chung, H. K.; Kim, C. J. The Antioxidative Properties of Mustard Leaf (Brassica Juncea) Kimchi Extracts on Refrigerated Raw Ground Pork Meat against Lipid Oxidation. Meat Sci. 2010, 84(3), 498–504. DOI: 10.1016/j.meatsci.2009.10.004.
- Biswas, A. K.; Chatli, M. K.; Sahoo, J. Antioxidant Potential of Curry (Murraya Koenigii L.) And Mint (Mentha Spicata) Leaf Extracts and Their Effect on Colour and Oxidative Stability of Raw Ground Pork Meat during Refrigeration Storage. Food Chem. 2012, 133(2), 467–472. DOI: 10.1016/j.foodchem.2012.01.073.
- Kaczmarek, A. M.; Muzolf-Panek, M.; Rudzińska, M.; Szablewski, T.; Cegielska-Radziejewska, R. The Effect of Plant Extracts on Pork Quality during Storage. Ital. J. Food Sci. 2017, 29, 644–656.
- Bedford, D.; Claro, J.; Giusti, A. M.; Karumathy, G.; Lucarelli, L.; Mancini, D.; Marocco, E.; Milo, M.; Yang, D. Biannual Report On Global Food Markets, Food Outlook. 2017. https://doi.org/ISSN1560-8182
- Sánchez-Moreno, C.; Larrauri, J. A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficienc Y of Polyphenols. J. Sci. Food Agric. 1998, 76(2), 270–276. DOI: 10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9.
- Benzie, I. F. F.; Strain, J. J. Ferric Reducing (Antioxidant) Power as a Measure of Antioxidant Capacity: The FRAP Assay. Methods Enzym. 1999, 299, 15–36.
- Singleton, V. L.; Rossi, J. A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16(3), 144 LP–158.
- Folch, J.; Lees, M.; Stanley, G. H. S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tisues. J. Biol. Chem. 1957, 226(1), 497–509.
- AOAC International. Peroxide value of oils and fats. In Official Methods of Analysis of AOAC International; Cunniff, P., Ed.; AOAC International: Gaithersburg, USA, 1999, vol. 2, chapter 41:9B.
- AOCS. Spectrophotometric Determination of Conjugated Dienoic Acid. In Official Methods and Recommended Practices of the American Oil Chemists’; AOCS Press: Champaign, IL, USA, 2003; Vol. 2009, pp 1–2.
- ISO 11357-6. Plastics — Differential Scanning Calorimetry (DSC) — Part 6. Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT), third ed., 2018, ISO, Switzelralnd.
- Mielnik, M. B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape Seed Extract as Antioxidant in Cooked, Cold Stored Turkey Meat. LWT Food Sci. Technol. 2006, 39(3), 191–198. DOI: 10.1016/j.lwt.2005.02.003.
- Schieberle, P.; Grosch, W. Quantitative Analysis of Aroma Compounds in Wheat and Rye Bread Crusts Using a Stable Isotope Dilution Assay. J. Agric. Food Chem. 1987, 35(2), 252–257. DOI: 10.1021/jf00074a021.
- Ellman, G. L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82(1), 70–77. DOI: 10.1016/0003-9861(59)90090-6.
- Przygodzka, M.; Zielińska, D.; Ciesarová, Z.; Kukurová, K.; Zieliński, H. Comparison of Methods for Evaluation of the Antioxidant Capacity and Phenolic Compounds in Common Spices. LWT - Food Sci. Technol. 2014, 58(2), 321–326. DOI: 10.1016/j.lwt.2013.09.019.
- El-Maati, M. F. A.; Mahgoub, S. A.; Labib, S. M.; Al-Gaby, A. M. A.; Ramadan, M. F. Phenolic Extracts of Clove (Syzygium Aromaticum) with Novel Antioxidant and Antibacterial Activities. Eur. J. Integr. Med. 2016, 8(4), 494–504. DOI: 10.1016/j.eujim.2016.02.006.
- Shan, B.; Cai, Y. Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53(20), 7749–7759. DOI: 10.1021/jf051513y.
- Xia, W.; Budge, S. M. Techniques for the Analysis of Minor Lipid Oxidation Products Derived from Triacylglycerols: Epoxides, Alcohols, and Ketones. Compr. Rev. Food Sci. Food Saf.. 2017, 16(4), 735–758. DOI: 10.1111/1541-4337.12276.
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A Review of Analytical Methods Measuring Lipid Oxidation Status in Foods: A Challenging Task. Eur. Food Res. Technol. 2013, 236(1), 1–15. DOI: 10.1007/s00217-012-1866-9.
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Zabalza, I. B.; Rodriguez-Estrada, M. T.; Novelli, E.; Fasolato, L. Effect of Phenols Extracted from a By-Product of the Oil Mill on the Shelf-Life of Raw and Cooked Fresh Pork Sausages in the Absence of Chemical Additives. LWT - Food Sci. Technol. 2017, 85, 89–95. DOI: 10.1016/j.lwt.2017.07.001.
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of Chitosan Nanoparticles Loaded with Cinnamon Essential Oil on the Quality of Chilled Pork. LWT - Food Sci. Technol. 2015, 63(1), 519–526. DOI: 10.1016/j.lwt.2015.03.049.
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant Activity of Black Currant (Ribes Nigrum L.) Extract and Its Inhibitory Effect on Lipid and Protein Oxidation of Pork Patties during Chilled Storage. Meat Sci. 2012, 91(4), 533–539. DOI: 10.1016/j.meatsci.2012.03.010.
- Carpenter, R.; O’Grady, M. N.; O’Callaghan, Y. C.; O’Brien, N. M.; Kerry, J. P. Evaluation of the Antioxidant Potential of Grape Seed and Bearberry Extracts in Raw and Cooked Pork. Meat Sci. 2007, 76(4), 604–610. DOI: 10.1016/j.meatsci.2007.01.021.
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The Effect of Dried Galangal Powder and Its Ethanolic Extracts on Oxidative Stability in Cooked Ground Pork. LWT - Food Sci. Technol. 2007, 40(2), 324–330. DOI: 10.1016/j.lwt.2005.08.008.
- Lorenzo, J. M.; Sineiro, J.; Amado, I. R.; Franco, D. Influence of Natural Extracts on the Shelf Life of Modified Atmosphere-Packaged Pork Patties. Meat Sci. 2014, 96(1), 526–534. DOI: 10.1016/j.meatsci.2013.08.007.
- Demeyer, D. I.; Vandekerckhove, P.; Moermans, R. Compounds Determining PH in Dry Sausage. Meat Sci. 1979, 3(3), 161–167. DOI: 10.1016/0309-1740(79)90033-0.
- O’Grady, M. N.; Carpenter, R.; Lynch, P. B.; O’Brien, N. M.; Kerry, J. P. Addition of Grape Seed Extract and Bearberry to Porcine Diets: Influence on Quality Attributes of Raw and Cooked Pork. Meat Sci. 2008, 78(4), 438–446. DOI: 10.1016/j.meatsci.2007.07.011.
