 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A simple microwave-assisted method for extracting antioxidant phenolic compounds from emblic (Phyllanthus emblica L.) fruits was developed and optimized with response surface methodology (RSM). The influence of process conditions including microwave power, irradiation time, ethanol concentration, and solvent-to-solid ratio on the extraction of total phenolic content was analyzed by using a second-order regression equation. The optimum extraction parameters were as follows: microwave power, 480 W; irradiation time, 29 s; ethanol concentration, 66%; liquid-to-solid ratio, 25 mL/g. Under these conditions, the recovery of total phenolic content was 133.58 ± 15.61 mg gallic acid equivalent/g dry weight (DW). The extracts were also analyzed using HPLC-ESI-TOF-MS to identify eight compounds including hamamelitannin, mucic acid-1,4-lactone-3-O-gallate, ellagic acid, and isocorilagin. To our knowledge, this is the first report of hamamelitannin in emblic fruit. The extracts exhibited strong antioxidant capacities in DPPH and ABTS+ radical scavenging assays and showed an excellent inhibition of lipid peroxidation and reducing property. These findings illustrated that the optimized microwave-assisted extraction method was efficient in extracting phenolic compounds with high antioxidant activity from emblic fruits. It was suggested that emblic fruits may be a new potential source as natural antioxidant agents applied in food industry.
Introduction
Reactive oxygen species (ROS) play important roles in either the initiation or progression of carcinogenesis by oxidative stress in the aerobic metabolism.[Citation1] The strong oxidative stress causes lipid peroxidation, DNA damage, and denaturation of proteins. Our body has developed sophisticated antioxidant systems against oxidative stress through the antioxidant nutrients.[Citation1] Antioxidants are a series of bioactive compounds that are able to capture free radicals to reduce risks of aging and chronic cardiovascular diseases.[Citation2] Synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tertiary butylhydroquinone (TBHQ) have been used for reducing oxidative stress.[Citation1] However, due to the potential toxicity and carcinogenicity, the uses of these synthetic antioxidants have raised significant concerns about the safety problems.[Citation3] As a consequence, more attention has been placed on exploiting natural and safe antioxidants from plant and agricultural by-products as health-promoting additives.[Citation4] There has been an increasing demand for phytochemicals as ingredients in food, pharmaceuticals, dietary supplements, and cosmetics.[Citation5] However, extraction methods and conditions can significantly affect the yield, quality, and bioactivity of phytochemicals from plant.[Citation2] To identify phytochemicals, simple, efficient and environment-friendly methods for extraction are needed.
Phyllanthus emblica L., commonly known as emblic, is one of the most important traditional medicinal plants from the family Euphorbiaceae. This species grows widely in tropical and subtropical areas of China, India, Indonesia, and the Malay Peninsula.[Citation6,Citation7] Emblic fruit is well accepted by consumers for its special taste and used extensively as an antioxidant, antiaging, anticancer, anti-inflammatory, antimicrobial, and immunomodulatory agent in many traditional medicinal systems, such as Chinese herbal medicine, Tibetan medicine, and Ayurvedic medicine.[Citation8–Citation12] Recent studies show that the fruit is rich in polyphenols, ascorbic acid, tannins, flavones, polysaccharides, and other bioactive substances.[Citation10–Citation20] Emblic fruit could be considered as plant source for natural antioxidants and nutraceutical or pharmaceutical ingredients due to the abundant existence of phenolic compounds. Previous studies of P. emblica were focused on phenolic identification, methods for the facile and high effective extraction and maintaining strong antioxidant activities from P. emblica fruits have not been reported.
Methods for extraction of phenolic compounds include microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), pressurized liquid extraction (PLE), and supercritical fluid extraction (SFE) from herbal medicine.[Citation4,Citation21,Citation22] Among them, MAE is a relatively simple and efficient alternative to conventional heating, boiling, or refluxing extraction techniques. MAE can offer shorter operation times, reduced both extraction time and solvent consumption, simplified manipulation and lower energy input.[Citation23] It also produces higher extraction rates of the target species and better results with lower costs.[Citation4] The mechanism of microwave-assisted extraction is attributed to heating polar solvents with microwave energy while in contact with solid samples, and to partition interest compounds between the sample and the solvent.[Citation24] Furthermore, the thermal degradation effects using microwave irradiation can be avoided while favoring the rapid desorption from matrices.[Citation25] The efficiency of the MAE extraction is influenced by many factors including duration of microwave irradiation, microwave power, solvent to solid ratio, and their interaction with each other. Thus, optimizing these parameters is required to extract a large amount of quality phenolic compounds.[Citation4,Citation22]
In order to study the potential industrial application of P. emblica as an antioxidant source, an MAE method for extracting polyphenols from P. emblica was developed, and the extraction conditions were optimized according to the enhancement of extraction yield along with maintaining antioxidant capacity by using response surface methodology (RSM). This MAE method was also compared with a conventional method for phenolics extraction. Moreover, the extracts were investigated using high-resolution high-performance liquid chromatography with mass spectrometry detection (HPLC-ESI-TOF-MS) to determine phenolic compositions. In vitro assays were designated to evaluate the antioxidant activity.
Materials and methods
Plant materials
Fruits were harvested from P. emblica trees grown in an orchard in Huian county, Fujian province of China (Southeast of China; north latitude 24°49′-25°15′, east longitude 118°38′-119°05′, altitude 29 m). Harvested fruits were immediately transported to the laboratory at Fujian Agriculture and Forestry University and dried in a forced-air oven at 40°C to constant weight, and then ground using an electric grinder (IKA model-A11, Staufen, Baden-Württemberg, Germany). The ground powders were passed through a standard 425 μm sieve and were collected and stored at 4°C in airtight bags until further use.
Reagents
All chemicals and solvents used in this study were of analytical grade. Gallic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and potassium persulfate were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Na2CO3, FeSO4, Folin–Ciocalteu’s phenol reagent, Na2HPO4, and HCl were purchased from China National Pharmaceutical Group Co. (Beijing, China).
Microwave-assisted extraction of total phenolic content
Phenolic compounds from powders of emblic fruits were extracted using a domestic microwave oven system (2450 MHz, Galanz Model G80F20CN2L-B8, Foshan, Guangdong, China). The apparatus was equipped with a digital control system for microwave power and irradiation time. The microwave oven was modified in order to condense the vapors generated during extraction from the sample. Different concentrations of ethanol in water were used as an efficient and safe solvent for the extraction of phenolic compounds.[Citation4]
The ground emblic fruit sample (2.0 g each) were used to test the following parameters in the microwave oven: extraction time (10–50 s), microwave power (80–720 W), ethanol concentration (10–90%), and liquid-to-solid ratio (10–40 mL/g). The influence of each parameter was analyzed in single-factor experiments. After MAE treatment, each extracted slurry was centrifuged at 8000 rpm for 10 min (5810R, Eppendorf AG., Germany), and supernatant was collected to a volumetric flask. Each trial was carried out five times. All extracts were stored at 4°C until further use.
Conventional solvent extraction of total phenolic content
Phenolic compounds in emblic fruits were also extracted using a conventional solvent extraction method described by Nampoothiri et al.[Citation10] Briefly, 2.000 g of powder was mixed with 70% ethanol (v/v) in a conical centrifugal tube and the mixture was kept at a temperature (27 ± 2°C) with stirring for 8 h to extract phenolic compounds. The extraction process was repeated till the solvent became colorless. After the conventional solvent extraction treatment, the supernatant was collected and stored at 4°C until further use.
Determination of Total Phenolic Content (TPC)
The amount of total phenolics in all extracts was determined by the Folin–Ciocalteu method with slight modifications.[Citation24] The TPC was expressed as mg of gallic acid equivalents (GAE) per gram of powder on dry weight (DW) basis.
Central composite design and optimization
On the basis of the single-factor experimental results, major influence factors and their levels were confirmed. A five-level (−2, −1, 0, +1, and +2) and four-variable (X1, X2, X3, and X4) central composite design (CCD) was performed to obtain a second-order model (EquationEq. 1)(1)
(1) to predict the content of total phenolics.[Citation21]The independent variables and their levels were microwave power (X1, 240–560 W), irradiation time (X2, 20–40 s), ethanol concentration (X3, 60–80% v/v), solvent to solid ratio (X4, 10–30 mL/g).
where Y represents the response function (in this case the TPC yield); βo is a constant coefficient; βі, βіi, βіj are regression coefficients for linear, quadratic, and interactive terms, respectively; and Xi and Xj represent the coded independent variables while k equals to the number of the tested factors (k = 4). All experiments were performed randomly in triplicate. Statistical analysis was performed using Design-Expert 8.0.6 software and fitted to a second-order polynomial regression model containing the coefficient of individual linear, quadratic and interactive terms. An analysis of variance (ANOVA) with 95% confidence level was then carried out for each response variable in order to test the model significance and suitability.
Identification of phenolic compounds by HPLC-ESI-TOF-MS
Separation of phenolic compounds was performed with Agilent 1200 series high-performance liquid chromatography (Agilent Technologies, Palo Alto, CA, USA) consisting of a vacuum degasser, autosampler, and a binary pump equipped with a Phenomenex Luna C18(2) analytical column (4.6 × 250 mm 5μm, Phenomenex Inc., Torrance, CA, USA) based on a method described by Taamalli et al.[Citation25] with some modifications. The mobile phases were water with acetic acid (0.5%) (phase A) and methanol (phase B), and the solvent gradient was changed according to the following conditions: 0–10 min, 5–30%B; 10–60 min, 30–45% B; 60–90 min, 45–60% B; 90–120 min, 60–90% B. The flow rate was set at 0.80 mL/min throughout the gradient. The column temperature was maintained at 25°C, and the injection volume was 10 μL.
To identify phenolic compounds, the HPLC system was coupled to an Agilent 6520 accurate-mass quadrupole time-of-flight (Q-TOF) LC/MS (Agilent Technologies, Santa Clara, CA, USA), electrospray ionization (ESI) was operated in the negative ion mode in the mass range 50 to 1000 (m/z). The optimum values of the ESI-MS parameters were: capillary voltage, +4.5 kV; drying gas temperature, 350°C; drying gas flow, 11.0 L/min; and nebulizing gas pressure, 40psi.
Determination of antioxidant activity
The antioxidative properties of the sample were evaluated by the scavenging activity against DPPH and ABTS radicals and the determination of anti-lipid peroxidation assay and reducing power according to the methods described by Chen et al.[Citation26], Dahmoune et al.[Citation4], and Huang et al.[Citation27], respectively.
Statistical analysis
All the experiments were carried out in triplicate, and the results were expressed as means ± SE (standard error). Statistical analyses were performed using the Statistical Product and Service Solutions version 19.0 software (IBM Corporation, Armonk, NY, USA). When significance occurred, means were separated by Fisher’s Least Significant Difference (LSD) at P< 0.05 or P< 0.01 level.
Results and discussion
Optimizing the TPC yield by microwave-assisted extraction using RSM
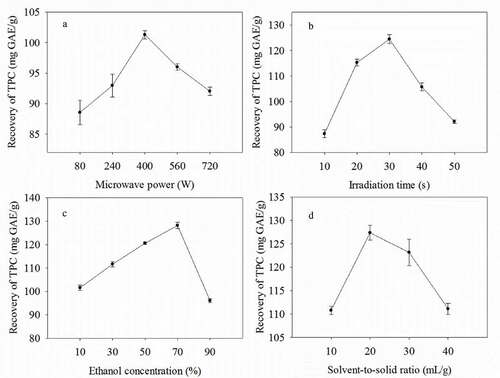
Based on the single-factor experimental results (), major influence factors and their levels were confirmed on the MAE process. The MAE of phenolic compounds from emblic fruits was optimized using response surface methodology (RSM). The experimental design is presented in where there were six replicates.
Table 1. Experimental design with the response surface methodology for total phenolic compounds (TPC) yield extracted from Phyllanthus emblica fruit using microwave-assisted extraction (MAE) method
Figure 1. The effect of microwave power (a), extraction/irradiation time (b), ethanol concentration (c), and liquid-to-solid ratio (d) on the total phenolic yield extracted from Phyllanthus emblica fruits (n = 3)
The regression coefficients of the intercept, linear, quadratic, and interaction terms of the model were calculated using a Design Expert program (version 8.0.6), and the factors and interactions were subjected to analysis of variance (ANOVA) for testing significance. Regression coefficients and ANOVA of the second-order polynomial models for the yield of TPC are summarized in . Two linear parameters, microwave power (X1), and liquid solvent to solid ratio (X4) were significant (P< 0.05), whereas irradiation time (X2) and ethanol concentration (X3) were not significant. All the quadratic parameters were highly significant at P< 0.01 level. The interactions X1X2, X1X3, X1X4,and X3X4 were also significant (P< 0.05). According to the yield of TPC, the model of final predictive equation was found to be as follows (EquationEq. 2(2)
(2) ):
Table 2. Estimated regression coefficients of the quadratic polynomial model and analysis of variance (ANOVA) for the experimental results of total phenolic contents extracted from Phyllanthus emblica fruit using microwave-assisted extraction (MAE) method
The ANOVA in showed that the quadratic polynomial model was highly significant (P< 0.0001) and sufficient to represent the actual relationship between the response and significant parameters. The model also showed statistically insignificant lack of fit at 95% confidence level, which indicated that the fitted models were considered adequate. Furthermore, the determination coefficient (R2) of 0.9352 and the adjusted determination coefficient () of 0.8748 for the model did not differ greatly in a good statistical model.[Citation4,Citation28]The low value of pure error suggesting that the model was reliable and reproducible which agrees with the previous data obtained from the ANOVA. The results indicated that the model should work well for the prediction of TPC yield in this MAE from the fruit of P. emblica.
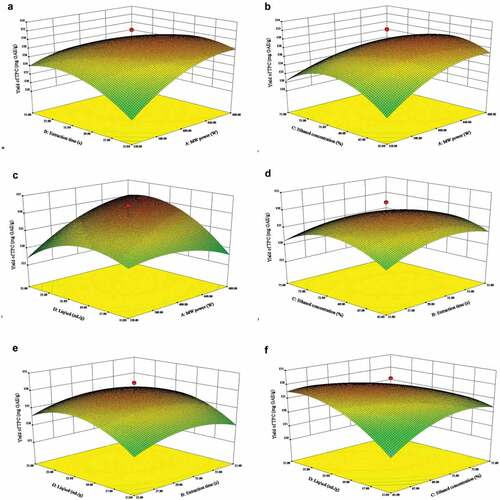
To investigate the interactive effects of the operational parameters and their mutual interaction on TPC yield, this study generated the two- and three-dimensional response surface profiles of multiple non-linear regression models (), respectively. The interactive effects of microwave power (X1) and irradiation time (X2) had a significant influence on the acquired ratio of TPC (P< 0.01). The microwave power (X1) and ethanol concentration (X3) had significant interactive effects (P< 0.05) on the extraction yield of TPC. The interactive effect of microwave power (X1) and liquid-to-solid ratio (X4) had a significant influence on the recovery of TPC (P< 0.01). The extraction yield of TPC gradually increased with the increase of irradiation time up to 32.5 s and ethanol concentration up to 69.5% and decreased slowly after reaching the critical values. When microwave power and ethanol concentration are at a certain value, the extraction of TPC increased with irradiation time increase. The recovery of TPC using microwave energy appears a highly significant function of the synergistic effect of ethanol concentration (X3) and liquid to solid ratio (X4) (P< 0.01).
Figure 2. Response surface plots of the total phenolic yield extracted from Phyllanthus emblica fruits using microwave-assisted extraction as a function of significant interactions among factors: (a) microwave power and extraction/irradiation time; (b) microwave power and ethanol concentration; (c) microwave power and liquid-to-solid ratio; (d) extraction/irradiation time and ethanol concentration; (e) extraction/irradiation time and liquid-to-solid ratio; and (f) ethanol concentration and liquid-to-solid ratio
Using the Design Expert 8.0.6 software, we were able to identify optimal conditions for MAE of TPC from emblic fruit: microwave power, 480 W; irradiation time, 29.06 s; ethanol concentration, 66.06%; and liquid-to-solid ratio, 25mL/g. The predicted extraction yield of TPC was 133.62 mg GAE/g DW at those conditions. To test the reliability of the model, emblic fruit was extracted using the MAE with the established parameters. A mean value of TPC from this experiment was 133.58 ± 15.61 mg GAE/g DW (n = 5), which was in close agreement with the predicted value and were not significantly different (P> 0.05) using a paired t-test. This experiment showed that the RSM model was valid. The confirmation suggested that a high yield of TPC can be extracted from emblic fruit using this model. The TPC yield from this study was considerably higher than it obtained using conventional solvent extractions (92 ± 2.66 mg GAE/g DW).[Citation10]Additionally, this extraction method was simple and fast.
Analysis of emblic fruits extracts by HPLC-ESI-TOF-MS
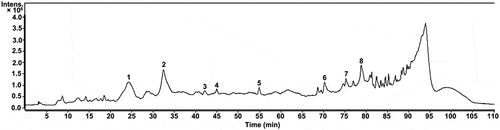
The HPLC has been commonly used for identifying the composition of plant extracts. However, the HPLC method was not satisfactory for complete characterization of compounds with high sensitivity. LC-MS had emerged as a more powerful tool for the determination of many compositions of extracts.[Citation10] The TOF-MS instrumentation had been shown to provide excellent mass resolution and mass accuracy and was the perfect choice for molecular formula determination.[Citation25] Thus, the HPLC-ESI-TOF-MS method was used to analyze the extracts derived from emblic fruits. A total of eight peaks were observed as shown in . The compositions were identified by their retention times and mass spectra as compared with reported data in the literature, which are summarized in . Among them, the peak area of compound 2 was the largest (7.2% of the total), followed by compounds 1 (5.3%), 8 (3.4%), 6 (2.8%), 5 (2.2%), 7 (2.1%), 3 (1.2%), and 4 (1.1%). By analyzing the MS data of the eight compounds, including retention time (TR), experimental m/z, molecular formula, error of the experimental m/z, and the MS2 fragments of the individual compounds, these compounds were successfully identified through reference to the Mass Bank MS database (https://massbank.eu/MassBank/index.html) and available literatures.
Table 3. The HPLC-ESI-TOF-MS identification and characterization of compounds isolated from Phyllanthus emblica fruit extracts by the optimized MAE method
Figure 3. Total ion current chromatogram of an emblic fruits extracts obtained using the optimized MAE method: (1) mucic acid-1,4-lactone-3-O-gallate, (2) hamamelitannin, (3) isocorilagin, (4) ethyl gallate, (5) methyl gallate, (6) ellagic acid, (7) quercetin-3-O-rhamnoside, and (8) an undefined compound
The mass spectra of compound 1 (TR = 24.790 min) showed a [M−H]− at m/z 343 and fragment anions at m/z169 and 191. According to the previous reports (Luo et al.[Citation15] and Zhang et al.[Citation16]), compound 1, producing the anion at m/z169, which was a characteristic fragment of galloyl group formed by the loss of a mucic acid group (191 Da), was identified as mucic acid-1,4-lactone-3-O-gallate.
The mass spectra of compounds 2 (TR = 32.367 min) showed [M−H]− at m/z483, and produced MS2characteristic fragment anions at m/z313, 271, 211, 169, and 125. The ionat m/z313 arises from the loss of a gallate unit (170 Da) from the [M−H]− at m/z483. The fragment ion at m/z271 is explained by the loss of a C2H2O from the latter fragment, while the fragment at m/z211 derives from the loss of C2H4O2 from the fragment at m/z271. The fragment ion at m/z169,typical mass in the negative mode of galloyl group, indicates the loss of a – C2H2Ogroup (m/z42) from the fragment at m/z211, and the fragment ion at m/z169 showed a loss of a carboxyl group (44 Da) producing the fragment ion at m/z125. Thus, the peak was identified as 2ʹ,5ʹ-di-O-galloyl-ᴅ-hamamelose (hamamelitannin), which was previously reported by Touriño et al.[Citation29] To our knowledge, this is the first time that this compound has been identified in P. emblica fruit. Quantification of the hamamelitannin content was calculated to 36 mg/100 ml in P. emblica fruit extracts by HPLC-DAD according to the methods described by Duckstein et al.[Citation30]
Compound 3 (TR = 41.720 min) showed a precursor anion at m/z 633 with four fragment anions at m/z 463, 301, and 169. The anion at m/z169showed the existence of galloyl group in Compound 3. The ion at m/z463 arises from the loss of a gallate unit (170 Da) from the [M−H]− at m/z633. According to Nampoothiriet al.[Citation10], the anion at m/z301 was a characteristic fragment of ellagic acid, produced through the loss of a – C6H10O5 group (m/z162) from the fragment at m/z463. Therefore, the peak was identified as isocorilagin, which was previously reported by Liu et al.[Citation14] and Luo et al.[Citation15]
The mass spectra of compounds 4 (TR = 45.055 min) and 5 (TR = 54.944 min) showed precursor anions ([M−H]−) at m/z 197and 183, and both showed two major fragment anions at m/z124 and 78. Through comparison of the MS2 fragmentation data with other studies (Touriñoet al.[Citation29]; Zhang et al.[Citation31]) and the Mass Bank MS database, compounds 4 and 5 were identified as ethyl gallate and methyl gallate. Of these compounds, ethyl gallate has been identified in leaves of Juglansregia by Zhang et al.[Citation31], and methyl gallate has been identified in leaves of Hamamelis virginiana by Touriñoet al.[Citation29]
The mass spectra of compound 6 (TR = 71.966 min) showed a precursor anion [M−H]− at m/z301 and fragment anions at m/z257(by the loss of one CO2, i.e.,−CO2), and 229 (−CO), respectively. Through comparison of the MS2 fragmentation data from Terminalia bellerica and Emblica officinalis.[Citation10] and the Mass Bank MS database, compounds 6 was identified as ellagic acid.
The mass spectra of compound 7 (TR = 75.742 min) showed a [M−H]− at m/z447with fragment anions (301, 271, and 151 m/z). Based on the Retro–Diels–Alder reaction (Chang and Wu[Citation32]), the fragment at m/z 151 which corresponds to the fragment of flavone aglycones, resulted from the separated heterocyclic ring (ring C) at positions C-2 and C-4. According to the previous reports (Taamalli et al.[Citation25]; Huang et al.[Citation33]; Yamazaki et al.[Citation34]), quercetin showing a [M−H]− at m/z301 produced a fragment of a −C7H3O4 group at m/z 151 through the loss of a −C8H6O3group via the Retro-Diels-Alder reaction, and the ion at m/z301 is diagnostic of quercetin derivatives resulting from the loss of a rhamnoside unit (m/z146) from the [M−H]− at m/z483.Therefore, compound 7 was identified as quercetin-3-O-rhamnoside.
The mass spectra of compounds 8 (TR = 78.86 min) exhibited precursor anion ([M−H]−) at m/z233 and fragment anions at m/z217, 163, and 75. The molecular formula was established to be C15H22O2 from EI-MS based on mass spectra [M+m/z234] data. The fragment ion at m/z217 is explained by the loss of a hydroxyl unit from the [M−H]− at m/z233. However, due to lack of the effective information in MS2 fragments and too many isomers of molecular formula C15H22O2, the compound in peak 8 was undefined.
We identified seven phenolic compounds in P. emblica fruit, such as mucic acid-1,4-lactone-3-O-gallate, hamamelitannin, isocorilagin, ethyl gallate, methyl gallate, ellagic acid, quercetin-3-O-rhamnoside. One of the original contributions of our work to the emblic fruits extraction is the identification of hamamelitannin, which was isolated from Hamamelis virginiana[Citation29] but not reported in the fruit of P.emblica.
Determination of antioxidant capacity
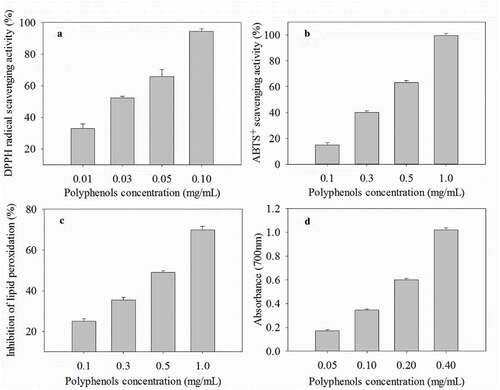
Different in vitro antioxidant methods had been used to analyzing antioxidant activities of plant extracts.[Citation27] The polyphenols extracted by the optimized MAE method exhibited strong dose-dependent scavenging activities based on the assay methods of DPPH radical (R2 = 0.99), ABTS radical cation (R2 = 0.98), its inhibition of lipid peroxidation capacity (R2 = 0.99), and reducing property (R2 = 0.99) (). Furthermore, the extract had antioxidant activity greater than either the control BHT or ascorbic acid (). Our result demonstrated that the emblic fruit extracts had strong antioxidant activity.
Table 4. The half maximal inhibitory concentration (IC50, mg/mL) of sample, BHT, and ascorbic acid in antioxidant activities assays
Figure 4. Antioxidant activities of polyphenols of Phyllanthus emblica fruit extracts isolated using the optimized microwave-assisted extraction method. (a) DPPH radical scavenging activity. (b) ABTS+ scavenging activity. (c) Inhibition of lipid peroxidation. (d) Reducing power. Data are represented as means ± SE (n = 3)
The HPLC-ESI-TOF-MS analysis of ethanolic extracts identified eight compounds as the main phenolics. The high antioxidant activity of these phenolics is likely related to their high degree of hydroxylation of aromatic rings, the arrangement of the hydroxyl group, as well as the number of ortho-hydroxyl groups and galloyl group, on benzene nucleus structure. More importantly, we identified hamamelitannin as a major compound in emblic fruit. It showed strong free radical scavengers against ABTS, DPPH and electron transfer, which could be due to the fact that there are seven hydroxyl groups bonded to the aromatic ring.[Citation29] The structure of phenolics possesses a number of hydroxyl groups which can lose a protonated hydrogen and then form strong coordination oxygen ion complexes with metal ion.
Additionally, these phenolics may be able to directly trap free radicals and/or act through metal chelation.[Citation17] It was reported that mucic acid-1,4-lactone-3-O-gallate, ellagic acid, and isocorilagin in methanolic extracts of emblic fruit could chelate ferrous ion and inhibit linoleic acid peroxidation.[Citation17] Furthermore, ethyl gallate,[Citation31] methyl gallate[Citation29] and quercetin-3-O-rhamnoside[Citation34] were reported to be responsible for the antioxidant activity of several extracts. The ortho-hydroxyl structures could also increase the antioxidant activity of phenolics because the ortho-quinone was easily formed.[Citation31,Citation35] As a result, the remarkable antioxidant activity of ethanolic extracts of P. emblica could be attributed to the presence of the high percentage of phenolic compounds, such as hamamelitannin, mucic acid-1,4-lactone-3-O-gallate, and ellagic acid, which have a large number of hydroxyl groups presented in their aromatic rings and ortho-hydroxyl structures of these phenolic compounds as well as possible synergistic effects of these compounds.
Conclusion
This study established an MAE method for extracting polyphenols with high antioxidant activities from P. emblica fruit using RSM. An optimal polyphenol yield of 133.58 mg GAE/g DW was achieved under the following optimum operating conditions: microwave power of 480 W, irradiation time of 29 s, ethanol concentration of 66%, and liquid-to-solid ratio of 25mL/g. HPLC-ESI-TOF-MS analysis identified eight phenolic compounds including hamamelitannin, which was a prominent compound that has not been reported before in emblic fruits. Moreover, the extract had significantly high antioxidant activities. The presence of hydroxylation and ortho-hydroxyl groups in their aromatic rings of phenolic compounds from emblic fruits were the most important factor contributing to antioxidant activities, nevertheless, the further study to identify the phenolic compounds from P. emblica and elucidate their antioxidant mechanisms are in progress. Results from this study showed that the optimized MAE method is simple and easy for use to effectively extract antioxidant phenolic compounds from emblic fruits. The information will contribute to the future application of P. emblica as raw materials in health-care food industry.
Additional information
Funding
References
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of Ultrasonic-Assisted Extraction of Pomegranate (Punica Granatum L.) Peel Antioxidants by Response Surface Methodology. Sep. Purif. Technol. 2012, 98, 16–23. DOI: 10.1016/j.seppur.2012.06.038.
- Samaram, S.; Mirhosseini, H.; Tan, C. P.; Ghazali, H. M.; Bordbar, S.; Serjouie, A. Optimisation of Ultrasound-Assisted Extraction of Oil from Papaya Seed by Response Surface Methodology: Oil Recovery, Radical Scavenging Antioxidant Activity, and Oxidation Stability. Food Chem. 2015, 172, 7–17. DOI: 10.1016/j.foodchem.2014.08.068.
- Carocho, M.; Ferreira, I. C. F. R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. DOI: 10.1016/j.fct.2012.09.021.
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of Microwave-Assisted Extraction of Polyphenols from Myrtus Communis L. Leaves. Food Chem. 2015, 166, 585–595. DOI: 10.1016/j.foodchem.2014.06.066.
- Da Silva, B. V.; Barreira, J. C. M.; Oliveira, M. B. P. Natural Phytochemicals and Probiotics as Bioactive Ingredients for Functional Foods: Extraction, Biochemistry and Protected-Delivery Technologies. Trends Food Sci. Technol. 2016, 50, 144–158. DOI: 10.1016/j.tifs.2015.12.007.
- Kim, H. Y.; Okubo, T.; Juneja, L. R.; Yokozawa, T. The Protective Role of Amla (Emblica Officinalis Gaertn.) Against Fructose-Induced Metabolic Syndrome in a Rat Model. Br. J. Nutr. 2010, 103, 502–512. DOI: 10.1017/S0007114509991978.
- Mishra, P.; Mishra, S.; Mahanta, C. L. Effect of Maltodextrin Concentration and Inlet Temperature during Spray Drying on Physicochemical and Antioxidant Properties of Amla (Emblica Officinalis) Juice Powder. Food Bioprod. Process. 2014, 92, 252–258. DOI: 10.1016/j.fbp.2013.08.003.
- Liu, X.; Zhao, M.; Wang, J.; Yang, B.; Jiang, Y. Antioxidant Activity of Methanolic Extract of Emblica Fruit (Phyllanthus Emblica L.) From Six Regions in China. J. Food Compost. Anal. 2008, 21, 219–228. DOI: 10.1016/j.jfca.2007.10.001.
- Liu, X.; Zhao, M.; Wu, K.; Chai, X.; Yu, H.; Tao, Z.; Wang, J. Immunomodulatory and Anticancer Activities of Phenolics from Emblica Fruit (Phyllanthus Emblica L.). Food Chem. 2012, 131, 685–690. DOI: 10.1016/j.foodchem.2011.09.063.
- Nampoothiri, S. V.; Prathapan, A.; Cherian, O. L.; Raghu, K. G.; Venugopalan, V. V.; Sundaresan, A. In Vitro Antioxidant and Inhibitory Potential of Terminalia Bellerica and Emblica Officinalis Fruits against LDL Oxidation and Key Enzymes Linked to Type 2 Diabetes. Food Chem. Toxicol. 2011, 49, 125–131. DOI: 10.1016/j.fct.2010.10.006.
- Liu, Q.; Wang, Y. F.; Chen, R. J.; Zhang, M. Y.; Wang, Y. F.; Yang, C. R.; Zhang, Y. J. Anti-Coxsackie Virus B3 Norsesquiterpenoids from the Roots of Phyllanthus Emblica. J. Nat. Prod. 2009, 72, 969–972. DOI: 10.1021/np800792d.
- Lv, J. J.; Wang, Y. F.; Zhang, J. M.; Yu, S.; Wang, D.; Zhu, H. T.; Cheng, R. R.; Yang, C. R.; Xu, M.; Zhang, Y. J. Anti-Hepatitis B Virus Activities and Absolute Configurations of Sesquiterpenoid Glycosides from Phyllanthus Emblica. Org. Biomol. Chem. 2014, 12, 8764–8774. DOI: 10.1039/C4OB01196A.
- Luo, W.; Zhao, M.; Yang, B.; Shen, G.; Rao, G. Identification of Bioactive Compounds in Phyllenthus Emblica L. Fruit and Their Free Radical Scavenging Activities. Food Chem. 2009, 114, 499–504. DOI: 10.1016/j.foodchem.2008.09.077.
- Liu, X.; Cui, C.; Zhao, M.; Wang, J.; Luo, W.; Yang, B.; Jiang, Y. Identification of Phenolics in the Fruit of Emblica (Phyllanthus Emblica L.) And Their Antioxidant Activities. Food Chem. 2008, 109, 909–915. DOI: 10.1016/j.foodchem.2008.01.071.
- Luo, W.; Wen, L.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Structural Identification of Isomallotusinin and Other Phenolics in Phyllanthus Emblica L. Fruit Hull. Food Chem. 2012, 132, 1527–1533. DOI: 10.1016/j.foodchem.2011.11.146.
- Zhang, Y. J.; Tanaka, T.; Yang, C. R.; Kouno, I. New Phenolic Constituents from the Fruit Juice of Phyllanthus Emblica. Chem. Pharm. Bull. 2001, 49, 537–540.
- Luo, W.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Antioxidant and Antiproliferative Capacities of Phenolics Purified from Phyllanthus Emblica L. Fruit. Food Chem. 2011, 126, 277–282. DOI: 10.1016/j.foodchem.2010.11.018.
- Yang, B.; Kortesniemi, M.; Liu, P.; Karonen, M.; Salminen, J.-P. Analysis of Hydrolyzable Tannins and Other Phenolic Compounds in Emblic Leafflower (Phyllanthus Emblica L.) Fruits by High Performance Liquid Chromatography–Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 8672–8683. DOI: 10.1021/jf302925v.
- Zhang, Y. J.; Nagao, T.; Tanaka, T.; Yang, C. R.; Okabe, H.; Kouno, I. Antiproliferative Activity of the Main Constituents from Phyllanthus Emblica. Biol. Pharm. Bull. 2004, 27, 251–255.
- Li, Y.; Chen, J.; Cao, L.; Li, L.; Wang, F.; Liao, Z.; Chen, J.; Wu, S.; Zhang, L. Characterization of a Novel Polysaccharide Isolated from Phyllanthus Emblica L. And Analysis of Its Antioxidant Activities. J. Food Sci. Technol. (Mysore). 2018, 55, 1–7.
- Şahin, S.; Aybastıer, Ö.; Işık, E. Optimisation of Ultrasonic-Assisted Extraction of Antioxidant Compounds from Artemisia Absinthium Using Response Surface Methodology. Food Chem. 2013, 141, 1361–1368. DOI: 10.1016/j.foodchem.2013.04.003.
- Hammi, K. M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of Ultrasound-Assisted Extraction of Antioxidant Compounds from Tunisian Zizyphus Lotus Fruits Using Response Surface Methodology. Food Chem. 2015, 184, 80–89. DOI: 10.1016/j.foodchem.2015.03.047.
- Yang, Y.; Li, J.; Zu, Y.; Fu, Y.; Luo, M.; Wu, N.; Liu, X. Optimisation of Microwave-Assisted Enzymatic Extraction of Corilagin and Geraniin from Geranium Sibiricum Linne and Evaluation of Antioxidant Activity. Food Chem. 2010, 122, 373–380. DOI: 10.1016/j.foodchem.2010.02.061.
- Pérez-Serradilla, J. A.; de Castro, M. D. Luque Microwave-Assisted Extraction of Phenolic Compounds from Wine Lees and Spray-Drying of the Extract. Food Chem. 2011, 124, 1652–1659. DOI: 10.1016/j.foodchem.2010.07.046.
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of Microwave-Assisted Extraction for the Characterization of Olive Leaf Phenolic Compounds by Using HPLC-ESI-TOF-MS/IT-MS2. J. Agric. Food Chem. 2012, 60, 791–798. DOI: 10.1021/jf204233u.
- Chen, J.; Wu, S.; Li, Y. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oil from Leaves of Alpinia Zerumbet ‘Variegata’. Res. J. Biotechnol. 2014, 9, 50–57.
- Huang, W.; Xue, A.; Niu, H.; Jia, Z.; Wang, J. Optimised Ultrasonic-Assisted Extraction of Flavonoids from Folium Eucommiae and Evaluation of Antioxidant Activity in Multi-Test Systems in Vitro. Food Chem. 2009, 114, 1147–1154. DOI: 10.1016/j.foodchem.2008.10.079.
- Karazhiyan, H.; Razavi, S. M. A.; Phillips, G. O. Extraction Optimization of a Hydrocolloid Extract from Cress Seed (Lepidium Sativum) Using Response Surface Methodology. Food Hydrocolloids. 2011, 25, 915–920. DOI: 10.1016/j.foodhyd.2010.08.022.
- Touriño, S.; Lizárraga, D.; Carreras, A.; Lorenzo, S.; Ugartondo, V.; Mitjans, M.; Vinardell, M. P.; Juliá, L.; Cascante, M.; Torres, J. L. Highly Galloylated Tannin Fractions from Witch Hazel (Hamamelis Virginiana) Bark: Electron Transfer Capacity, in Vitro Antioxidant Activity, and Effects on Skin-Related Cells. Chem. Res. Toxicol. 2008, 21, 696–704. DOI: 10.1021/tx700425n.
- Duckstein, S. M.; Stintzing, F. C. Investigation on the Phenolic Constituents in Hamamelis Virginiana Leaves by HPLC-DAD and LC-MS/MS. Anal. Bioanal. Chem. 2011, 401, 677–688. DOI: 10.1007/s00216-011-5111-3.
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant Phenolic Compounds from Walnut Kernels (Juglans Regia L.). Food Chem. 2009, 113, 160–165. DOI: 10.1016/j.foodchem.2008.07.061.
- Chang, C.-L.; Wu, R.-T. Quantification of (+)-Catechin and (−)-Epicatechin in Coconut Water by LC–MS. Food Chem. 2011, 126, 710–717. DOI: 10.1016/j.foodchem.2010.11.034.
- Huang, H.; Sun, L.; Yang, L.; Zhou, J.; Yin, P.; Li, K.; Xue, Q.; Li, X.; Liu, Y. Assessment of the Bioactive Phenolic Composition of Acer Truncatum Seed Coat as a Byproduct of Seed Oil. Ind. Crops Prod. 2018, 118, 11–19. DOI: 10.1016/j.indcrop.2018.03.030.
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant Activity of Japanese Pepper (Zanthoxylum Piperitum DC.) Fruit. Food Chem. 2007, 100, 171–177. DOI: 10.1016/j.foodchem.2005.09.036.
- Choi, J. S.; Chung, H. Y.; Kang, S. S.; Jung, M. J.; Kim, J. W.; No, J. K.; Jung, H. A. The Structure–Activity Relationship of Flavonoids as Scavengers of Peroxynitrite. Phytotherapy Res. 2002, 16, 232–235. DOI: 10.1002/ptr.828.
